Prenatal exposure to lipopolysaccharide results in increases in blood pressure and body weight in rats1
Introduction
Cardiovascular disease ranks among the leading causes of morbidity and mortality in adult populations in most countries in the world. Significant progress in understanding the etiology of cardiovascular disease has come from recent recognition that inflammation plays a key role in its development[1]. Most of the studies now focus on the role of inflammation in some cardiovascular diseases, such as atherosclerosis[2], and ischemic heart disease[3] or heart failure[4,5]. Among these diseases, hypertension is a major risk factor and its underlying pathogenetic mechanisms are still not well elucidated. What we know about hypertension is only that its pathogenesis is a complex and multifactorial phenomenon affected by genetic predisposition, metabolism, and environment. Now there is accumulating clinical evidence showing that inflammation is also related to hypertension to some extent, which was also discovered in some cardiovascular diseases. Sesso and colleagues[6] reported a positive relationship between increased serum levels of the C-reactive protein and the risk of development of incident hypertension in participants of the Women’s Health Study. A total of 20525 women were followed up prospectively for a median of 7.8 years, during which time approximately one fourth of the women acquired elevated blood pressure. Those with higher levels of the C-reactive protein were more likely to develop hypertension. As we know, C-reactive protein levels in the upper ranges of the normal distribution are widely believed to reflect a state of low-grade chronic inflammation. Therefore, the association between higher C-reactive protein levels and new-onset hypertension led us to focus our attention on the relationship between inflammation and hypertension. We wondered whether inflammation participated in the pathogenesis of hypertension.
Bautista et al[7] discovered an elevated level of inter-leukin-6 (IL-6) in a study of hypertensive patients. Mean-while, Chae et al[8] found the relationship between this cytokine level and the values of blood pressure. More interestingly, Samuelsson[9] showed that prenatal exposure to IL-6 resulted in hypertension and increased hypothalamic-pituitary-adrenal (HPA) axis activity in adult rats. It has been commonly accepted that IL-6 is a pro-inflammatory factor which is produced by various cells of the organism, including monocytes, macrophages, fibroblasts, and endothelial cells. Thus, prenatal exposure to IL-6 might induce maternal systemic inflammation[10]. Moreover, systemic inflammatory response during pregnancy represents a form of stressful event for the fetus. Nevertheless, there are some data suggesting that IL-6 possesses anti-inflammatory properties[11]. Is hypertension in offspring induced single-handedly by IL-6 or by maternal systemic inflammation? This hypothesis has not been tested. Therefore, we chose lipopolysaccharide (LPS) from Gram-negative bacteria to act as a non-specific immunostimulant to induce maternal systemic inflammation. LPS has been shown to stimulate the HPA axis via the release of cytokines including IL-6[12,13]. Prenatal exposure to LPS results in increased basal plasma corticosterone levels and a reduction in the number of central glucocorticoid receptors, which are important factors contributing to the regulation of blood pressure. Therefore, we speculated that prenatal exposure to LPS would probably cause hypertension in offspring. One probable way through which inflammation caused hypertension might be via maternal inflammation in utero, which can affect the development of the offspring organs.
Materials and methods
Animals Nulliparous, time-mated Sprague-Dawley (SD) rats were purchased from the Animal Center of the Third Military Medical University (Chongqing, China). All the rats had ad libitum access to both standard laboratory rat chow and tap water and were caged individually in a room under constant temperature (24 °C) and a 12 h/12 h light/dark cycle until parturition. Pups were raised with a lactating mother until 4 weeks of age, and thereafter they lived in cages with 3 rats per cage. All surgical and experimental procedures were carried out in accordance with institutional animal care guidelines.
