Neocartilage formation from predifferentiated human adipose derived stem cells in vivo1
Introduction
As defects of hyaline cartilage have been known to have poor capacity of healing spontaneously, current treatments do not result in complete regeneration of the original hyaline architecture[1–3]. Use of the tissue engineering technique could be a promising way of regenerating the articular lesions. This technique combines isolated cells, biomaterials, and environmental factors in order to produce new cartilage tissue with the typical characteristics of hyaline cartilage. As far as the choice of seeding cells is concerned, autologous cells are generally preferred to avoid the immunological rejection. Autologous cultured chondrocytes transplantation has been used in treatment of osteochondral defects[4,5]; however, the technique was restricted clinically because the procurement of many chondrocytes caused harm to the healthy cartilage. Long time culture for expansion in vitro will induce chondrocyte dedifferentiation[6]. As an alternative to chondrocytes, many researches have focused on bone marrow-derived mesenchymal stem cells (MSC) as a source of chondrogenic cells for cartilage repair[7–10]. Unfortunately, MSC also have problems, such as donor site morbidity, lower cell numbers, and limited differentiation potential as the age of the donor increases[11].
Recent studies have shown that human adipose tissue contain multipotential cells, termed processed lipoaspirate or human adipose derived stem cells (hASC)[12]. Isolation of single hASC cell populations (clones) illustrates that they have at least a trilineage potential to form bone, cartilage, and fat. Immunofluorescence and flow cytometry analysis demonstrated that these cells are similar to MSC, but have distinct differences[13]. Adipose tissue is easily obtained with relatively little discomfort, carrying a lower donor-site morbidity and is available in larger numbers[14,15]. Therefore, it seems that stem cells from adipose tissue can be a feasible choice as the seeding cells in constructing tissue engineered cartilage.
Cytokines that belong to the transforming growth factor (TGF) beta superfamily are thought to play important roles in the chondrogenic differentiation of stem cells. Although data is plentiful on the contribution of TGF beta1 to hASC chondrogenesis[12,13,16–18], reports on the role of TGF beta2 and its expression in these cells are scarce. Previous experimental results have strongly suggested that TGF beta2 was involved in chondrogenesis[19–22]. In the present study, we sought to demonstrate the chondrogenesis of hASC induced by human TGF (hTGF) beta2 both in vitro and in vivo.
The goals of our study are (i) to investigate whether chondrogenesis of hASC can be obtained by combining pellet culture and hTGF beta2 treatment in vitro; and (ii) to determine whether the differentiated hASC can maintain the chondrogenic phenotype and produce neocartilage in vivo.
Materials and methods
Harvest of hASC After informed consent from all patients and approval from the Ethics Committee of Peking university, Third hospital, leftover subcutaneous adipose tissue was obtained form 7 patients with femoral neck fracture (mean age 66 years, range 56–78) undergoing routine total hip joint replacement. To isolate hASC, adipose tissue was dissected and washed extensively with phosphate-buffered saline (PBS) to remove contaminating debris and red blood cells, then digested with 0.1% type I collagenase (Sigma-Aldrich, St Louis, MO, USA) at 37 °C and shaken at 170 rpm for 45 min. Enzyme activity was neutralized with Dulbecco’s modified Eagle’s medium (DMEM, GibcoBRL, Grand Island, NY, USA) containing 10% fetal bovine serum(FBS) and centrifuged at 800×g for 10 min to obtain a high-density cell pellet and remove the mature adipocytes. The pellet was resuspended in DMEM and filtered through a 100 µm nylon mesh to remove cellular debris. After incubation overnight at 37 °C in 5% CO2 in DMEM supplemented with 10% FBS, 100 units/mL sodium penicillin, and 100 µg/mL streptomycin, the plates were washed extensively with PBS to remove residual non-adherent red blood cells. The culture medium was changed every 2–3 d. To expand cell numbers, dense cell plaques were trypsinized and re-plated until they formed confluent monolayers that were then trypsinized and passaged into duplicate flasks.
Flow cytometry analysis hASC grown for 5 passages were characterized by flow cytometry. 1×106 cells were trypsinized and centrifuged at 1500 r/min for 5 min. The cells were then washed with PBS and fixed in 3% paraformaldehyde for 30 min at 4 °C for blocking. Unlabeled monoclonal mouse anti-human CD45 and CD29 were purchased from Pharmingen (San Diego, CA, USA). Fluorescein isothio-cyanate (FITC)-labeled mouse anti-human CD14 and CD90 were purchased from Serotec (Oxford, UK).
The following procedure was performed for staining with CD45 and CD29: the cells were incubated with the primary antibodies for 60 min. After wash, the cells were incubated with FITC-conjugated goat antimouse immunoglobulin for 30 min. CD14 and CD90 staining was performed in a single step during which cells were incubated with FITC-labeled CD14 and CD90 for 60 min. Cell fluorescence was evaluated by flow cytometry (FACSCalibur, BD Biosciences, Mountain View, CA, USA) with an excitation of 488 nm and analyzed with CELLQuest software (BD Biosciences, San Jose, CA, USA). Mouse isotype antibodies served as the controls (BioLegend, San Diego, CA, USA).
Pellet culture For the preparation of each pellet, hASC of passage 5 were trypsinized, counted and resuspended in the chondrogenic DMEM containing 10 ng/mL hTGF beta2, 1×10-7 mol/L dexamethasone, 6.25 µg/mL insulin, and 50 µg/mL ascorbate-2-phosphate. Aliquots of 1×106 cells were spun down at 500×g in 15 mL conical tubes. Pellets were divided into 2 experimental groups (hTGF beta2-treated and untreated controls). The cells were cultivated at 37 °C in a humidified atmosphere with 5% CO2 for 14 d by changing the medium every 2 d. The cells in the monolayer culture were also used as the negative control.
Release of cells from pellets After 14 d of induction, the cells were digested with trypsin for 5 min and then with 0.2% crude type II collagenase (Life Technologies, Rockville, Maryland, USA) in DMEM for 1 h. Released cells were reseeded onto the prepared coverslips. Twenty-four hours after seeding, the cells were fixed in 4% paraformaldehyde and prepared for histological and immunohistochemical analysis.
