Effects of gestrinone on uterine leiomyoma and expression of c-Src in a guinea pig model
Introduction
Uterine leiomyoma, a smooth muscle tumor arising from the uterus, occurs in about 20%–30% women over the age of 30, and is the most frequently occurring tumor in gynecology[1]. Although the vast majority of leiomyomas are benign, these tumors could induce severe pain, excessive bleeding, infertility, repetitive pregnancy loss, and other pregnancy-related complications. As a result, uterine leiomyomas are ranked as the major cause for hysterectomy, accounting for a third of all hysterectomies[2,3]. Unfortunately, the underlying geneses of uterine leiomyoma are poorly understood. Estradiol benzoate (E2) and progesterone (P) have generally been regarded as the major factors responsible for the development of the leiomyoma, since the growth of uterine leiomyoma is associated with menstrual cycle and the fluctuation of steroid hormone level[4,5]. Estradiol receptors (ER) and progesterone receptors (PR), as members of a superfamily of nuclear receptors that function as ligand-modulated transcriptional regulators, are ligands for steroid hormone. ER and PR are also regarded as predominant regulators in the promotion of endometrium proliferation and uterine leiomyoma[6]. In classical theory, steroid hormones act through the interaction of their receptors with specific steroid responsive elements to regulate the transcription of target gene networks. This process causes hormone-dependent cell proliferation[7,8]. There is recent evidence suggesting that the development of leiomyomas may also be attributed to genetic and environmental influences[6]. Several genes and/or growth factors, such as transforming growth factor, epidermal growth factor, and insulin-like growth factor have been demonstrated to be differentially expressed in human tissues and primary-cultured leiomyoma cells compared with normal myometrium[9–12]
c-Src, belonging to Src family kinases, was first recognized as a proto-oncogene with intrinsic protein tyrosine kinase activity[13,14]. c-Src plays a vital role in the regulation of both normal and oncogenic processes, including prolifera-tion, differentiation, survival, motility, and angiogenesis[15,16]. Elevated c-Src protein levels and kinase activity in several human cancers have been reported since the early 1980s, including tumors in the colon, breast, and ovaries. The protein level of c-Src is significantly higher in carcinomas than in normal matched tissues[17–21]. Recently, Migliaccio’s et al demonstrated that c-Src takes a critical role in hormone-dependent tumor progression[22]. c-Src activity is stimulated rapidly by estradiol, progestin, and androgens in breast and prostate cancer cells and is involved in the nontranscriptional action of estradiol[23,24]. Src is downstream of classical sex-steroid receptors, such as ER, PR, and androgen receptors. These sex steroid receptors can activate Src. Signaling via Src plays an important role in ER activation. Estradiol controls the expression of a cell-cycle regulator through the activated signaling pathway, which triggers DNA synthesis and cell proliferation[22,23]. The loss of the c-Src tyrosine kinase is correlated with defects in ductal development as well as in uterine and ovarian development. The mammary gland, ovarian, and uterine tissues derived from the c-Src-deficient animals displayed a dramatic development delay[25]. This evidence indicates that c-Src may be a critical target gene for steroid hormone action.
Since uterine leiomyoma is considered to be steroid-hormone dependent, whether Src plays a role in the development of uterine leiomyoma is questionable. It would help to better understand the underlying causes of uterine leiomyoma that lead to more targeted strategies to treat the hormone-dependent tumor. Therefore, we investigated the expression of c-Src kinase in a uterine leiomyoma of a guinea pig model. To understand the association between the activity of c-Src and the formation of the uterine leiomyoma, the protein expression of phospho-416Src autophosphorylation site, Tyr416, and increases in c-Src activity were also assayed.
Medical treatment of leiomyomas relies on drugs that inhibit ovarian steroids and induce a hypoestradiolic state that causes atrophy of the tumor at the same time. Gestrinone is a synthetic trienic 19-norsteroid compound with potent anti-estradiolic and antigonadotropic properties. The compound also inhibits gonadotropin secretion and ovulation, which prevents the occurrence of menstruation[26]. Gestrinone was developed originally as a long-acting contraceptive. Because of its marked inhibitory effects on ER and PR in the endometrium and uterus, gestrinone has also been clinically applied to treat endometriosis and uterine leiomyoma with success[27,28]. Marca et al demonstrated that gestrinone significantly increased blood flow impedance of the uterine artery[29]. In the present study, we further investigated the effect of gestrinone on the development of uterine leio-myoma, and the expression of c-Src, phospho-416Src, ER, and PR in the guinea pig model.
Materials and methods
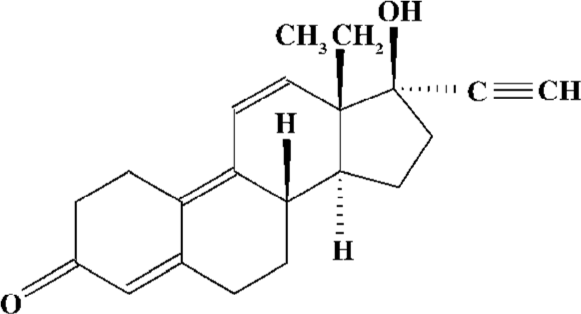
Materials Gestrinone microparticle (13β-ethyl-17α-ethynyl-17β-hydroxy- gona-4,9,11-trien-3-one) is a patent of Prof Qing-hua CHEN (Department of Pharmaceuticals, Shanghai Institute of Pharmaceutical Industry, Shanghai, China) and was generously gifted to us. The structure of gestrinone is shown in Figure 1. E2 was purchased from Shanghai General Pharmacy Company (Shanghai, China). The c-Src rabbit polyclonal antibody and the anti-ER mouse monoclonal antibody were purchased from Lab Vision Co (Lab Vision Co, Fremont, CA, USA). The mouse anti-phospho-Src (Tyr416) monoclonal antibody was from Upstate Co (Lake Placid, NY, USA). The PR mouse monoclonal antibody was from Novocastra Laboratories Ltd (Newcastle, UK). The avidin-biotin-peroxidase complex (ABC) kit was the product of Sino-American Biotechnology Co (Shanghai, China). Trizol was purchased from Gibco-BRL (Grand Island, NY, USA). An enhanced chemiluminescence (ECL) detection kit for horseradish peroxidase (HRP) was purchased from Pierce (Rockford, IL, USA). The magnetic average phase enzyme immunoassay system (MGI) was the product of Serono Inc (Geneva, Switzerland). Image processing was done with Image-Pro Plus® Version 5.1.0 software (Media Cybernetics Inc, Silver Spring, MD, USA).
