Cytotoxic T lymphocyte response induced by an improved synthetic lipopeptide vaccine against cervical cancer
Introduction
Cervical cancer is one of most common cancers in women. Human papillomaviruses (HPV), particularly HPV-16, are associated with most cervical cancers[1,2]. HPV oncogenic proteins, E6 and E7, are expressed in most HPV-containing cervical cancers and are constitutively expressed in cervical carcinoma cells. They are the only viral proteins whose expression is required for the maintenance of the transformed state[3–5]. Compared with E6, E7 has stronger immunogenicity[6], therefore, E7 is an attractive target for the specific immunotherapy of cervical carcinoma.
Cytotoxic T lymphocyte (CTL) response is an important defense mechanism against tumors[7,8]. CTL recognize immunogenic peptides derived from cellular proteins presented to the cell surface in the content of MHC class I molecules. Many studies demonstrate that immunization with antigenic peptides alone is not sufficient to elicit an efficient CTL response[9]. Studies have shown that helper T lymphocyte (Th) immunity is also considered vital for the CTL induction[10–13]. Lipopeptide formulations have been successfully used to induce CTL responses and have recently gained considerable interest and are a promising vaccine candidate[14–16]. Moreover, the peptide of the antigen HPV E786–93 is restricted to human leukocyte antigen (HLA)-A*2010, the most common HLA class I molecule, which makes it a good candidate for vaccination[17]. The TT830–843 peptide, derived from tetanus toxoid, was chosen as a source of T cell help because of its promiscuous binding to human MHC class II antigens and its promiscuous recognition by T cells[18]. A dipalmitic lipopeptide could induce epitope-specific CTL in phase I clinical studies[19,20], but it is difficult to be synthesized and purified. So we constructed a monopalmitic lipopeptide (Table 1).
Full table
We evaluated the ability of the lipopeptide to provoke in vivo- and in vitro-specific CTL responses in HLA-A*0201Kb transgenic (Tg) mice and peripheral blood mononuclear cells (PBMC) preparations from MHC-matched healthy donors. We also studied the presentation of peptides in T2 cells and dendritic cell (DC) maturation induced by peptides.
Our findings show that the lipopeptide can induce a strong CTL response, the mechanisms of which are associated with an increase in the stability and persistence of the antigenic complex formed with the lipopeptide and in the production of interleukin (IL)-12 by DC. These findings suggest that the lipopeptide against cervical cancer can be considered as a more effective vaccine type for a human.
Materials and methods
Peptide synthesis and characterization The HLA-A*0201-restricted the CTL epitope derived from the HPV oncogenic protein, E7, referred to here as the P3 peptide, was used in this study (Table 1)[21]. The lipopeptide construct is referred to here as the P1 lipopeptide. An unlipidated version denoted P2 was used as the control peptide (Table 1).
All peptides were synthesized on an ABI 431A instrument using standard Fmoc technology (PE Applied Biosystems, Foster city, CA, USA)[22]. The solid support was Wang resin (Sigma, St Louis, MO, USA). The Fmoc protecting the group was removed by 20% peperidine in N-methylpyrrolidone (NMP). To synthesize the lipopeptide, a Boc-Lys-(Fmoc)-OH group was incorporated at the N-terminal extremity, which allowed the selective removal of the Fmoc group by 20% peperidine in NMP. Then the cold peptide-resin was placed into the cold cleavage mixture (0.25 mL ethanedithiol, 0.25 mL water, 9.5 mL trifluoroacetic acid). The reaction mixture was stirred at room temperature for 1.5 h. Then these peptides were removed from the resin support and precipitated by adding cold diethyl ether and lyophilized. These crude peptides were purified by RP-HPLC. All peptides were checked for homogeneity by analytical RP-HPLC and for identity by an electrospray mass spectrometer. The purity was >85% for all peptides. The peptides were dissolved in DMSO at a concentration of 20 g/L and stored at -20 °C.
Cells lines Human transporter associated with antigen processing (TAP)-deficient T2, cervical carcinoma Caski cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in culture according to the supplier’s specifications. A stable transfectant of the jurkat human cell line that expresses the chimeric class I molecule, A*0201Kb, was kindly provided by Dr Jehad CHARO (Max Delbruck Center for Molecular Medicine, Berlin, Germany). The cell lines were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA) with glutamax containing 10% fetal calf serum (FCS), antibiotics, N-2-hydroxyethyl-piperazine-N-2-ethanesulfonic acid (HEPES), and 0.4 g/L G418 (Gibco, USA).
Animal immunization procedures and generation of CTL HLA-A*0201Kb Tg mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mice were bred and maintained in specific, pathogen-free facilities. Groups of 8-12 week-old HLA-A*0201Kb female Tg mice (5 mice per group) were immunized with synthetic peptide +/– incomplete Freund’s adjuvant (IFA). Synthetic peptides were injected with 50 nmol per mice at the base of the tail for subcutaneous route. Transgenic mice were boosted 2 weeks later with the same synthetic peptide. After 14 d, the mice were killed and the spleens were aseptically removed. Splenic single cell suspensions were produced by teasing the organs through a sterile nylon mesh as previously described[21]. The splenic suspensions (3×106) from the immunized mice were cocultured with 1 µg/mL P3 peptide in 5% CO2 at 37 °C. Cytotoxic effector populations were harvested after 5 d of in vitro culture.