RT-PCR analysis The cell pellets were treated with Trizol reagent (Invitrogen, Carlsbad, CA, USA). Extracted cellular RNA was dissolved in RNase-free water. Reverse transcription was carried out using the Kit (SuperScriptIII, Invitrogen, USA). 1 µg RNA was used for the first strand cDNA synthesis in a total volume of 20 µL. The regimen for PCR was 5 min at 94 °C followed by the appropriate number of cycles of 5 s at 94 °C, 30 s at the proper annealing temperature for each primer pair, and 30 s at 72 °C, with a final 7 min extension at 72 °C. The reaction was also carried out with GAPDH as the housekeeping gene. Amplified PCR products were analyzed by ethidium bromide staining after gel electrophoresis. The primers for the following human cDNA are indicated in Table 1.
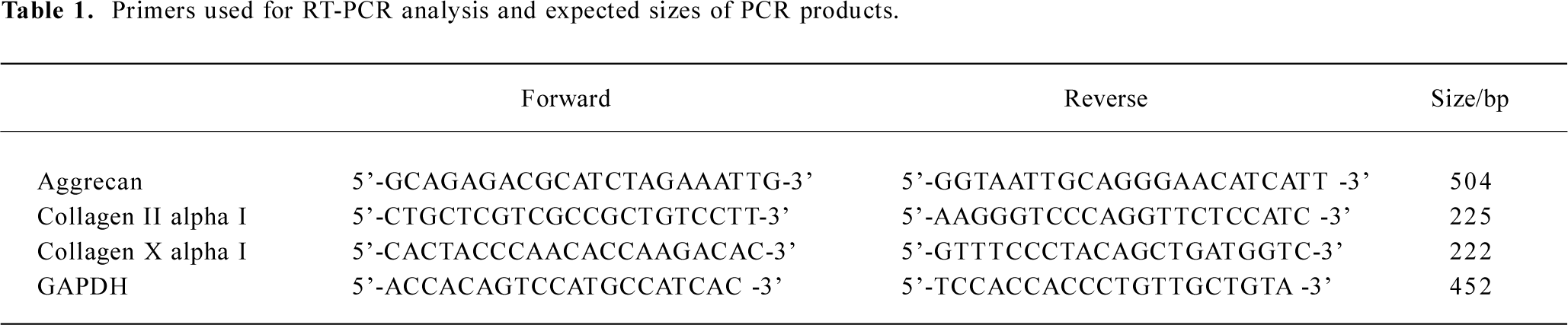
Full table
Western blot analysis Six pellets from variant conditions were washed twice with PBS and lysed in the lysis buffer [20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L ethylene diamine tetra acetic acid (EDTA), 1 mmol/L ethylene glycol tetra acetic acid (EGTA), and 1 mmol/L phenyl-methanesulfonyl fluoride (PMSF)]. After centrifugation at 18 000×g for 20 min, the supernatant was collected and protein concentrations were determined by bicinchoninic acid (BCA) assay (Pierce, Rockford, USA). Equal protein samples were separated by electrophoresis in 10% polyacrylamide gels (Bio-Rad, Hercules, CA, USA) and electrophoretically transferred to a supported nitrocellulose membran (Amersham Pharmacia, Biotech UK Limited, UK). The membranes were blocked in 5% non-fat milk diluted with Tris-buffered saline containing 0.1% Tween-20 (TBST) for 2 h and incubated overnight at 4 °C with mouse monoclonal antibodies against type II collagen, type X collagen, and β-actin (Sigma-Aldrich, St Louis, MO, USA) at a dilution of 1:500 in blocking buffer, respectively. The membranes were then washed 3 times for 10 min each with TBST and incubated for 1 h in the dark with the appropriate IRDyeTM 800-conjugated secondary antibodies (L1-COR Bioscience Inc, USA) prepared in TBST/5% non-fat milk. The signal was detected using the Odyssey Imaging System (L1-COR Bioscience Inc, USA).
Preparation of alginate beads The cells released from the induced cell pellets were prepared for alginate beads. hASC were resuspended in 1.2% low-viscosity alginate (Sigma-Aldrich, USA) solution in 0.9% NaCl at a concentration of 1×106, 5×106 and 1×107 cells/mL, respectively. Alginate beads were formed by expressing 50 µL cell suspension through a 16-gauge needle into a bath of 102 mmol/L CaCl2. The beads were allowed to gel for 10 min at room temperature. Once hardened, they were washed 3 times with 0.9% NaCl, and overlaid with culture medium (DMEM containing 10% FBS, 100 units/mL sodium penicillin, and 100 µg/mL streptomycin). The alginate beads were prepared and implanted 24 h later for the in vivo study.
Animal surgeries For the in vivo evaluation, 12 4–6-week-old female BALB/C nude mice (Peking University Experimental Animal Center, Beijing, China) were used. All animal experiments were performed in accordance with the institutional animal guidelines. Before implantation, the mice were anesthetized with ketamine (50 mg/kg) administered intraperitoneally using the sterile technique. Subcutaneous pockets were created on the dorsum of each mouse. Cell pellets after induction were cautiously placed inside the pockets. Each pocket received 4 alginate beads with the same implanted cell concentration. The location of each implant was randomized and recorded. The skin pockets were closed using Vecryl suture.
Cell pellets were harvested 2 or 4 weeks after surgery; alginate beads were explanted 12 weeks after in vivo culture. The animals were sacrificed with an inflation of CO2 atmosphere; the constructs were carefully removed and fixed in 4% paraformaldehyde for at least 24 h.
Histological and immunohistochemistry analysis The cell pellets and explants harvested from the nude mice were fixed, dehydrated, and subsequently embedded in paraffin. The sections (5 µm thick) were stained with HE. Chondrogenesis was evaluated with toluidine blue staining and immunohistochemical analysis for type II collagen. Briefly, following deparaffinization and rehydration, the sections were incubated for 10 min with 0.1% toluidine blue (Sigma-Aldrich, St Louis, MO, USA) in 0.1 mol/L sodium acetate buffer (pH 4.0) to visualize the tissue proteoglycans. For the immunohistochemical analysis of type II collagen, the sections were incubated for 10 min with newly diluted 3% H2O2 solution to destroy internal peroxidases, followed by trypsin digestion for 10 min. After digestion, the slides were incubated for 20 min with normal goat serum for blocking. The slides were then immunoblotted with a goat anti-human polyclonal antibody against type II collagen (Santa Cruz, CA, USA) overnight at 4 °C. Incubation with biotin-labeled rabbit anti-goat IgG for 30 min was followed by a 10 min incubation with horseradish peroxide-conjugated streptavidin (SP kit, Vector Laboratories, Burlingame, CA, USA). After several washes, antibody binding was visualized using diaminoben-zidine and the nuclei were counterstained with hematoxylin. The reaction was stopped by immersion in water. HASC grown on the coverslips were fixed and carried the same procedure to detect the chondrogenic phenotype. Specimens were processed using identical protocols, but without the primary antibodies were used as the negative control; human articular cartilage was used as a positive control.