Animals Dunkin-Hartley female guinea pigs (3 months old, weighing 350–400 g) were purchased from the Sino-British Experiment Animal Lab Co (Shanghai, China). The animals were treated in accordance with protocols approved by the Laboratory Animal Ethics Committee at the Shanghai Institute of Planned Parenthood Research. All animals were housed: 5 in 1 cage under a 12 h/12 h light/dark cycle with free access to food and water. Each animal was weighed once a week and fed 50 g cabbage twice a week for vitamin C supplementation.
Model establishment and treatment Sixty-five female guinea pigs were divided into 5 groups. Group 1 consisted of normal control animals and group 2, castrated animals. After bilateral oophorectomized, animals were bred as group 1; Group 3, model animals. After bilateral oophorectomy, the animals were treated intramuscularly with E2 100 µg/d twice per week from week 1 through to week 16. The bilateral oophorectomized animals in groups 4 and 5 were treated intramuscularly with E2 at 100 µg/d alone twice per week from week 1 through to week 6, and then in combination with subcutaneous administration of gestrinone microparticle at 2 or 4 mg/kg once biweekly from week 7 through to week 16. Anesthetics were administered with 3% pentobarbital sodium during oophorectomy. At the end of the sixteenth week, blood was sampled from the animals’ hearts. The animals were killed by ether inhalation. Body weight was measured before killing. After killing, the uteri were dissected, weighed, and then fixed immediately in 10% neutral formalin or liquid nitrogen. The collected blood was centrifuged, and sera were frozen at -20 °C until the time of assay.
Histological examination The formalin-fixed tissues were embedded in paraffin wax and sliced into 4 µm thick pieces using a rotary microtome, and then stained with hematoxylin-eosin (HE) for histological examination under light microscope.
Serum E2 and P assays Serum estradiol and progesterone levels were assayed using MGI with the Serono magimu-zyme-III analyzer (Serono Inc, Geneva, Switzerland) at a wavelength of 550 nm.
Immunohistochemical staining The paraffin-fixed sections were baked overnight at 55 °C. Sections were deparaf-finized with xylene and rehydrated with ethanol in gradient. Endogenous peroxidase activity was blocked by incubating the slides with 0.3% H2O2 in methanol for 10 min. Antigen retrieval was performed with 10 mmol/L citric acid buffer (pH 6.0) for a total of 10 min in a microwave oven. Non-specific binding was blocked by incubating the specimens in 0.1 mol/L Tris-HCl (pH 7.6) containing 10% goat serum for 1 h. The antigen was then detected using c-Src, anti-ER, or anti-PR primary antibodies at a 1:100 dilution with blocking buffer as diluents and incubated in a moist chamber at 37 °C for 1 h. After washing with phosphate buffered saline (PBS), the sections were incubated with biotinylated goat-anti-mouse IgG followed by HRP conjugated streptavidin biotin peroxidase complex (SABC) according to the manufacturer’s protocol. The resulted sections were then stained with 3,3'diaminobenzidine tetrahydrochloride (DAB). As a negative control to assess potential nonspecific staining, 0.01 mol/L PBS was used instead of primary antibodies, and no staining was observed. Finally, the sections were dehydrated and sealed with mounting media for visualization by light microscopy. The dark brown staining of nuclei or cytoplasm was regarded as a positive reaction. Immunohistochemistry was semiquantitatively evaluated using the immunoreactive score (IRS). The IRS was obtained by the product of intensity of immunostaining (none=0; weak=1; moderate=2; and strong=3) and the percentage of positive cells (none=0; <10%=1; 10%–50%=2; 51%–80%=3; and >80%=4)[30,31]. The number of positive cells was recorded with Image-Pro Plus® Version 5.1.0 software (Media Cybernetics Inc). One section was examined for 1 animal. Statistical analysis was performed on the average number of positive cells of 10 sections per group.
Protein isolation and analysis Protein extracts were prepared by grinding 100 mg of frozen tissue to powder with liquid nitrogen in a mortar and pestle. The resulted proteins were added with 1 mL extraction buffer (100 mmol/L Tris-HCl pH 7.5, 0.5 mmol/L dithiothreitol, 2 mmol/L phenylmethylsulfonyl fluoride, 5 mmol/L EDTA, 500 mmol/L NaCl, and 0.1% Triton X-100), and then centrifuged at 4 °C, 12 000×g for 15 min. The supernatant were collected and stored at -70 °C. The protein concentration was measured using the bicinchoninic acid (BCA) method (Pierce, Rockford, IL, USA). Samples were diluted to 1 µg/µL with extraction buffer. An aliquot of 20 µg was mixed with an equal volume of SDS-PAGE loading buffer (50 mmol/L Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 5% β-mercaptoethanol, 0.002% bromophenol blue), and heated at 95 oC for 5 min before being loaded onto 8% SDS-PAGE (30% acrylamide:bisacrylamide 29:1) for electrophoresis in TBE buffer (Tris-Borate-EDTA). To perform the Western blot analysis, the separated proteins were electrophoretically transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were incubated with the primary antibody in 3% BSA (albumin bovin fraction V)-PBS (1:600) at 37 °C for 2 h. After washing with 0.1% Tween 20-PBS, the membranes were incubated with peroxidase-conjugated secondary antibody IgG in 3% BSA-PBS (1:2000) at 37 °C for 1 h. The blot was developed with ECL according to the manual and was exposed to X-film. The relative levels of protein were semiquantitatively determined with Image-Pro Plus® Version 5.1.0 software (Media Cybernetics Inc). The relative levels of protein were obtained by taking the ratio of the band intensity of c-Src, phospho-416Src, ER, or PR to β-actin. Statistical analysis was performed on the average protein level of 5 animals per group.
Statistical analysis Data were presented as mean±SEM. Various kinds of indexes between the control group and administrated groups were analyzed by one-way ANOVA test with SPSS 10.0 software (version 10.0 for Windows; SPSS, Chicago, Illinois, USA). P<0.05 was considered statistically significant.
Results
Weights and coefficients of uterine tissues At the end of 16 weeks, there was no visible change in the guinea pigs’ body weight, the absolute and the relative uterine weights between the control and the castrated group. Compared with the control group, there was a significant decrease of body weight in the model and the gestrinone treated group (P<0.05). However, there was a marked increase of the absolute and the relative uterine weights in the model group (P<0.05). In the gestrinone-treated group, the absolute and the relative weights of uterine tissues were lower than that of the model group, and the organ coefficients in the 4 mg/kg gestrinone group were much lower than that of the 2 mg/kg gestrinone-treated group (P<0.05) (Table 1).