Prenatal LPS exposure After 1 week of acclimation, the dams were randomly divided into 2 groups. Each group contained 8 pregnant rats. On their 8th, 10th, and 12th gestational days, the dams (n=8) received ip injections of 0.79 mg/kg LPS (Escherichia coli 026:B6, Sigma, St Louis, MO, USA) dissolved in 1 mL sterile saline. The control dams (n=8) received sterile saline only. Gestation lasted for 20–22 d. Two treated dams and 1 control dam did not deliver any pups. After birth, the litters were counted and weighed. The pups were, after recording the birth weight, randomly chosen to be included in this study (males: controls, n=12; LPS-treated, n=12; females: controls, n=12; LPS-treated, n=12) within 1 week. All of the chosen pups were redistributed within the same treatment group of dams so that each group had 3 males and 3 females per lactating mother. They were left undisturbed until 4 weeks of age when they were weaned.
Blood pressure measurement Arterial blood pressure was measured in conscious rats using the tail-cuff method (ML125, PowerLab, ADInstruments, Castle Hill, Australia)[14]. Before the measurement, the animals were placed inside a warming chamber (about 34 °C) for 15 min. The aim of the procedure was to calm the animals and dilate the tail blood vessels. Then the rats were placed in plastic restrainers. It was ensured that Perspex restraint cages were selected to fit the animal comfortably. The rats were placed in the Perspex cylinder restraint cage and the depth was adjusted forwards and backwards within the tube to restrict movement. The tube was kept in a proper position to prevent the animal from turning around. A cuff with a pneumatic pulse sensor was attached to the tail and was positioned at the proximal end of the tail. The rats were allowed to habituate to this procedure for 7 d before the experiments. The active site of the pulse transducer was located on the ventral surface of the tail, directly below the caudal artery. The transducer was positioned directly following the tail cuff. Maximum sensitivity was achieved when the artery was positioned above the most sensitive position on the transducer. Systolic blood pressure (SBP) appeared when the cuff pressure corresponded to the restoration of the first caudal artery pulse. Arterial blood pressure was measured at least 3 times for each animal.
Body weight The offsprings’ body weights were regularly monitored once every 2 weeks during the experiments from the age of 4 to 24 weeks.
Food intake When the rats reached 15 weeks of age, food consumption for each cage was recorded once a day. Each cage contained 3 rats which were presented with the same amount of food, and their intake was measured the following day by subtracting the uneaten food. This was done during 1 week and was calculated as food intake in grams per rat per day as well as in grams per body weight per day.
Adipose tissue weight At the end of the 24th week, the rats were killed by decapitation. The epididymal, parametrial, mesenteric, retroperitoneal, inguinal, and around the kidney adipose tissues were rapidly excised and weighed.
Serum level of leptin The blood samples were obtained by decapitation. Then they were allowed to clot for half an hour at room temperature before centrifuging at 3000×g for 20 min. The serum was removed and stored at -20 °C until radioimmunoassay (Linco Research Company, St Charles, Missouri, USA).
Statistical analysis All data were expressed as mean± SD. The means were evaluated by Student’s unpaired t-test. P<0.05 was considered to be statistically significant.
Results
Dams and litters There were no significant differences in the number of progeny per dam [9.7±3.6 (5–15) and 9.0±2.9 (5–13) pups/dam for treated (n=6) and control (n=7) dams, respectively; P=0.72]. Meanwhile, no significant differences were discovered for the ratio of male births to total births in each litter [LPS-treated, 0.51±0.08 (0.40–0.60); controls, 0.49±0.11 (0.40–0.67); P=0.61]. The body weights of the newborn pups did not differ much between the LPS and control groups [male pups: 6.1±0.9 (4.8–7.7) and 6.3±0.8 (5.1–7.7) g; P=0.67; female pups: 6.0±0.8 (4.7–7.4) and 6.2±0.7 (5.1–7.6) g; P=0.26].
Blood pressure measurement It was found that all offspring with prenatal exposure to LPS had elevated SBP from the age of 6 to 24 weeks. As the offspring rats grew older, the blood pressure of those with prenatal exposure to LPS began to rise and was higher than that of the controls. The difference between these 2 groups become more significant (123.40±9.07 vs 109.35±5.89 mmHg, 120.66±5.52 vs 108.98±4.21 mmHg, male and female rats, respectively, compared with the same gender controls at the end of 20th week; P<0.01), although the SBP in all offspring had not reached the standard level of hypertension in SD rats (Figure 1).