Results
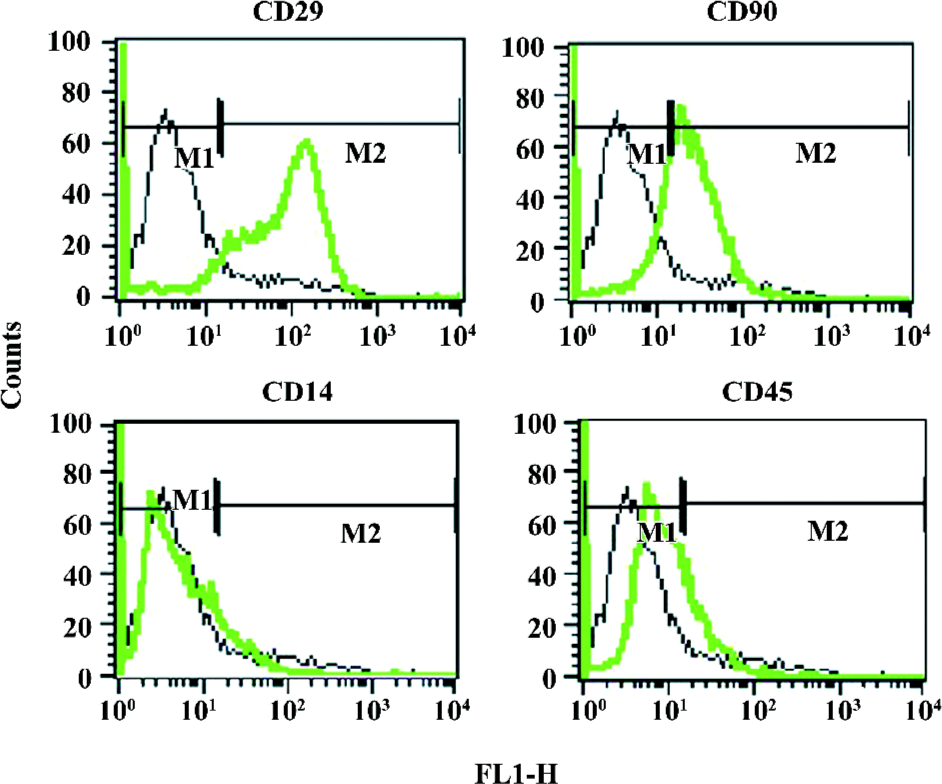
Characterization of hASC from subcutaneous adipose tissue Within 2–3 passages after the initial plating of the primary culture, hASC appeared as a monolayer of large, flat cells. They approached confluence and assumed a more spindle-shaped, fibroblastic morphology. Flow cytometry analysis of hASC of passage 5 demonstrated that the cells lacked CD14 and CD45, but the majority expressed CD29 and CD90 (Figure 1).
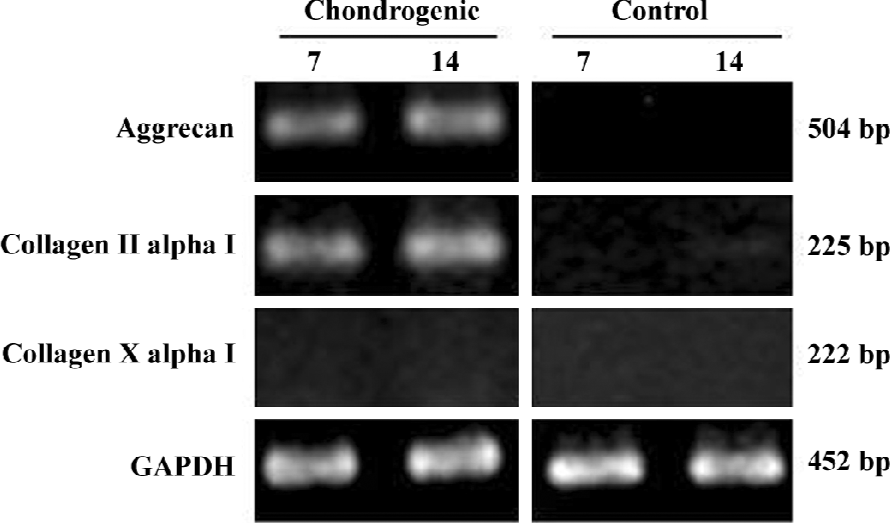
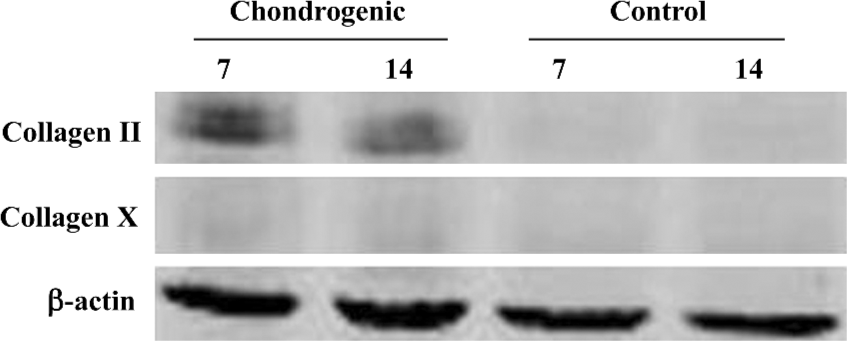
Chondrogenic differentiation of hTGF beta2-treated hASC in the pellet culture in vitro Chondrogenic marker genes were analyzed by RT-PCR; the analysis using total RNA was performed at 7 and 14 d after induction. As shown in Figure 2, the expression of the chondrocyte maker genes, type II collagen, and aggrecan were detected after 7 d of induction. The expression continued throughout the culture period. Type X collagen mRNA transcription, which indicates the presence of hypertrophic chondrocytes, was not observed during the culture periods in any of the groups. Collagen type II production was also demonstrated by Western blot analysis at 7 and 14 d time points (Figure 3).