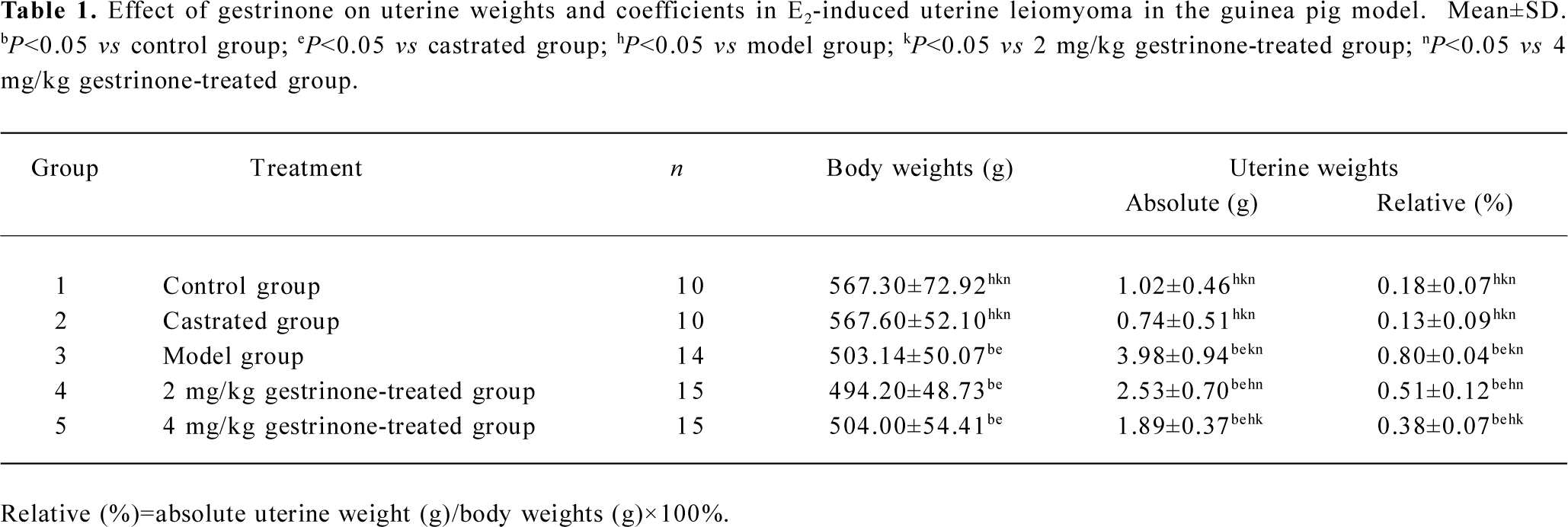
Full table
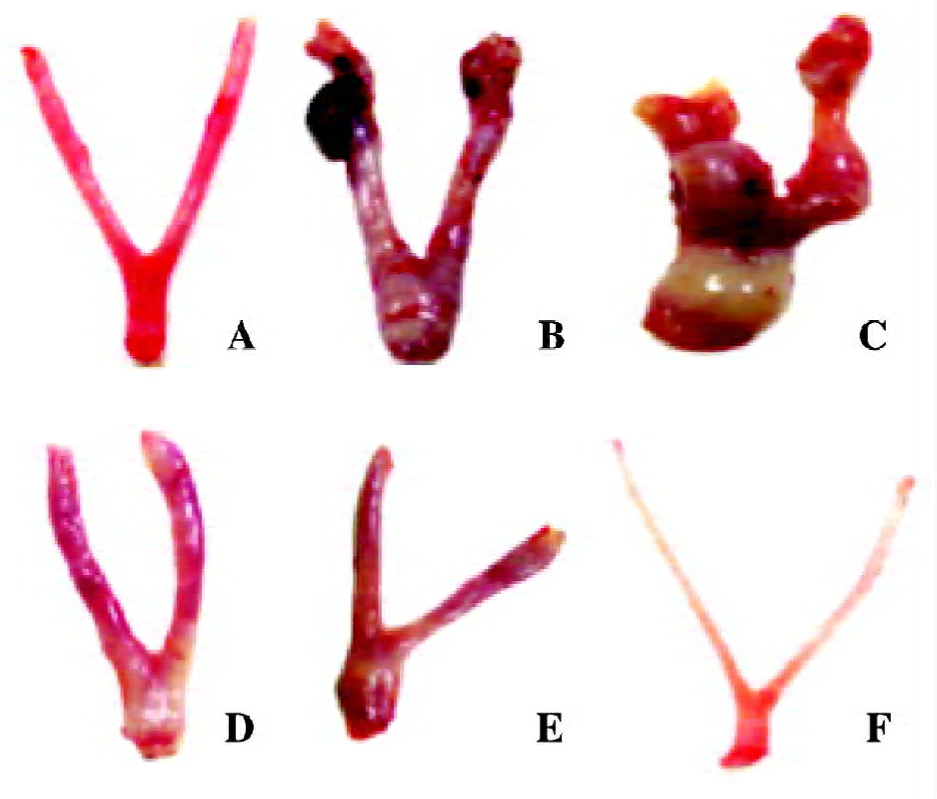
Morphological changes of uterine tissues Necropsy and pathological analysis were performed to examine whether there were leiomyoma features in the animals. Visually, there was no node or cystitis tumor observed in the myometrium of the control and the castrated animals. In the model animals, the uteri became enlarged, proliferative, and had a thick nodular or cystiform appearance. The nodules were generally found on the uterine horn, the uterine body, or the cervico-uterine junction. The general appearances of nodes were of a solitary, firm, gray mass or cystiform full of fluid, appearing as subserosa or intramural myometrium. In the 2 and 4 mg/kg gestrinone-treated animals, there were no nodes or severe hyperplastic myometrium observed. The appearances were similar to those of the control animals (Figure 2).