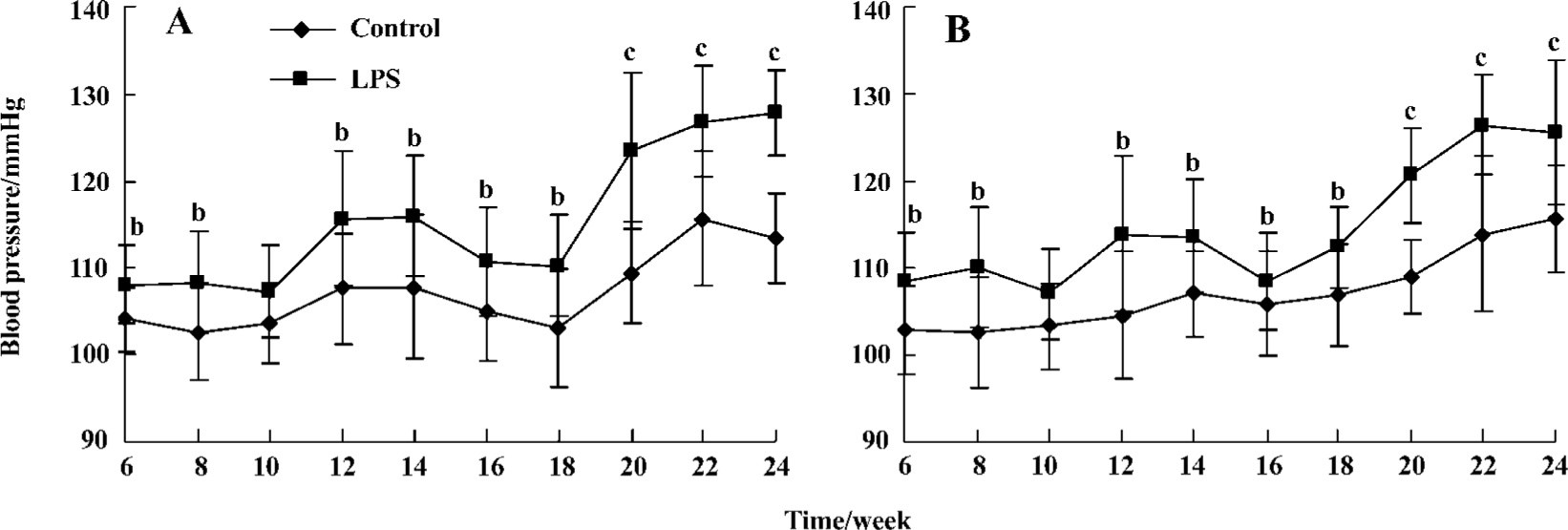
Body weight In the male offspring, the total body weights had statistical significance since the age of 6 weeks (114.95±4.92 g vs 107.95±7.19 g; P<0.05). However, there was a statistically significant difference since the age of 14 weeks in the female offspring (230.47±7.94 g vs 212.86±7.66 g; P<0.01) (Figure 2).