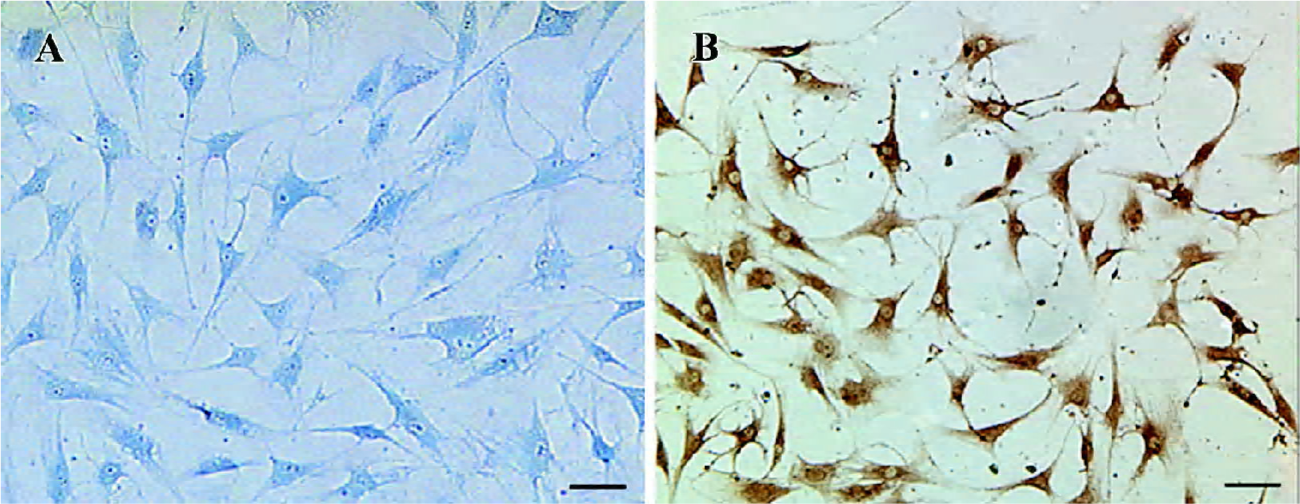
Two weeks after induction, positive clone staining with toluidine blue confirmed glycosaminoglycan (GAG) biosynthesis in the cell pellets. The presence of type II collagen was demonstrated by immunohistochemistry staining (Figure 4). When the cell pellets were digested into single cell suspension and reseeded in the monolayer, the ASC displayed a polygonal and triangle morphology compared with the primary spindle shape (Figure 5A, 5B). The pellets with control medium and hASC cultured in monolayer culture did not show any obvious evidence of chondrogenesis. In all immunohistochemical analyses, specimens examined with secondary antibody alone failed to demonstrate any immunoreactive protein.
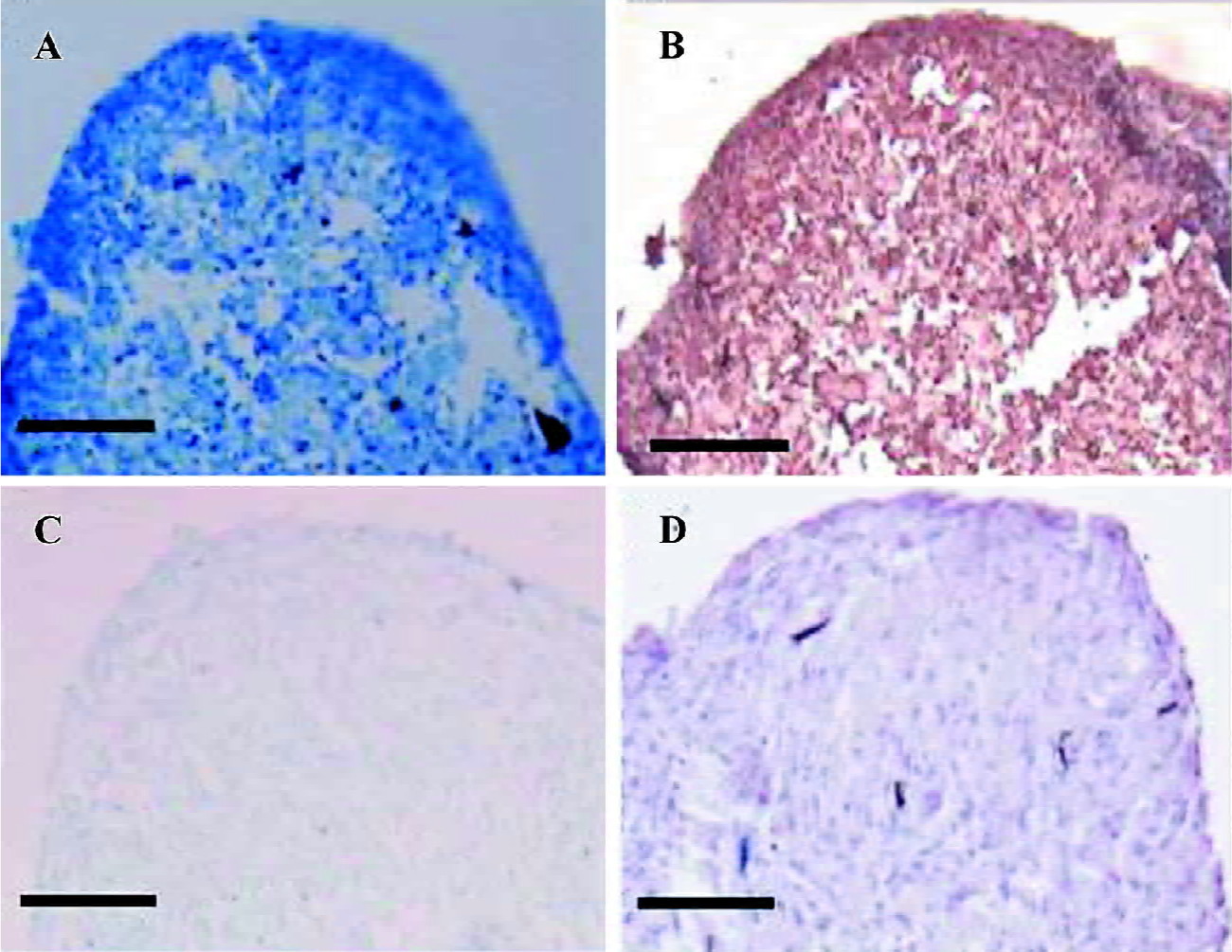
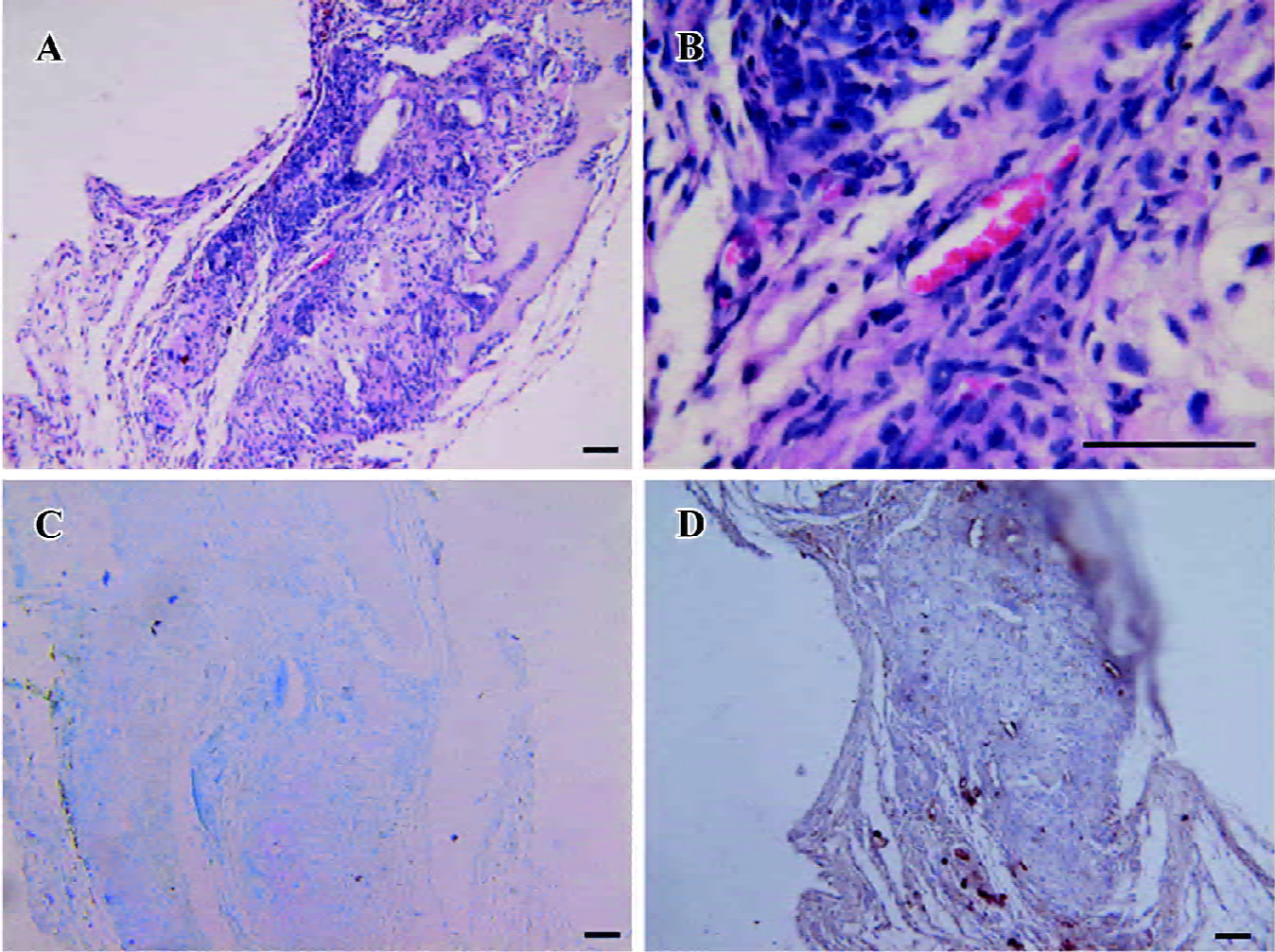
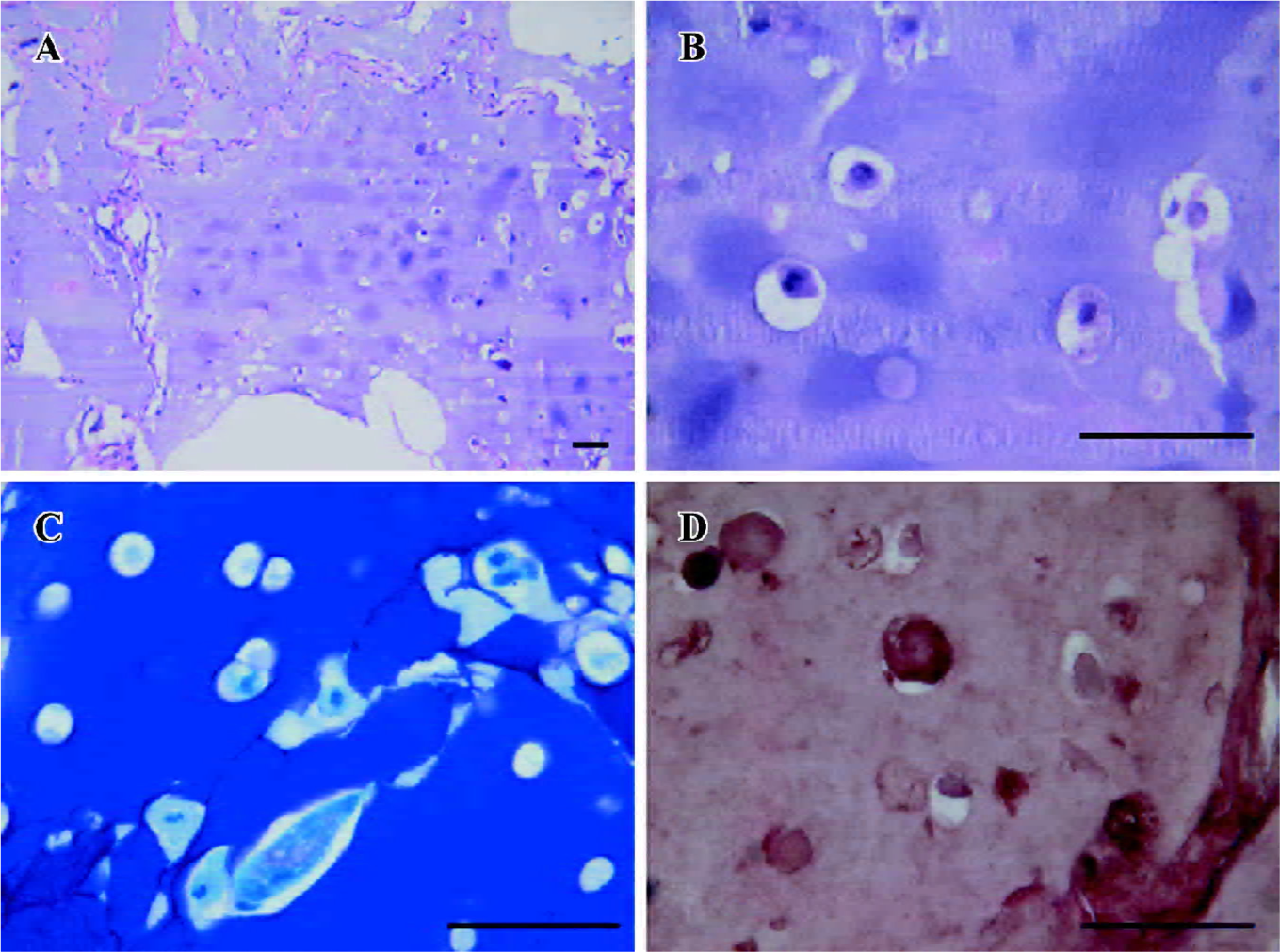
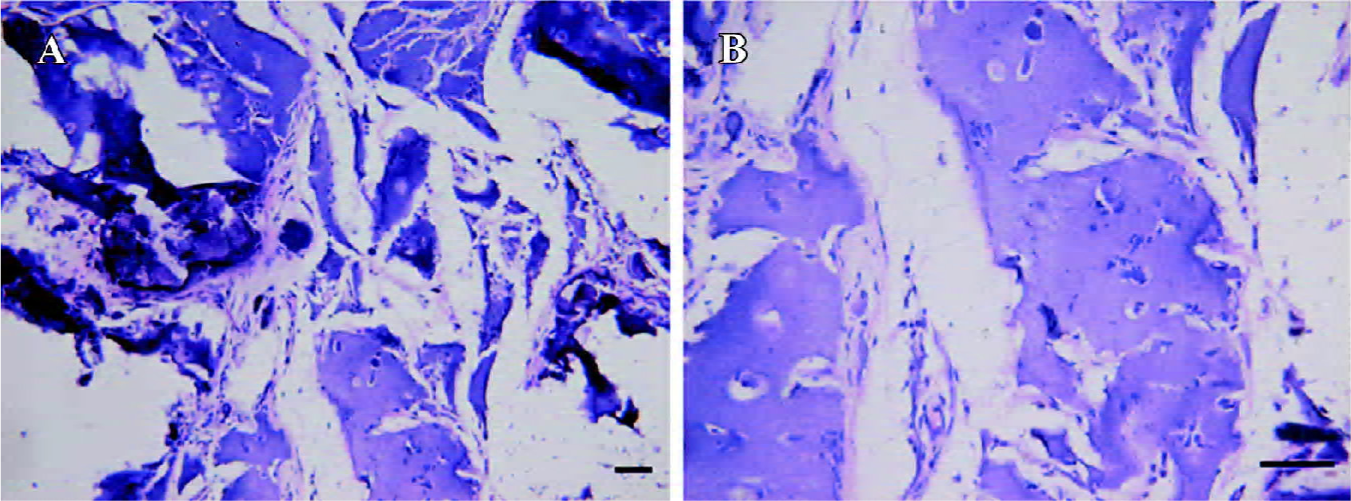
Predifferentiated hASC embedded in alginate gel produced neocartilage in vivo The cell pellets retrieved at 2 weeks became much smaller than their original size. More-over, invasions of fibrous tissues and angiogenesis were found (Figure 6A, 6B). The specimens were weakly stained with toluidine blue (Figure 6C) and immunohistochemistry against type II collagen (Figure 6D) as compared with the pellets before implantation. Four weeks after in vivo culture, no implants were identified macroscopically at the site of subcutaneous implantation, and neither cartilage nor bone formation was detected at histological examination.
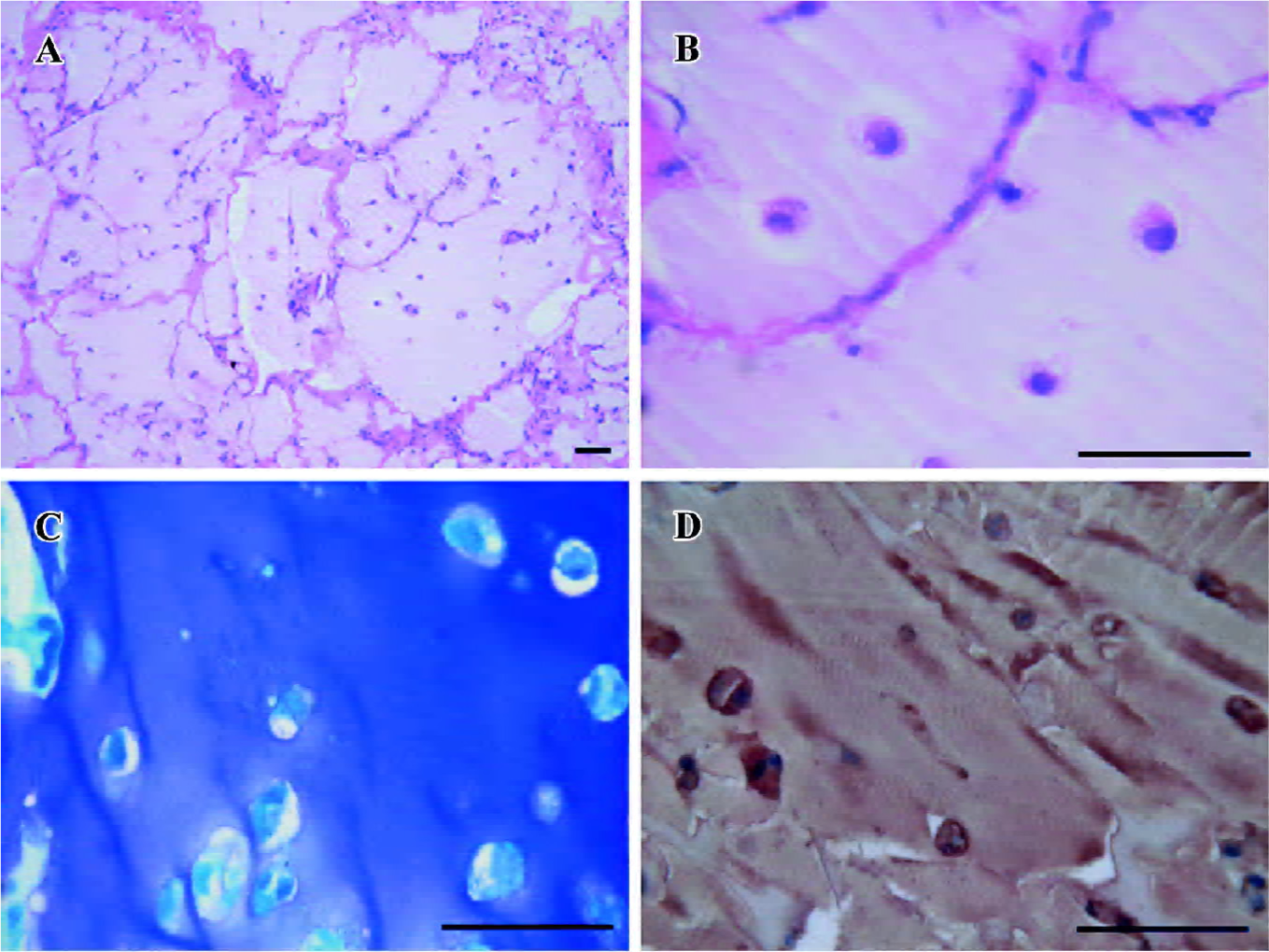
Cartilage formation of the predifferentiated hASC embedded in alginate gel was demonstrated by histological and immunohistochemistry examination. HE staining revealed that hASC appeared to be round and encased in lacunae (Figures 8B, 9B). Toluidine blue staining was positive and consistent with the presence of GAG around the cells (Figures 8C, 9C). The presence of collagen type II was revealed by positive staining of immunohistochemistry with collagen type II specific antibody (Figures 8D, 9D). The quality of neocartilage was obviously influenced by the implanted cell concentration. When the implanted cell concentration was 1×106/mL, large