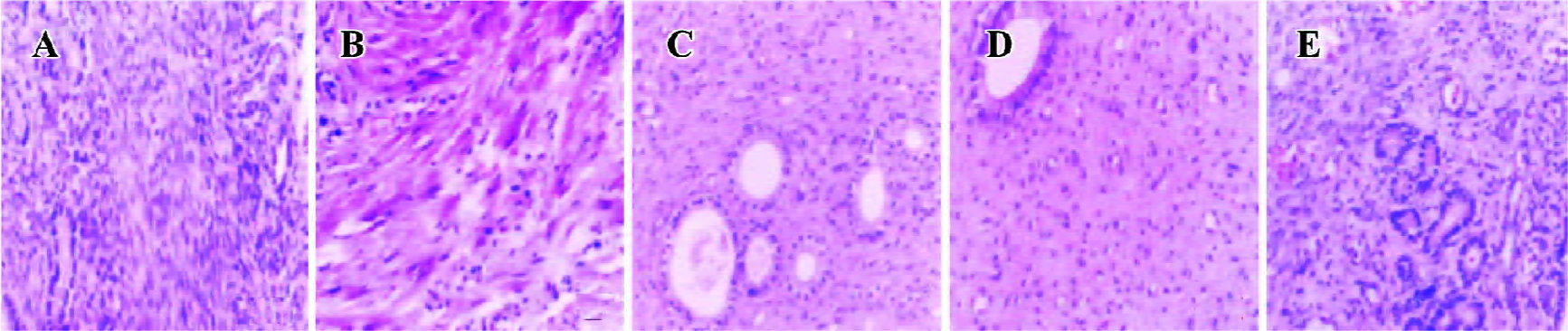
Histologically, proliferative features were demonstrated both in the leiomyoma and the myometrium of model animals. The majority of cell nuclei showed elongated, rod-like, or oval-round shape with a deep nuclear stain and grew in parallel arrays, exhibiting a typical, smooth, muscle-like appearance. A few spindle-shaped cells were also observed. The glandular cavity was enlarged. The muscle fiber showed rarefaction and misalignment, exhibiting paliform or fan-shape. In the castrated animals, the smooth muscle cells lined up densely, and the glandular cavity was atrophic. In the 2 and 4 mg/kg gestrinone-treated animals, the uterine tissues were thin. The smooth muscle cells lined up in order like those of the control animals (Figure 3).
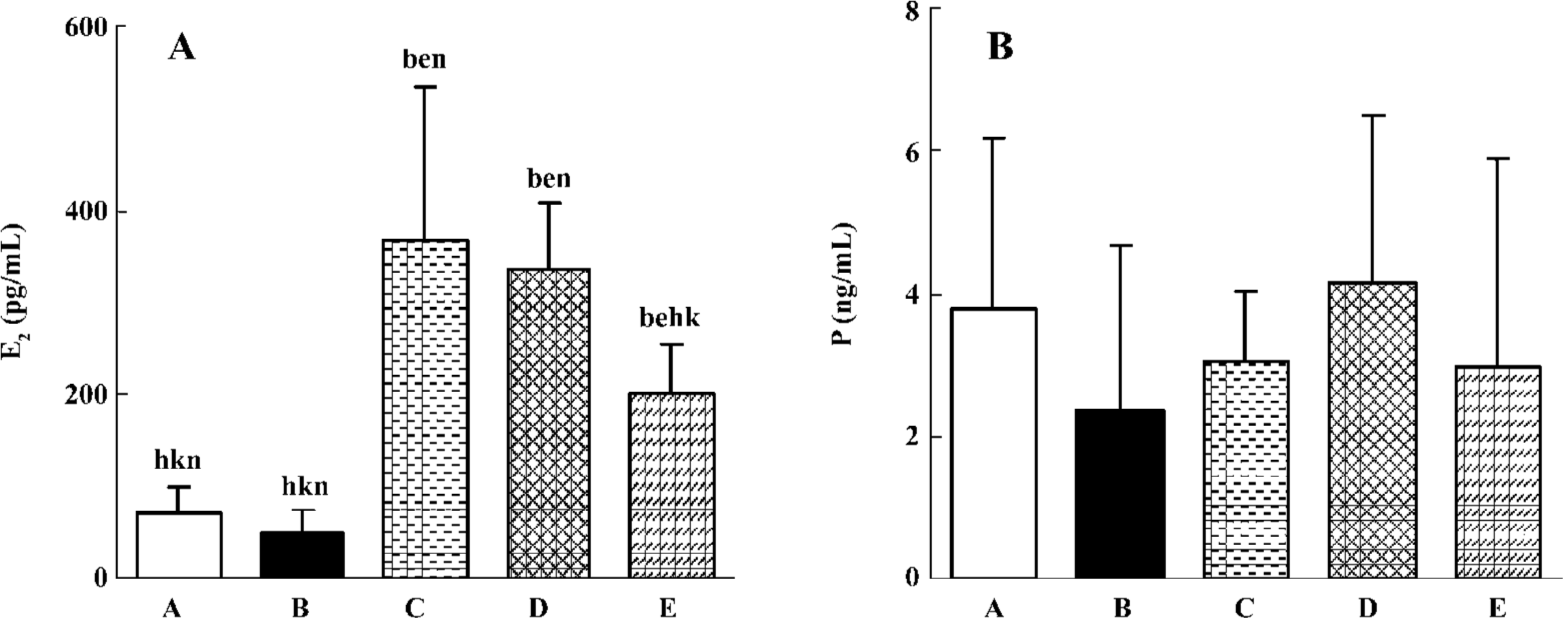
Serum hormone concentrations In the E2-treated model group, there was a visible increase in the serum E2 level than that of the control and the castrated groups (P<0.05). Compared with the model group, there was no significant decrease in the serum E2 level in the 2 mg/kg gestrinone-treated group (P>0.05), and there was an obvious decrease in the serum E2 level in the 4 mg/kg gestrinone-treated group (P<0.05). There was no significant fluctuation of serum P in the control, castrated, model, and 2 and 4 mg/kg gestrinone-treated groups (P>0.05) (Figure 4).