Generation of CTL in healthy donors The CTL induction in vitro was performed in accordance with the previously described procedures[23,24]. In brief, the PBMC of 3 HLA-A*0201 healthy donors from the blood bank of the Third Military Medical University (Chongqing, China) were first obtained with centrifugation at a Ficoll-Paque density gradient and then cultured in RPMI-1640 medium supplemented with 10% FCS, 100 U/mL penicillin, and 0.1 g/L streptomycin. These cells were pulsed by the synthetic peptides, respectively, at a final concentration of 10 µmol/L. The PBMC were restimulated every 7 d with fresh medium containing these peptides. Recombinant human interleukin (IL)-2 (Sigma, USA) at a concentration of 30 IU/mL was added to the culture medium on d 1 after every stimulation. On d 6 after the third round of restimulation, the cells were harvested and tested by cytotoxicity assay.
Cytotoxicity assay The cytotoxic activity of the cell culture was determined by a standard 4 h chromium release assay[25]. To measure the peptide-specific responses, T2 cells and jurkat A*0201Kb cells were loaded with 10 μmol/L peptide at 37 °C for 1 h. Peptide-plused T2 cells, jurkat A*0201Kb cells, and the Caski cell line were labeled with 3.7 MBq Na251CrO4 for 1.5 h at 37 °C. Target cells (104/ well) were transferred into a round-bottomed, 96-well plate. Varying numbers of CTL were added to give a final volume of 200 µL and incubated for 4 h at 37 °C. At the end of the assay, the supernatant was harvested and counted. The percentage of specific lysis was calculated as follow: (experimental release -spontaneous release)/(maximal release-spontaneous release)×100. Spontaneous and maximal release were determined in the presence of medium or 1% Triton X-100, respectively.
Cell isolation and generation of DC from PBMC The generation of DC from PBMC was performed as described previously[26]. In brief, peripheral blood was collected from health volunteers (n=3) and fractionated over Ficoll-Paque by a standard procedure. To derive DC, PBMC were seeded (2×106/mL) into 6-well plates in RPMI-1640 medium supplemented with 100 units/mL penicillin and 0.1 g/L streptomycin. After 2 h of incubation at 37 °C, nonadherent cells were removed and the adherent cells were cultured in RPMI-1640 medium supplemented with 10% FCS, 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sigma, USA), 500 U/mL IL-4 (Sigma, USA), 100 U/mL penicillin, and 0.1 g/L streptomycin for 4 d at 37 °C. Immature DC were incubated in vitro for 48 h with P1, P2, or P3 peptides. Medium-treated, immature DC and tumor necrosis factor (TNF)-α-stimulated DC were included as negative and positive controls, respectively. The phenotype of DC was analyzed by flow cytometry.
Interferon (IFN)-γ and IL-12 detection IFN-γ secretion in stimulated in vitro (IVS) culture supernatants and IL-12 in immature DC cultures were measured by a commercially available, two-site sandwich ELISA (Diaclone, Besancon, France) according to the manufacturer’s instructions. Collection of the supernatant was performed at 72 h after IVS. DC were pulsed with P1, P2, or P3 peptides and incubated with autologous PBMC in RPMI 1640 medium. After 48 h of culture, IL-12 concentrations in the supernatant were determined. Recombinant IFN-γ and IL-12 were used for the preparation of a standard curve. Each sample was tested in duplicate.
Peptide-binding assay To determine whether synthetic peptides could bind to HLA-A*0201 molecules, peptide-induced HLA-A*0201 upregulation in T2 cells was examined according to a protocol described previously[27]. Briefly, T2 cells were incubated with 10 µmol/L peptides and 5 µg/mL human β2-microglobulin (Sigma, USA) in serum-free Iscove’s Modified Dulbecco’s medium for 18 h at 37 °C. The expression of HLA-A*0201 on T2 cells was then determined using anti-HLA-A*0201 mAb B B7.2 followed by a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Becton Dickinson, Mountain View, CA, USA). The samples were analyzed on a FACScan Calibur (Becton Dickinson, USA).
Stability of HLA-A*0201-peptide complexes on cell surface[22] The T2 cells were incubated with 10 µmol/L peptides as described for the peptide-binding assay. After 18 h incubation at 37 °C, the T2 cells were washed. At the indicated times, they were stained with mAb B B7.2 and analyzed by flow cytometry.
Flow cytometry Standard flow cytometric analysis was used to assess surface expression of various markers using the following mAb directly conjugated with either phycoerythrin (PE) or FITC: FITC-CD40, FITC-CD80, FITC-CD83, and PE-HLA-DR (Pharmingen, San Diego, CA, USA). IgG isotype-matched control antibodies were used in all of the experiments. After staining, the cells were washed and fixed in 1% paraformaldehyde before analysis on a FACScan Calibur (Becton Dickinson, USA).