amounts of fibrous tissues and angiogenesis invaded into the constructs, and only scattered islands of cartilage were observed (Figure 7A, 7B). When the cell concentration was 5×106/mL, the integrity of the gel was separated by relatively limited invaded tissues (Figure 8A). Little fibrous tissues were detected when the implanted cell concentration reached 1×107/mL and the constructs displayed a typical cartilage structure (Figure 9A). In immunohistochemistry staining, the control sections processed using identical protocols, but without primary antibodies, showed negative labeling in all cases.
Discussion
Chondrogenic potential of hASC makes them a possible source of seeding cells for cartilage tissue engineering. In the present study, hASC were successfully isolated from the human subcutaneous adipose tissue. Although we could not confirm that all harvested cells were stem cells, it is possible that small amounts of pericytes, endothelial cells, and smooth muscle cells were included. An examination of expressed surface proteins revealed the presence of CD29 and CD90, but a small fraction of the cells expressed CD14 and CD45. A similar profile is found in human bone marrow stromal cells[23].
Our study demonstrated that hASC could be induced into chondrocyte-like phenotype combining pellet culture system and hTGF beta2 treatment in vitro. However, this predifferentiation did not guarantee ectopic cartilage formation in vivo unless the alginate gel was used as the cell carry scaffold, which is an appropriate 3-D scaffold, and certain implanted cell concentrations were required for the in vivo cartilage formation of the predifferentiated hASC.
TGF beta2 is regarded as an “activator”-like molecule in chondrogenic pattern formation[22]. Researchers also revealed that TGF beta2 treated dedifferentiated chondro-cytes expressing GAG and collagen type II and recovered their cartilage phenotype[24,25]. Perichondrium-derived progenitor cells cultured in medium with insulin growth factor-I (IGF-I) plus TGF beta2 produced a cartilage-specific matrix and showed evidence of chondrogenic potential[26]. Wang reported that transfection with pcDNA3.1(+)/hTGF beta2 stimulated both the