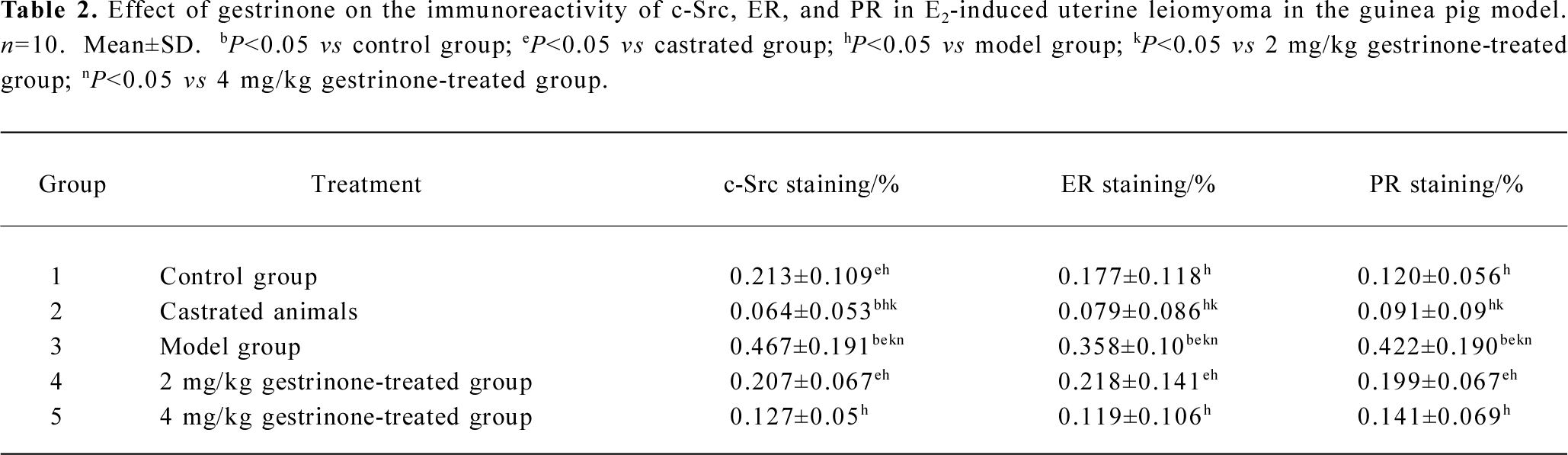
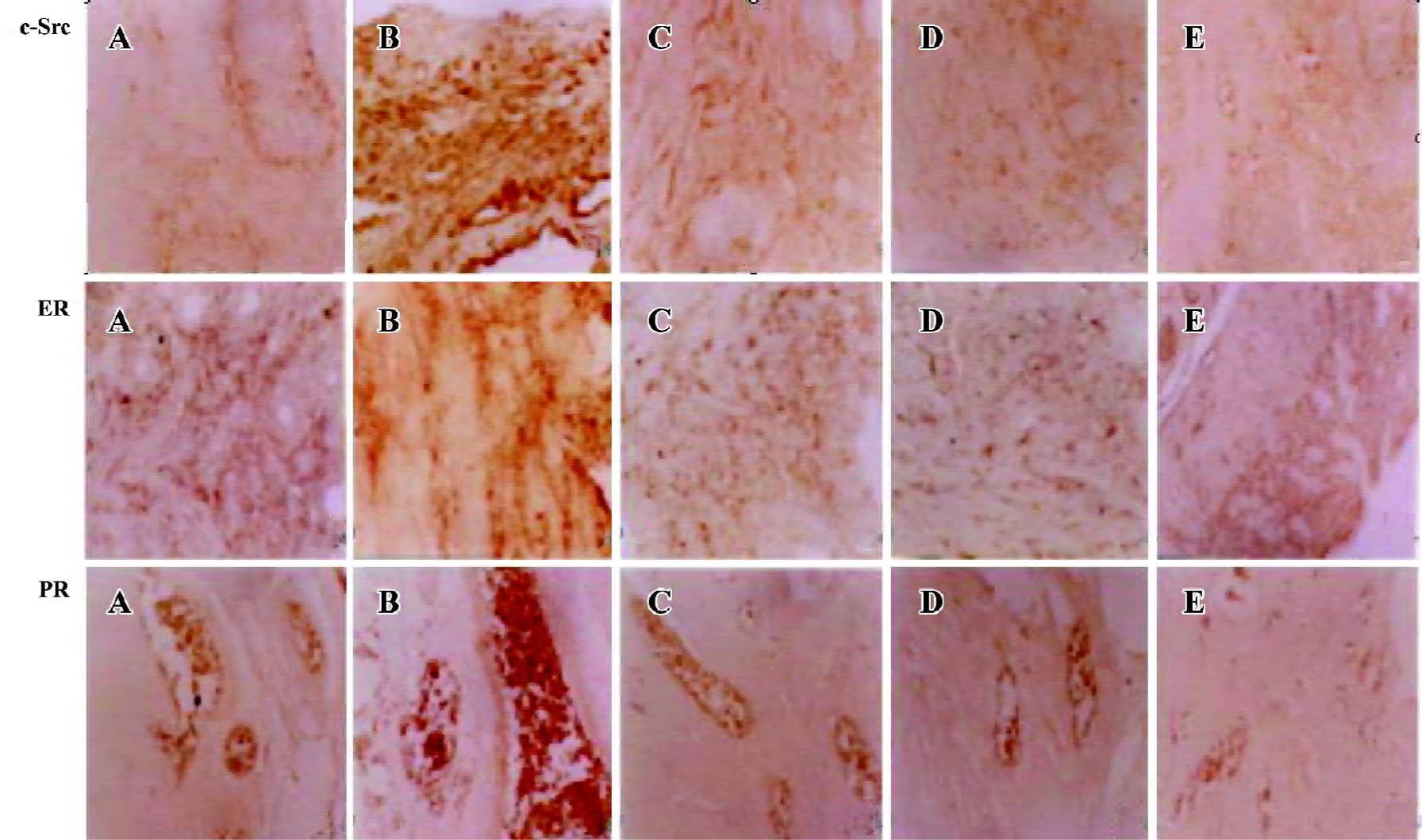
Immunohistochemical expression of ER, PR, and c-Src The presence of ER, PR, and c-Src were confirmed by immunohistochemical analysis in the myometrium of the control, castrated, model, 2 and 4 mg/kg gestrinone-treated animals. The expression of c-Src and ER distributed both in the cytoplasm and nucleus. The staining of PR was located in the nucleus. In the control and the castrated groups, the coloration of c-Src, ER, and PR were stained light brown. By contrast, the coloration of c-Src, ER, and PR in the model group were dark brown and the positive staining areas were extended. In the 2 and 4 mg/kg gestrinone-treated groups, the coloration of c-Src, ER, and PR was lighter than that of the model group. The number of positive uterine cells was less than those in the model group (Table 2) (Figure 5).