Statistical analysis The Student’s t-test was performed to evaluate the significance of the results. P values less than or equal to 0.05 were considered significant.
Results
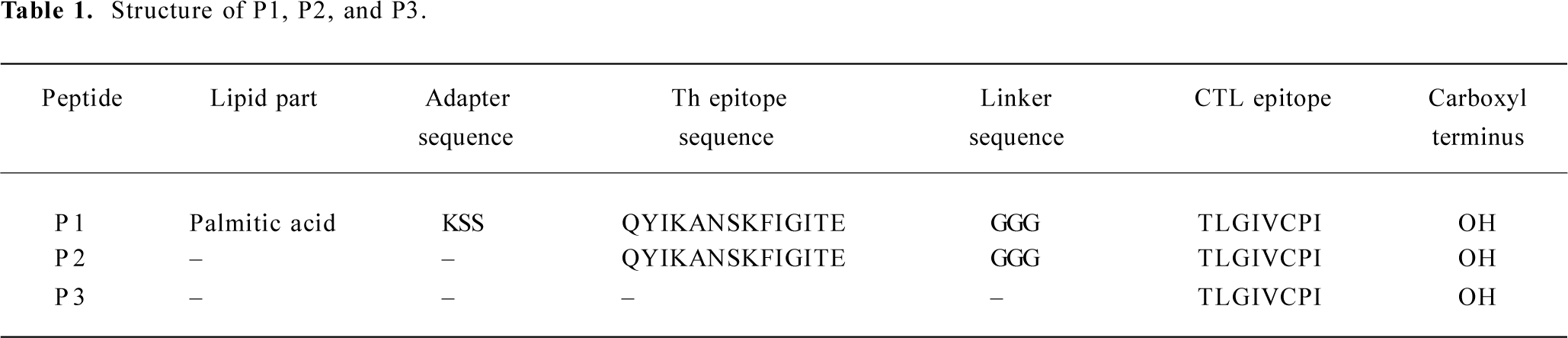
Immunological effect in transgenic mice after vaccination with peptides To assess their in vivo immunogenicity, HLA-A*0201Kb Tg mice were immunized with each of the peptide in IFA. Splenocytes from immunized mice were restimulated in vitro and tested for IFN-γ production and CTL reactivity. CTL from P1-immunized mice showed strong IFN-γ production, an indicator of CTL reactivity. CTL from P1-immunized mice efficiently lysed jurkat A*0201Kb cells added with the E786–93 peptide, but did not lyse jurkat A*0201Kb cells alone. These results indicated that the cytotoxicity of induced CTL was peptide specific. CTL from P2-immunized mice also showed modest IFN-γ production and cytotoxicity. In contrast, immunization with the P3 peptide in IFA was shown to have no specific reactivity (Figure 1).
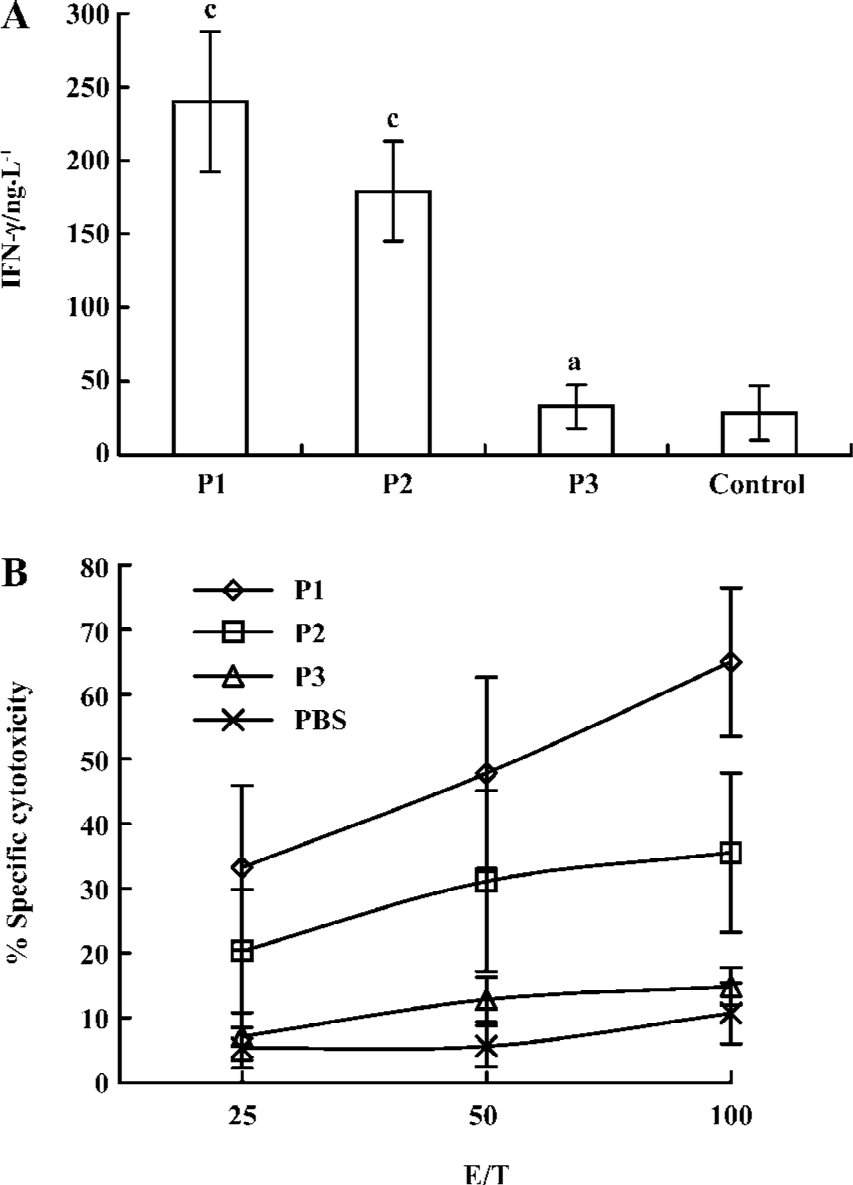