short- and long-term induction of bone marrow-derived mesenchymal progenitor cells into a chondrogenic lineage[27]. All these findings indicate that TGF beta2 plays important roles in chondrogenic pattern forma-tion; however, there have been few reports on the influence of hTGF beta2 on the chondrogenic differentiation of hASC. In this study, chondrogenic induction of the hASC in pellet culture induced by hTGF beta2 was confirmed by (i) the upregulation of mature chondrocyte markers, collagen type II, and aggrecan 7 d after induction revealed by RT-PCR analysis; (ii) the positive staining of toluidine blue specific for highly sulphated proteoglycans characteristic of cartilaginous extracellular matrix (ECM); (iii) the collagen type II production revealed by Western blot analysis and positive staining of immunohistochemistry the principal constituents of cartilage ECM; and (iiii) the single cell released from the cell pellets that appeared to be polygonal and triangular after being reseeded in monolayer. Collagen type II and aggrecan are believed to be specific to articular cartilage which are known to be expressed during chondrogenesis of mesenchymal progenitor cells[28,29]. The results presented here implicated that hTGF beta2 treatment could initiate or enhance the chondro-lineage differentiation of hASC in pellet culture in vitro. One interesting finding in the study was that after culture in the monolayer with chondrogenic media, hASC tended to aggregate and formed small spheroids, but no signs of chondrogenic differentiation were detected. These combined findings demonstrate that the condensed environment of pellet culture and bioactive hTGF beta2 both contribute to the chondrogenesis of hASC in vitro.
Transplantation of multipotent adult stem cells implies risks because of their uncertain differentiation ability. Predifferentiation in vitro has been regarded as a useful step for inducing specific lineage commitment and reducing the risk of undesired tissue formation. To determine the stability of chondrocyte-like phenotype of hASC obtained in vitro, the cell pellets after induction were implanted in subcutaneous pockets of the nude mice. The results revealed that they rapidly