Full table
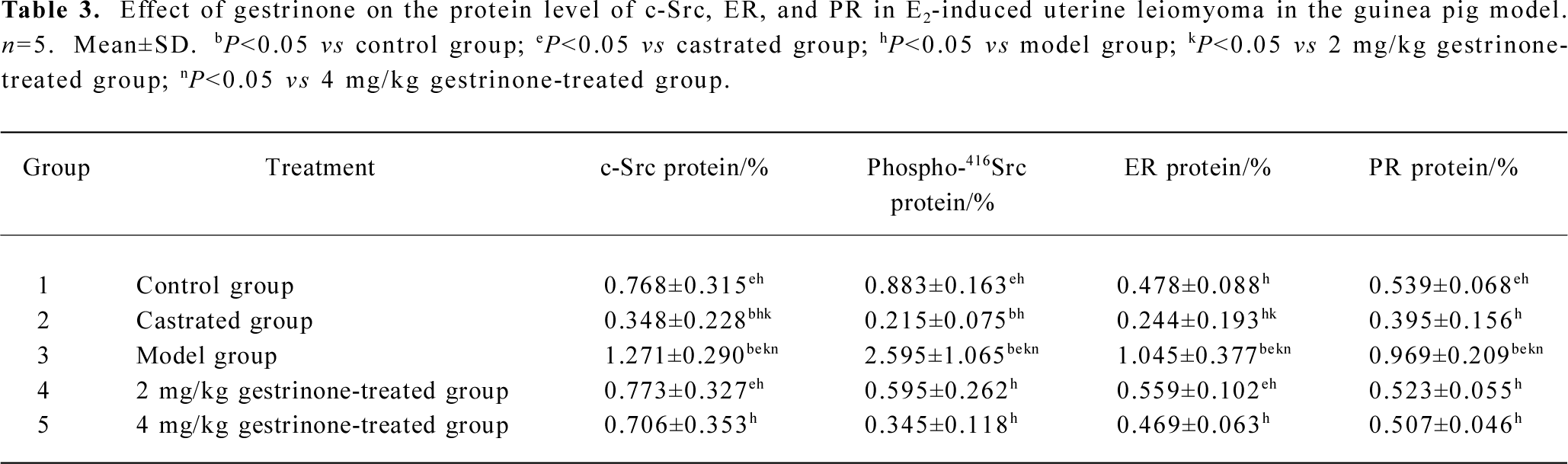
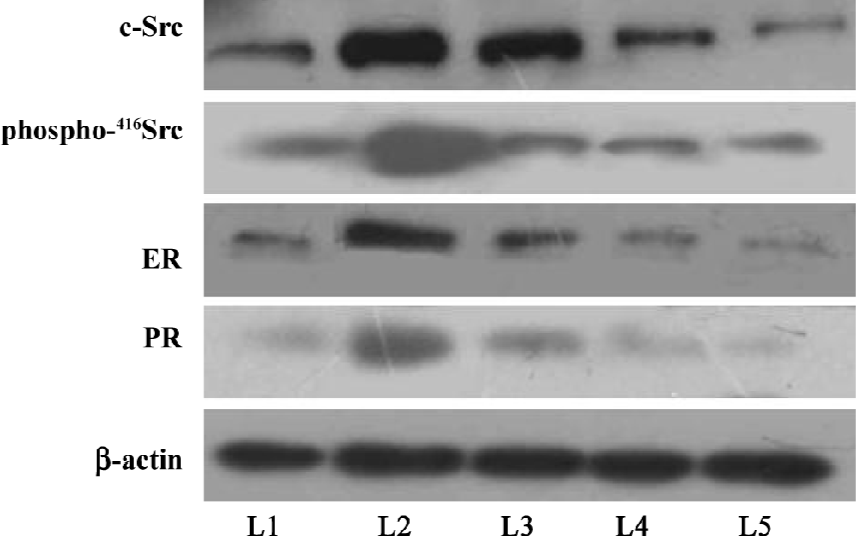
Protein expressions of c-Src, phospho-416Src, ER, and PR Western blots were performed to analyze the protein level of c-Src, phospho-416Src, ER, and PR. The protein expressions of c-Src, phospho-416Src, ER, and PR were detectable in all of the groups. The relative protein expressions of c-Src/β-actin, phospho-416Src/β-actin, ER/β-actin, and PR/β-actin were significantly higher in the model group than those of the control group (P<0.05). Compared with the control group, the relative protein expression of c-Src/β-actin and phospho-416Src/β-actin was lower in the castrated group (P<0.05), and there was no significant decrease in ER/β-actin and PR/β-actin protein expression (P>0.05). In the 2 and 4 mg/kg gestrinone-treated groups, the relative protein expressions of c-Src/β-actin, phospho-416Src/β-actin, ER/β-actin, and PR/β-actin were significantly lower than those of the model group (P<0.05), and there was no marked quantitative difference between the 2 and 4 mg/kg gestrinone-treated group (Table 3) (Figure 6).
Full table
Discussion
Uterine leiomyoma is the most common benign tumor in women. The cause of human uterine leiomyoma has not been fully revealed. As a tumor developed from an estrogen target organ (uterus), uterine leiomyoma is traditionally considered an estrogen-associated tumor[32]. In some clinical observations, surprisingly, there is no positive connection found between E2 concentration and the genesis of leiomyoma. Patients with uterine leiomyoma have similar plasma E2 and P levels to those of normal controls[6,33]. The development and growth of leiomyomas may involve a multistep cascade[34]. In pharmacological research, nevertheless, one of the effective methods for developing uterine leiomyoma is to administer a guinea pig with E2 for a long period of time. Studies on the guinea pig model of uterine leiomyoma provide insights into unraveling molecular mechanisms that regulate leiomyomas, and are widely used for preclinical drug screening assays. Approximately 8% of aged guinea pigs develop spontaneous leiomyomas, and ovariectomy in young animals combined with high-dose estrogen supplementation causes the development of uterine and abdominal leiomyomas with high frequency[35,36].
In this study, therefore, we induced uterine leiomyoma in guinea pigs with an E2 injection twice a week for 4 months[35,37] with a modified E2 injection frequency. During the experiment, one animal in the model group died due to pneumonia and the others lived healthily to the end of the experiment. At the end of the 16 weeks, all of the animals underwent necropsy. In the model group, the overall appearance of the myometrium was enlarged and proliferative. Pronounced nodes were observed in some model animals (8 of 14). The nodes were located on the uterine horn, the uterine body, or the cervico–uterine junction and exhibited intramural myoma (7 of 14), subserosal myoma (1 of 14), and uterine necrosis (2 of 14). There was a remarkable increase of the absolute and relative uterine weights in the model group than that of the control and castrated groups (Table l). Histologically, the myometrium of all of the model animals appeared proliferative and consisted of a mass of typical smooth muscle cells and a few fibroblasts. It exhibited typical features of leiomyoma, even if there was no pronounced nodule present. There was no nodule found in the abdominal cavities of the model group. According to Porter and Fujii et al[35,37], however, an intramuscularly injection of E2 3 times a week for 3 months would induce multiple nodules in the abdominal cavities, including the uterine horn, peritoneum, spleen, pancreas, and omentum. The marked disparity of the results may be attributed to the different treatment frequency of E2, since we obtained a similar result to that of Porter and Fujii et al when following their protocol in a preliminary experiment.