Induction of CTL by the lipopeptide without an adjuvant To further explore the potential of the lipopeptide for human vaccination, we investigated whether the lipid part of the lipopeptide construct could bypass the requirement for IFA. Separate groups of Tg mice were immunized either with the lipopeptide or with an equimolar amount of the unlipidated version. Peptide-specific CTL responses were examined in the spleens of Tg mice. CTL from lipopeptide-immunized mice efficiently lysed jurkat A*0201Kb added with the E786–93 peptide, but not the unlipidated analogue P2. These data indicated that the lipopeptide could efficiently induce specific CTL responses in vivo without the need for an adjuvant (Figure 2).
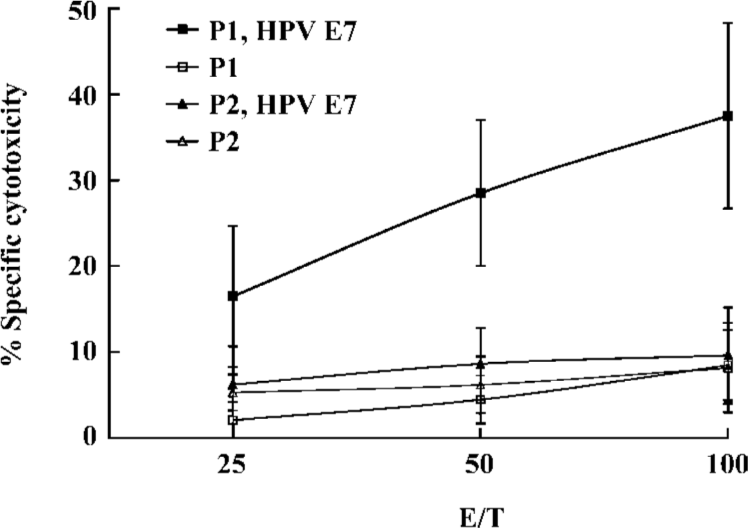
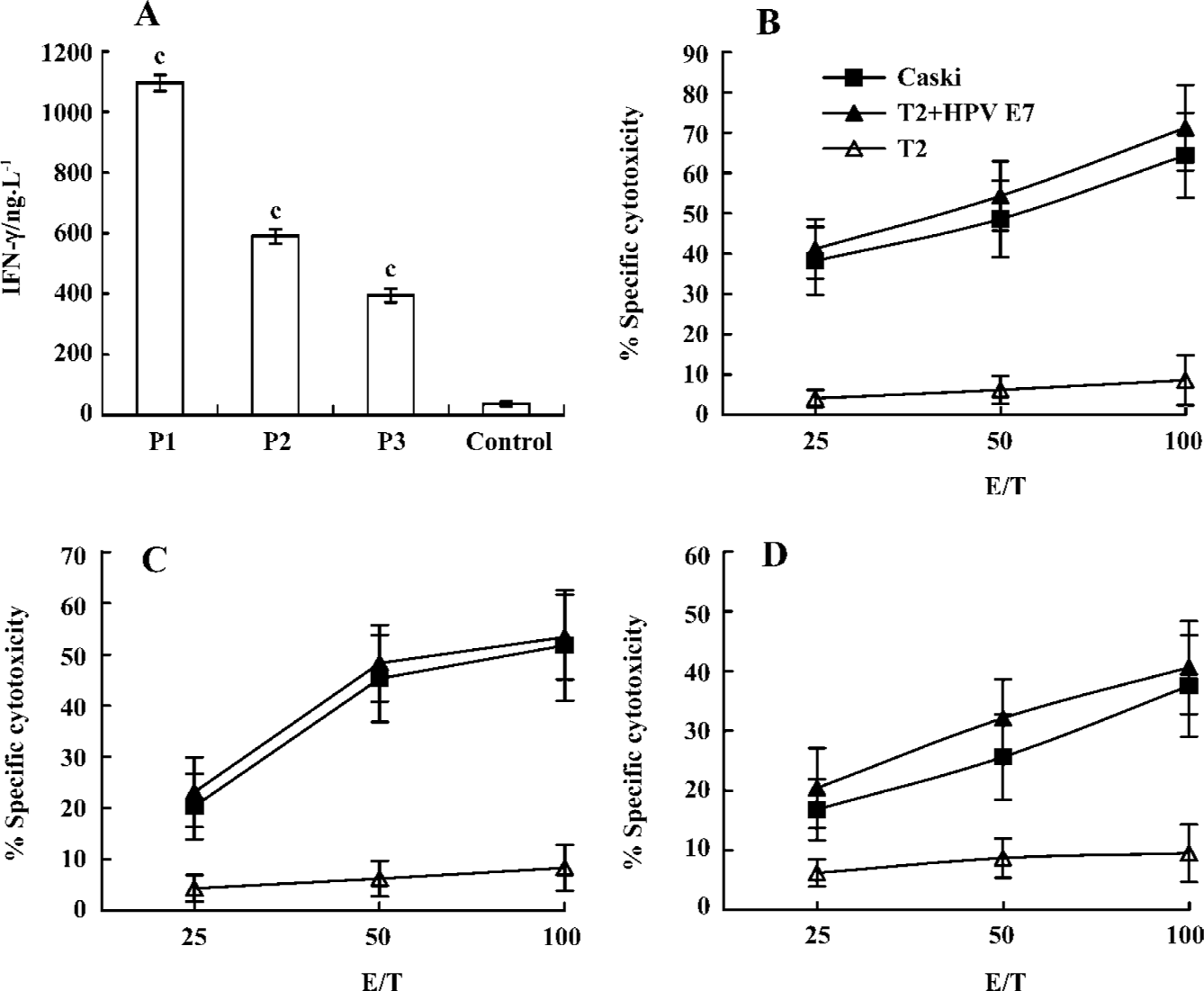
In vitro generation of peptide-specific CTL from healthy human donors To investigate the capacity of peptides to mobilize a human CTL repertoire, specific CTL were generated in vitro using healthy HLA-A*0201+donor PBMC with peptides. As shown in Figure 3, CTL induced with P1, P2, and P3 peptides lysed T2 cells pulsed with E786–93 and produced strong IFN-γ, but did not lyse T2 cells alone. Moreover, CTL induced by the P1 peptide elicited the strongest cytotoxic activity. To analyze the ability of specific CTL to lyse the endogenously HPV E7-expressing tumor