lost the chondrocyte phenotype and could not resist the fibrous tissues and angiogenesis invasion. This was also reported by De Bary who found that in vitro-differentiated mesenchymal stem cells from the synovial membrane could not form ectopic stable cartilage in vivo[30]. It was implied that predifferentiation in vitro was not sufficient to guarantee stable lineage commitment and restriction of differentiation; however, hASC embedded in alginate gel kept their ability of synthesizing aggrecan and collagen type II after several months in in vivo culture. These differences may be explained by the biophysical environment which alginate gel provides. Alginate itself constitutes a relatively containing environment, and the cells in alginate hydrogel were embedded in lacunae like chondrocytes being surrounded by specific ECM. This 3-D environment may be superior in keeping the chondrogenic phenotype compared with the pellet culture system in vivo. Another significant demonstration in this study is that certain cell density is required for the cartilage formation ability of the predifferen-tiated hASC in vivo. A low implanted concentration (1×106/mL) in the alginate gel could not resist the fibrous tissues invasion. Alginate gel integrity was separated into pieces by large amount of fibrous tissues, and as the cell density increased, less invasion was observed, and the structure of the neocartilage became similar to the native cartilage. The results implied that a specific threshold level was required for in vivo cartilage tissue formation by the predifferentiated hASC in alginate gel. High quality cartilage was obtained only when the cell concentration reached a definite level. Based on the results in our study, an implanted cell concentration of 1×107/mL or higher was recom-mended. Alginate hydrogels have been extensively used as a biomaterial scaffold in chondrogenic induction of stem cells and to regenerate cartilage tissue[17,18,31–36]; however, as far as the future clinical application is concerned, alginate gel alone as a cell carrier scaffold cannot stand mechanical loading nor surgical handling; an ideal biomaterial is required to provide initial strength to the graft.