In a preliminary experiment, we compared ovariectomized animals with non-ovariectomized animals, both treated with E2. The result showed that there was slight proliferation on the myometrium and no nodes were observed in the non-ovariectomized animal. The leiomyoma was better developed in the ovariectomized animals. In order to equalize the original hormones of the tested animals and induce uterine leiomyoma successfully, we utilized oophorectomized guinea pigs in the present study. In the castrated animals, the serum E2 level significantly decreased when the ovary was excised. There was a remarkable increase in the serum E2 level of the model group after E2 treatment. However, there was no appreciable fluctuation of serum P observed. Furthermore, there was a higher expression of ER and PR in the myometrium in the model group than the control group. This evidence indicates that E2 could increase the level of ER and PR in the myometrium, and that ER and PR play more important roles during the formation of uterine leiomyoma. Our observation is consistent with the previous reports that high concentrations of ER and PR in the myometrium contributes to the development of uterine leiomyoma[6,38,39]. Compared with the control group, we demonstrated that there was no marked decrease in immunoreactivity and protein expression of ER and PR in the castrated group in the present study. There was an overexpression of ER and PR in the uterine leiomyoma of the guinea pig model.
Clinically, a gestrinone tablet or capsule is administered orally at a dose of 2.5–5 mg twice weekly for at least 6–12 months for the treatment of leiomyoma. As an alternative for those patients who developed gastric intolerance to steroids and as a practical means to extend the action and bypass the liver to increase the bioavailability of the drug, a gestrinone microparticle was engineered by Prof CHEN. The commercial product is expected to be available from Beijing Zizhu Pharmaceutical Co Ltd (Beijing, China). In the present study, we administered the guinea pig model with the gestrinone microparticle once biweekly at 2 or 4 mg/kg for 10 weeks. Visually, no node was observed in the 2 and 4 mg/kg gestrin-one-treated groups. In a dose-dependent manner, the gestrinone microparticle decreased the serum E2 level, the absolute, and the relative uterine weights. Histologically, the myometrium was thin and the smooth muscle cells lined up in order, as that of the control animals. Gestrinone 2 mg/kg markedly reduced the immunoreactivity and protein expression of ER and PR. There was no significant difference in immunoreactivity and protein expression of ER and PR between the 2 and 4 mg/kg gestrinone-treated groups. The result indicated that gestrinone effectively reduced the proliferation of uterine smooth muscles and suppressed the formation of uterine leiomyoma in the guinea pig model. Gestrinone 2 mg/kg is as effective as 4 mg/kg in inhibiting the formation of uterine leiomyoma.
Recently, c-Src has been considered one of critical factors in the genesis of breast and prostate cancers[23,24]. Evidence shows that in E2 responsive tissues such as breast and endothelia, the biological function of ER involves not only the activation of estradiol-responsive elements, but also induces rapid activation of various signaling molecules and signal transduction pathways, which are regarded as “nongenomic” or “non-transcriptional signaling[40,41]. Migliaccio et al reported that in breast cancer cell lines, upon activation by E2, ER stimulate a mitogenic signaling network, such as Src/Ras/Erk and the PI3-K/AKT pathway, known to be engaged by growth factors[24,42–45]. In these signaling pathways, Src has been identified as a crucial molecule downstream of ERα and mediates E2 rapid action[22]. Activation of this signal pathway leads to S-phase entry of the cells and is responsible for the proliferative effect against apoptosis[42,23]. In the opinion of Migliaccio et al, Src appears to be an initial target of sex steroid hormone action in breast and prostate cancer[22].
In order to understand whether Src takes a role in the development of uterine leiomyoma, we examined the expression of c-Src in uterine leiomyoma in the guinea pig model. An immunohistochemical assay demonstrated that c-Src was expressed in the nucleus and cytoplasm of the myometrium. Compared with the control group, we found that there was lower immunoreactivity of c-Src in the castrated group, and there was significant increase in immunoreactivity of c-Src in the uterine leiomyoma of the guinea pig model induced by E2 (Figure 5). In the Western blot analysis, the protein expression of c-Src was weaker in the castrated group than that the control group. The protein expression of c-Src was stronger in the model group than that of the control and castrated groups (Figure 6). These results indicate that overexpressed c-Src plays an important role in the formation of uterine leiomyoma. Furthermore, we also demonstrated that gestrinone 2 and 4 mg/kg markedly downregulated cSrc protein in the myometrium, which may be attributed to the suppression of the formation of uterine leiomyoma in the guinea pig model. There was no marked difference in immunoreactivity and protein expression of c-Src between the 2 and 4 mg/kg gestrinone-treated groups.
Regulation of c-Src activity is complicated. Phosphorylation of Tyr527, at the carboxy-terminal end of c-Src, results in a decrease of c-Src kinase activity. In contrast, phosphorylation of the autophosphorylation site, Tyr416, increases c-Src activity[46,47]. To understand the relationship between c-Src and the formation of uterine leiomyoma, we assayed the protein level of phospho-416c-Src among the groups. The results demonstrated that the level of phospho-416c-Src was upregulated in the model group than the other groups. Gestrinone 2 and 4 mg/kg significantly downregulated the protein level of cSrc and phospho-416c-Src. There was no marked difference between the 2 and 4 mg/kg gestrinone-treated groups. The results indicated that the activity of c-Src was enhanced in the model of uterine leiomyoma, and gestrinone decreased the activity of c-Src.
On the basis of these findings, for the first time, we propose that c-Src plays a significant role in the development of uterine leiomyoma. The upregulation of Src and phospho-416Src indicate that the activity of c-Src is augmented in the leiomyoma model. c-Src is associated with the formation of uterine leiomyoma in the guinea pig model. There is significant downregulation of cSrc and phospho-416Src protein after gestrinone 2 and 4 mg/kg treatment. Similar to ER and PR, c-Src may be a vital factor contributing to the growth of uterine leiomyoma. Gestrinone markedly suppressed the growth of uterine leiomyoma induced by E2 in the guinea pig model. Gestrinone inhibited not only the expression of ER and PR, but also the protein expression of c-Src and phospho-416Src in the leiomyoma model of guinea pigs. Although the related mechanism is still to be elucidated, these results help us to further understand the underlying causes of uterine leiomyoma and provide a clue for a possible therapeutic treatment with gestrinone in depressing the formation of uterine leiomyoma via targeting the signaling pathway.
Acknowledgment
The authors greatly appreciate the help of Mrs Zhi-fang ZHAO in carrying out the animal experiments.