cell, the HPV E7-positive HLA-A*0201-expressing cervical cancer cell line, Caski, was used as the target cell in a standard 51Cr-release assay. As shown in Figure 3, CTL were able to efficiently lyse Caski tumor cells (Figure 3).
Peptide-binding affinity to HLA-A*0201 molecules Peptide-binding affinity to HLA-A*0201 molecules was analyzed by a binding assay on the TAP-deficient, human T2 cell line. The stabilization assay was based on the fact that in T2 cells, a large proportion of MHC class I molecules are devoid of endogenous peptides and are unstable at 37 °C. This could be prevented by the addition of exogenous peptides that bind within the MHC class I Ag-presenting groove, stabilizing the 3-D structure of the complex. According to our results, stabilization of MHC class I on T2 cells could be obtained with the lipopeptide (Figure 4).
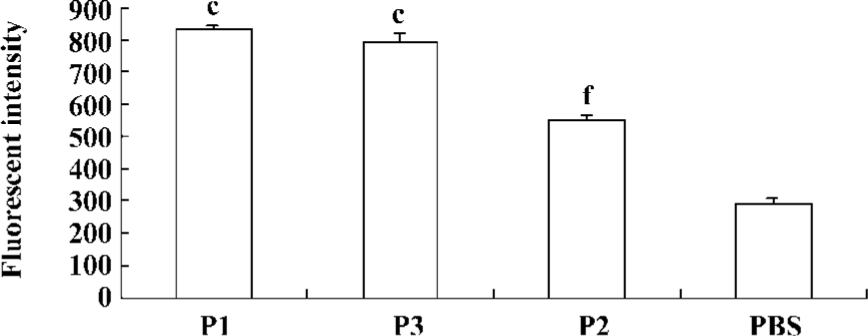
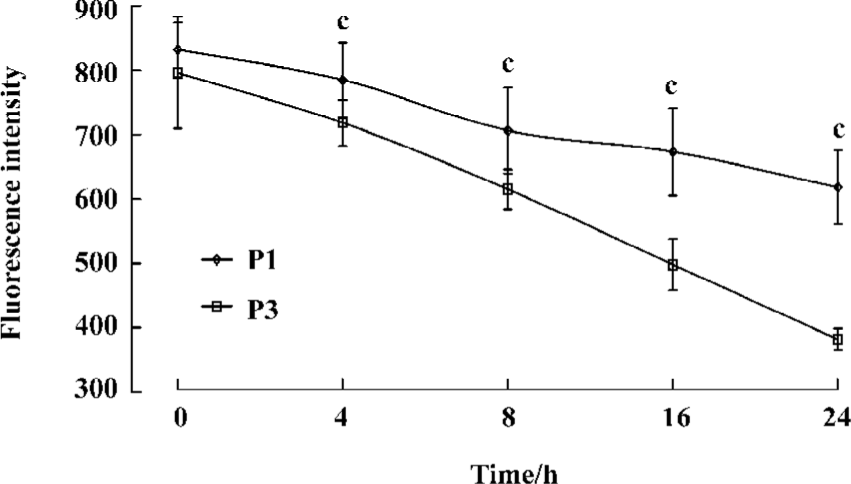
Complex stability assay The kinetics of complex dissociation was evaluated by measuring the time necessary for the complex to disappear from the cell surface. Our observations revealed an increase in the persistence of the antigenic complex formed with the P1 peptide (Figure 5).