Chondrogenesis of stem cells may therefore be determined by the microenvironment of the cells, including secreted factors, integral membrane proteins, and the extracellular matrix[37]. In this regard, subcutaneous implantation actually is not an optimal site for cartilage formation. Wakitani[38] et al demonstrated that osteochondral progenitor cells from rabbit bone marrow, when transplanted into an osteochondral defect, formed bone in the side adjacent to the bone marrow and generated new cartilage in the side abutting joint cavity. The author pointed out that the diarthrodial joint provided a good microenvironment for cartilage formation. The fate of in vitro predifferentiated hASC seeded in alginate gel when implanted in the diarthro-dial joint cavity is unknown; however, high quality tissue-engineered cartilage is expected.
In summary, our study demonstrated chondrogenesis of hASC in vitro could be induced by combining pellet culture and hTGF beta2 treatment. Predifferentiated hASC embedded in alginate gel had the ability of producing neocartilage in vivo. The results implied important functional considerations for autologous hASC in the treatment of articular cartilage injuries. However, animal models of joint surface defect repair are needed to evaluate potential use of hASC and their phenotypic behavior within the articular cartilage microenvironment.
References
- Beiser IH, Kanat IO. Subchondral bone drilling: a treatment for cartilage defects. J Foot Surg 1990;29:595-601.
- O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am 1998;80:1795-812.
- Gilbert JE. Current treatment options for the restoration of articular cartilage. Am J Knee Surg 1998;11:42-6.
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889-95.
- Brittberg M, Nilsson A, Lindahl A, Ohlsson C, Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res 1996.270-83.
- Hunziker EB. Articular cartilage repair: problems and perspec-tives. Biorheology 2000;37:163-4.
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 1998;238:265-72.
- Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 2001;268:189-200.
- Martin I, Shastri VP, Padera RF, Yang J, Mackay AJ, Langer R, et al. Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. J Biomed Mater Res 2001;55:229-35.
- Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, et al. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng 2004;32:911-23.
- Banfi A, Bianchi G, Notaro R, Luzzatto L, Cancedda R, Quarto R. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng 2002;8:901-10.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7:211-28.
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279-95.
- Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 2003;5:362-9.
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem 1997;64:278-94.
- Huang JI, Zuk PA, Jones NF, Zhu M, Lorenz HP, Hedrick MH, et al. Chondrogenic potential of multipotential cells from human adipose tissue. Plast Reconstr Surg 2004;113:585-94.
- Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun 2002;290:763-9.
- Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 2004;25:3211-22.
- Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development 1991;111:131-43.
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 1997;124:2659-70.
- Schofield JN, Wolpert L. Effect of TGF-beta 1, TGF-beta 2, and bFGF on chick cartilage and muscle cell differentiation. Exp Cell Res 1990;191:144-8.
- Miura T, Shiota K. TGFbeta2 acts as an “activator” molecule in reaction-diffusion model and is involved in cell sorting phenomenon in mouse limb micromass culture. Dev Dyn 2000;217:241-9.
- Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 2004;14:311-24.
- van Osch GJ, Marijnissen WJ, van der Veen SW, Verwoerd-Verhoef HL. The potency of culture-expanded nasal septum chondrocytes for tissue engineering of cartilage. Am J Rhinol 2001;15:187-92.
- van Osch GJ, van der Veen SW, Verwoerd-Verhoef HL. In vitro redifferentiation of culture-expanded rabbit and human auricular chondrocytes for cartilage reconstruction. Plast Reconstr Surg 2001;107:433-40.
- van Osch GJ, van der Veen SW, Burger EH, Verwoerd-Verhoef HL. Chondrogenic potential of in vitro multiplied rabbit perichondrium cells cultured in alginate beads in defined medium. Tissue Eng 2000;6:321-30.
- Wang WG, Lou SQ, Ju XD, Xia K, Xia JH. In vitro chondrogenesis of human bone marrow-derived mesenchymal progenitor cells in monolayer culture: activation by transfection with TGF-beta2. Tissue Cell 2003;35:69-77.
- Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol 2001;189:275-84.
- Stokes DG, Liu G, Dharmavaram R, Hawkins D, Piera-Velazquez S, Jimenez SA. Regulation of type-II collagen gene expression during human chondrocyte de-differentiation and recovery of chondrocyte-specific phenotype in culture involves Sry-type high-mobility-group box (SOX) transcription factors. Biochem J 2001;360:461-70.
- De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum 2004;50:142-50.
- Ma HL, Chen TH, Low-Tone Ho L, Hung SC. Neocartilage from human mesenchymal stem cells in alginate: implied timing of transplantation. J Biomed Mater Res A 2005;74:439-46.
- Paige KT, Cima LG, Yaremchuk MJ, Schloo BL, Vacanti JP, Vacanti CA. De novo cartilage generation using calcium alginate-chondrocyte constructs. Plast Reconstr Surg 1996;97:168-78.
- Fragonas E, Valente M, Pozzi-Mucelli M, Toffanin R, Rizzo R, Silvestri F, et al. Articular cartilage repair in rabbits by using suspensions of allogenic chondrocytes in alginate. Biomaterials 2000;21:795-801.
- Chubinskaya S, Huch K, Schulze M, Otten L, Aydelotte MB, Cole AA. Gene expression by human articular chondrocytes cultured in alginate beads. J Histochem Cytochem 2001;49:1211-20.
- Chang SC, Rowley JA, Tobias G, Genes NG, Roy AK, Mooney DJ, et al. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. J Biomed Mater Res 2001;55:503-11.
- Lin Y, Luo E, Chen X, Liu L, Qiao J, Yan Z, et al. Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J Cell Mol Med 2005;9:929-39.
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science 2000;287:1427-30.
- Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 1994;76:579-92.