References
- Cooper NP, Okolo S. Fibroids in pregnancy–common but poorly understood. Obstet Gynecol Surv 2005;60:132-8.
- Myers ER, Barber MD, Gustilo-Ashby T, Couchman G, Matchar DB, McCrory DC. Management of uterine leiomyoma: what do we really know? Obstet Gynecol 2002;100:8-17.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988-1990. Obstet Gynecol 1994;83:549-55.
- De Leo V, Morgante G, La Marca A, Musacchio MC, Sorace M, Cavicchioli C, et al. A benefit-risk assessment of medical treatment for uterine leiomyomas. Drug Saf 2002;25:759-79.
- Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. J Obstet Gynecol 1995;172:14-8.
- Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: A review. Environ Health Perspect 2003;111:1037-54.
- Edwards DP, Boonyaratanakornkit V. Rapid extranuclear signaling by the estradiol receptor (ER): MNAR couples ER and Src to the MAP kinase signaling pathway. Mol Interv 2003;3:12-5.
- McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 2002;108:465-74.
- Yeh J, Rein M, Nowak R. Presence of messenger ribonucleic acid for epidermal growth factor (EGF) and EGF receptor demonstrable in monolayer cell cultures of myometria and leiomyoma. Fertil Steril 1991;56:997-1000.
- Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab 1998;83:2192-8.
- Luo X, Ding L, Xu J, Chegini N. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-beta. Endocrinology 2005;146:1097-118.
- Englund K, Lindblom B, Carlstrom K, Gustavsson I, Sjoblom P, Blanck A. Gene expression and tissue concentrations of IGF-I in human myometrium and fibroids under different hormonal conditions. Mol Hum Reprod 2000;6:915-20.
- Hunter T, Sefton BM. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA 1980;77:1311-5.
- Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol 2001;2:467-75.
- Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell 2004;6:209-14.
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 1997;13:513-609.
- Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene 2000;19:5636-42.
- Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, et al. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol 2003;88:73-9.
- Bolen JB, Veillette A, Schwartz AM, DeSeau V, Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci USA 1987;84:2251-5.
- Verbeek BS, Vroom TM, Adriaansen-Slot SS, Ottenhoff-Kalff AE, Geertzema JG, Hennipman A, et al. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J Pathol 1996;180:383-8.
- Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer and metastasis reviews 2003;22:337-58.
- Migliaccio A, Castoria G, Di Domenico M, De Falco A, Bilancio A, Auricchio F. Src is an initial target of sex steroid hormone action. Ann N Y Acad Sci 2002;963:185-90.
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, et al. Sex steroid hormones act as growth factors. J Steroid Biochem Mol Biol 2003;83:31-5.
- Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, et al. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 1998;17:2008-18.
- Kim H, Laing M, Muller W. c-Src-null mice exhibit defects in normal mammary gland development and ERα signaling. Oncogene 2005;24:5629-36.
- Mora G, Faundes A, Pastore U. Clinical evaluation of an oral progestin contraceptive, r-2323, 5mg, administered at weekly intervals. Contraception 1974;10:145-57.
- Nieto A, Tacuri C, Serra M, Keller J, Cortes-Prieto J. Evaluation of gestrinone after surgery in treatment of endometriosis. Gynecol Obstet Invest 1997;43:192-4.
- Bromham DR, Booker MW, Rose GL, Wardle PG, Newton JR. Updating the clinical experience in endometriosis — the European perspective. Br J Obstet Gynaecol 1995;102 Suppl 12:12-6.
- La Marca A, Giulini S, Vito G, Orvieto R, Volpe A, Jasonni VM. Gestrinone in the treatment of uterine leiomyomata: effects on uterine blood supply. Fertil Steril 2004;82:1694-6.
- Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estradiol receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987;8:138-40.
- Munstedt K, Steen J, Knauf AG. Steroid hormone receptors and long term survival in invasive ovarian cancer. Cancer 2000;89:1783-91.
- Li S, McLachlan JA. Estrogen-associated genes in uterine leiomyoma. Ann N Y Acad Sci 2001;948:112-20.
- Dawood MY, Khan-Dawood FS. Plasma insulin-like growth factor-I, CA-125, estrogen, and progesterone in women with leiomyomas. Fertil Steril 1994;61:617-21.
- Andersen J, Grine E, Eng CL, Zhao K, Barbieri RL, Chumas JC, et al. Expression of connxin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol 1993;169:1266-76.
- Porter KB, Tsibris JC, Nicosia SV, Murphy JM, O’Brien WF, Rao PS, et al. Estrogen-induced guinea pig model for uterine leiomyomas: do the ovaries protect? Biol Reprod 1995;52:824-32.
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science 2005;308:1589-92.
- Fujii S, Nakashima N, Okamura H, Takenaka A, Kanzaki H, Okuda Y, et al. Progesterone-induced smooth muscle-like cells in the subperitoneal nodules produced by estrogen. Experimental approach to leiomyomatosis peritonealis disseminata. Am J Obstet Gynecol 1981;139:164-72.
- Adams AD, Hild-Petito S, Donnelly K, Fazleabas AT. Regulation of estradiol (E) and progestin (p) receptors (R) in leiomyomas during the cycle and leuprolide acetate treatment. In: Proceedings of the Forty-Ninth Annual Meeting of the American Fertility Society, October 11–14 1993; Quebec, Canada. Montreal: American Fertility Society, 1993. S134.
- Katzenellenbogen BS. Dynamics of steroid hormone receptor action. Annu Rev Physiol 1980;42:17-35.
- Watson CS, Gametchu B. Membrane-initiated steroid actions and the proteins that mediate them. Proc Soc Exp Biol Med 1999;220:9-19.
- Nemere I, Zhou LX, Norman AW. Nontranscriptional effects of steroid hormones. Receptor 1993;3:277-91.
- Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J 1996;15:1292-300.
- Ballare C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, et al. Two domains of the progesterone receptor interact with the estradiol receptor and are required for progesterone activation of the c- Src/Erk pathway in mammalian cells. Mol Cell Biol 2003;23:1994-2008.
- Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, et al. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J 2001;20:6050-9.
- Castoria G, Barone MV, Di Domenico M, Ametrano D, Migliaccio A, Auricchio F. Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J 1999;18:2500-10.
- Cartwright CA, Eckhart W, Simon S, Kaplan P. Cell transformation by pp6Oc-src mutated in the carboxy-terminal regulatory domain. Cell 1987;49:83-91.
- Morgan DO, Kaplan JM, Bishop JM., Varmus HE. Mitosis specific phosphorylation of pp60 c-src by p34cdc2 associated protein kinase. Cell 1989;57:775-86.