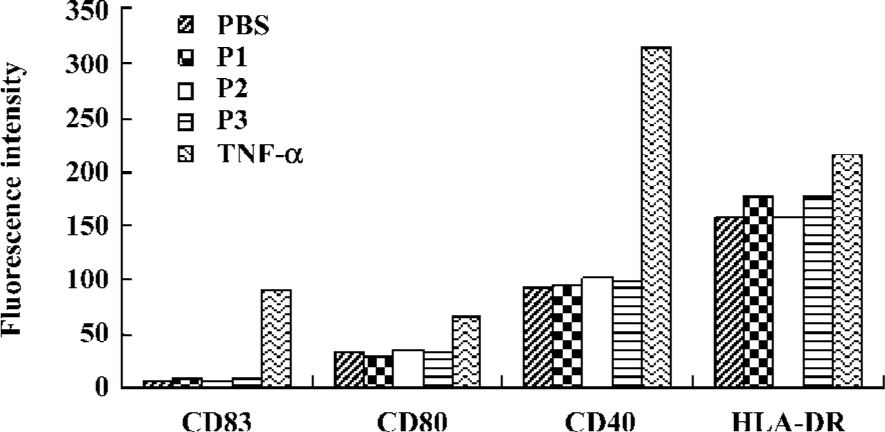
DC maturation induced by peptides To check whether the addition of peptides induce DC maturation, the DC were incubated further for 48 h with or without peptides. TNF-α was used as a maturation control. The mean surface fluorescence intensity of molecules involved in the antigen presentation and in costimulation was not increased, and CD83 expression was not induced by the peptides (Figure 6).
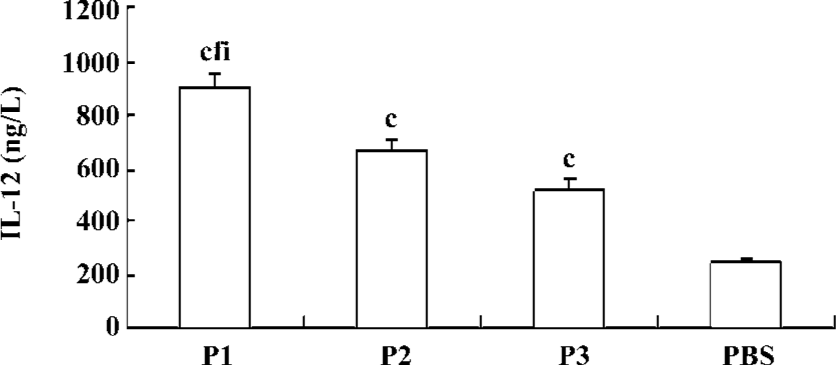
IL-12 secretion of DC induced by peptides To check whether the exposure of DC to peptides increases IL-12 secretion, the DC were pulsed with P1, P2, or P3 peptides and incubated with autologous PBMC in RPMI 1640 medium. After 48 h of culture, IL-12 concentrations in the supernatant were determined. There was an upregulation of production of IL-12 following incubation of DC with P1, P2, or P3 peptides, and the effect of the P1 peptide was the strongest (Figure 7).
Discussion
Traditional immunization includes vaccines that use live attenuated organisms, inactivated organisms, whole proteins, and naked DNA. From a practical and safe standpoint, however, live, attenuated vaccines raise issues related to manufacturing and safety that may preclude their widespread use. Peptide-based approaches offer several potential advantages over conventional whole proteins or naked DNA in terms of purity, lot-to-lot consistency, cost of production, and a high specificity in eliciting immune response[15,28–31]. This technology has proven to be an extremely useful strategy to avoid potentially harmful immune responses. However, immunization with antigenic peptides alone is not sufficient to elicit an efficient CTL response[9]. It is important for peptide-based vaccine development to improve the immunogenicity of peptides.
Our study showed P2 containing Th and CTL epitopes could induce CTL response, but P3 only containing the CTL epitope could not do so in Tg mice. The result showed the necessity for the help effect of Th cells in the induction of CTL, which was in agreement with recent studies[12,32]. Our study also showed that P1 containing Th, CTL, and lipid part had already induced the strongest CTL response, which showed that linkage of Th, the CTL epitope, and lipid part could improve the immunogenicity of the CTL epitope. Of particular interest was the finding that peptide-specific CTL were able to lyse Caski tumor cells, which increased the possible clinical use of the lipopeptide as a cancer vaccine.
We investigated whether the lipopeptide could bypass the need for IFA. Our study confirmed that a lipopeptide alone could induce CTL response, and demonstrated that such an immunogen bypassed the requirement for an adjuvant. The toxicity of a lipopeptide was low and a patient could easily tolerate the toxicity, so the lipopeptide might be a good vaccine for human use[15,16,33].
Loing and colleagues reported that the lipopeptide could gain access into the MHC-loading compartment of T2 cells without the help of the TAP transport system, and the TAP-deficient T2 cells could specifically present the lipopeptide to CD8+ T cells[22]. Our data indicated that the lipopeptide could be processed and presented by T2 cells. Moreover, studies have shown that the lipopeptide could also be presented by powerful antigen-presenting cells (APC)[34,35], which provide an additional advantage to the lipopeptide construct in terms of immunogenicity potential.
IL-12 forms a link between innate resistance and adaptive immunity and plays an essential role in antitumor immune response. IL-12 has been shown to favor a Th1 response and to enhance the generation of CTL[36]. Our results showed that the peptide could induce the production of IL-12 of DC, which was one of the mechanisms of the lipopeptide enhancing the immunogenicity of CTL epitope peptides.
DC are the only APC capable of activating naive lymphocytes, resulting in the initiation of protective immune responses. In the absence of strong DC activation, naive T cell will differ and die. Only properly matured DC will deliver the signals required for full memory and/or effector CTL induction. In other words, immature DC tolerate while mature DC immunize[37,38]. Our results showed that the lipopeptide could not directly induce the maturation of DC, but the lipopeptides could activate Th and enhance the expression of the CD40 ligand (CD40L) on Th. Then CD40L on Th interacted with CD40 on DC, by which DC was activated[39].
In our observation, the binding affinity of peptide vaccines to the HLA molecules and their stability, as well as levels of IL-12 production from DC by peptide vaccines appear to be important for induction of CTL responses. In our study, however, P3 peptides showed a higher binding affinity to HLA molecules, as compared to P2 peptides. In contrast, P2 peptides displayed a higher CTL activity than P3 peptides. This discrepancy in the importance of the peptide binding affinity vs CTL induction might be explained by the possibility that P2 peptides (Th plus CTL epitopes) might get a help from Th cells for improved CTL induction, as compared to P3 peptides (CTL epitope alone).
In conclusion, the lipopeptide can induce a strong CTL response, the mechanisms of which are associated with an increase in the stability and persistence of the antigenic complex formed with the P1 peptide and in the production of IL-12 of DC. These findings suggest that the lipopeptide against cervical cancer can be considered a more effective vaccine type for a human. Moreover, the lipopeptide can be synthesized in pure form, providing the ability to develop rapidly this type of vaccine at a low cost.
References
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12-9.
- Ljubojevic S. The human papillomavirus vaccine. Acta Dermatovenerol 2006;14:208.
- Govan VA. Strategies for human papillomavirus therapeutic vaccines and other therapies based on the E6 and E7 oncogenes. Ann N Y Acad Sci 2005;1056:328-43.
- Hudson JB, Bedell MA, McCance DJ, Laiminis LA. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol 1990;64:519-26.
- Chen LP, Thomas EK, Hu SL, Hellstrom I, Hellstrom KE. Human papillomavirus type 16 nucleoprotein E7 is a tumor rejection antigen. Proc Natl Acad Sci USA 1991;88:110-4.
- De Bruijn ML, Schuurhuis DH, Vierboom MP, Vermeulen H, de Cock KA, Ooms ME, et al. Immunization with human papillomavirus type 16 (HPV16) oncoprotein-loaded dendritic cells as well as protein in adjuvant induces MHC class I-restricted protection to HPV16-induced tumor cells. Cancer Res 1998;58:724-31.
- Melief CJ. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv Cancer Res 1992;58:143-75.
- Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol 1991;49:281-355.
- Bona CA, Casares S, Brumeanu TD. Towards development of T-cell vaccine. Immunol Today 1998;19:126-33.
- Bevan MJ. Helping the CD8+ T-cell response. Nat Rev immunol 2004;4:595-602.
- Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, et al. Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood 1999;93:4309-17.
- BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology 2002;106:113-21.
- Zwaveling S, Ferreira Mota SC, Nouta J, Johnson M, Lipford GB, Offringa R, et al. Established human popillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol 2002;169:350-8.
- BenMohamed L, Gras-Masse H, Tartar A, Daubersies P, Brahimi K, Bossus M, et al. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur J Immunol 1997;27:1242-53.
- Gahery-Segard H, Pialoux G, Charmeteau B, Sermet S, Poncelet H, Raux M, et al. Multiepitopic B- and T-cell responses induced in humans by a human immunodeficiency virus type I lipopeptide vaccine. J Virol 2000;74:1694-703.
- Livingston BD, Crimi C, Grey H, Ishioka G, Chisari FV, Fikes J, et al. The hepatitis B virus-specific CTL responses induced in humans by lipopeptide vaccination are comparable to those elicited by acute viral infection. J Immunol 1997;159:1383-92.
- Shieh DC, Lin DT, Yang BS, Kuan HL, Kao KJ. High frequency of HLA-A*0207 subtype in chinese population. Transfusion 1996;36:818-21.
- Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class I and promiscuous recognition by T cells. Eur J Immunol 1989;19:2237-42.
- Steller MA, Gurski KJ, Murakami M, Daniel RW, Shah KV, Celis E, et al. Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin Cancer Res 1998;4:2103-9.
- Muderspach L, Wilczynski S, Roman L, Bade L, Felix J, Small LA, et al. A phase I trial of a human papillomavirus(HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res 2000;6:3406-16.
- Ressing ME, Sette A, Brandt RM, Ruppert J, Wentworth PA, Hartman M, et al. Human CTL epitopes encoded by human papillomavirus type16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol 1995;154:5934-43.
- Loing E, Andrieu M, Thiam K, Schorner D, Wiesmuller KH, Hosmalin A, et al. Extension of HLA-A*0201-restricted minimal epitope by N-palmitoyl-lysine increases the life span of functional presentation to cytotoxic T cells. J Immunol 2000;164:900-7.
- Tahara K, Takesako K, Sette A, Celis E, Kitano S, Akiyoshi T. Identification of a MAGE-2-encoded human leukocyte antigen-A24-binding synthetic peptide that induces specific antitumor cytotoxic T lymphocytes. Clin Cancer Res 1999;5:2236-41.
- Zhu B, Chen Z, Cheng X, Lin Z, Guo J, Jia Z, et al. Identification of HLA-A*0201-restricted cytotoxic T lymphocyte epitope from TRAG-3 antigen. Clin Cancer Res 2003;9:1850-7.
- Wei WZ, Ratner S, Shibuya T, Yoo G, Jani A. Foreign antigenic peptides delivered to the tumor as targets of cytotoxic T cell. J Immunol Methods 2001;258:141-50.
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony stimulating factor plus interleukin 4 and downregulated by tumour necrosis factor alpha. J Exp Med 1994;179:1109-18.
- Oh S, Terabe M, Pendleton CD, Bhattacharyya A, Bera TK, Epel M, et al. Human CTLs to wild-type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Res 2004;64:2610-8.
- Berzofsky JA, Ahlers JD, Derby MA, Pendleton CD, Arichi T, Belyakov IM. Approaches to improve engineered vaccines for human immunodeficiency virus and other viruses that cause chronic infections. Immunol Rev 1999;170:151-72.
- Paul WE. Can the immune response control HIV infection? Cell 1995;82:177-82.
- Gras-Masse H. Single-chain lipopeptide vaccines for the induction virus-specific cytotoxic T cell responses in randomly selected populations. Mol Immunol 2001;38:423-31.
- Diamond DJ, York J, Sun JY, Wright CL, Forman SJ. Development of a candidate HLA-A*0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood 1997;90:1751-67.
- Mortara L, Gras-Masse H, Rommens C, Venet A, Guillet JG, Bourgault-Villada I. Type I CD4+ T cell help is required for induction of anti-peptide multispecific cytotoxic T lymphocytes by a lipopeptidic vaccine in rhesus macaques. J Virol 1999;73:4447-51.
- Bessler WG, Mittenbuhler K, Esche U, Huber M. Lipopeptide adjuvants in combination treatment. Int Immunopharmacol 2003;3:1217-24.
- Andrieu M, Loing E, Desoutter JF, Connan F, Choppin J, Gras-Masse H, et al. Endocytosis of an HIV-derived lipopeptide into human dendritic cells followed by class I-restricted CD8+ T lymphocyte activation. Eur J Immunol 2000;30:3256-65.
- Andrieu M, Desoutter JF, Loing E, Gaston J, Hanau D, Guillet JG, et al. Two human immunodeficiency virus vaccinal lipopeptides follow different cross-presentation pathways in human dendritic cells. J Virol 2003;77:1564-70.
- Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol 1995;154:5071-9.
- Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigen. Immunol Rev 2004;199:9-26.
- Mao YX, Chen YJ, Ge Y, Ma HB, Yu JF, Wu HY, et al. Recombinant human B7-H4 expressed in Escherichia coli inhibits T lymphocyte proliferation and IL-2 secretion in vitro. Acta Pharmacol Sin 2006;27:741-6.
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 1998;393:474-8.