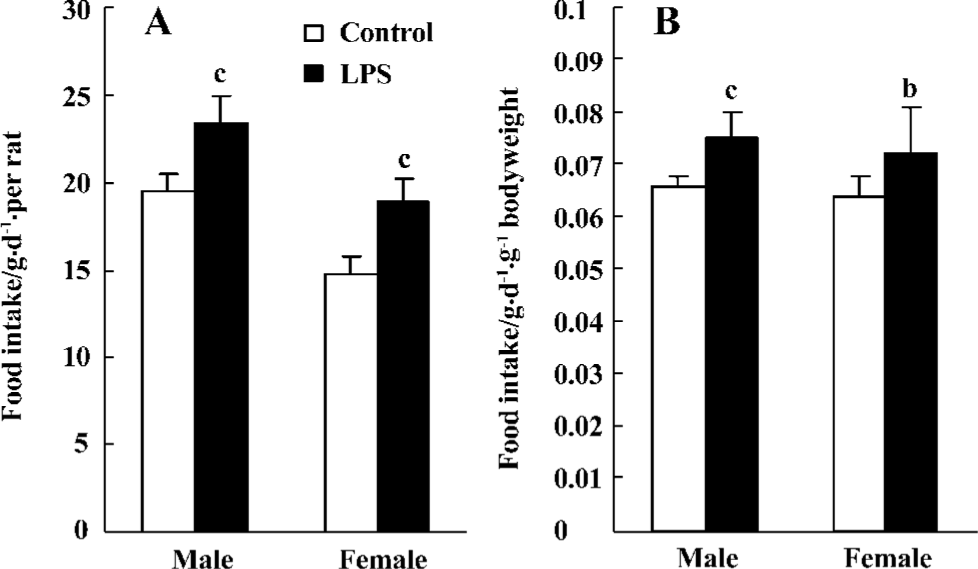
Food intake The food intake of each rat increased in 15-week-old male LPS offspring compared with the control offspring [23.4±1.6 (21.6–25.9) and 19.6±1.0 (18.2–21.0) g/day, respectively; P<0.01]. The food intake of each female offspring was [19.0±1.3 (17.6–20.9) and 14.7±1.1 (13.2–16.0) g/day, respectively; P<0.01]. The food intake, measured by per body weight per day, in the male offspring was [0.075±0.005 (0.067–0.085) and 0.066±0.002 (0.063–0.073) g·d-1·g-1 bodyweight, respectively; P<0.01] and that of the female offspring was [0.072±0.009 (0.061–0.087) and 0.064±0.004 (0.057–0.070) g·d-1·g-1 bodyweight, respectively; P<0.05](Figure 3).
Adipose tissue weight The total weight of various tissues, including epididymal, retroperitoneal, mesenteric, inguinal, and around the kidney fat depots in male offspring and parametrial instead of epididymal fat depots in female offspring 24 weeks of age are shown in Table 1. All the fat depots weight in the abdomen were significantly heavier in LPS offspring, regardless of sex, than that of the controls (P<0.01).
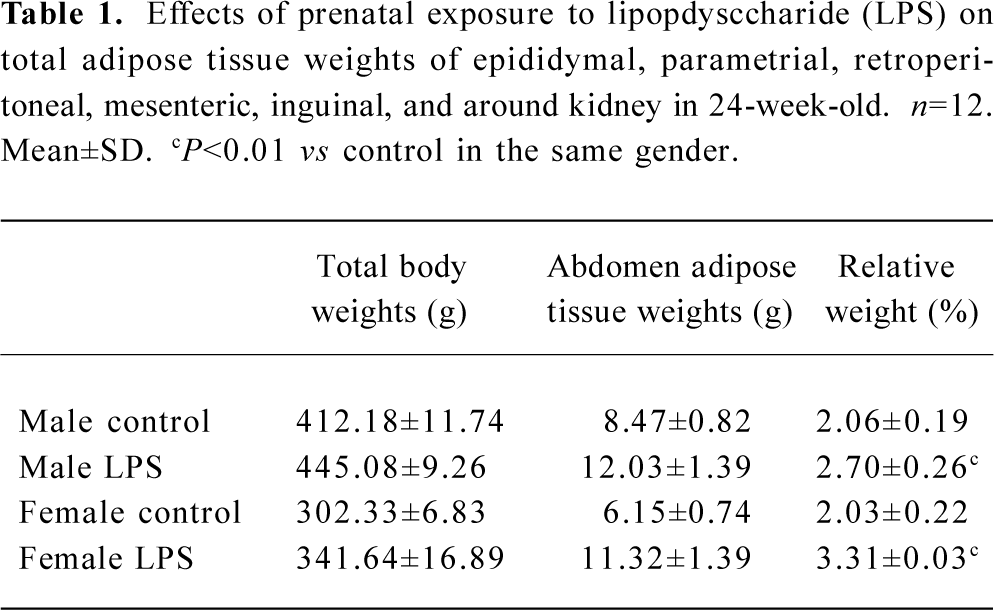
Full table
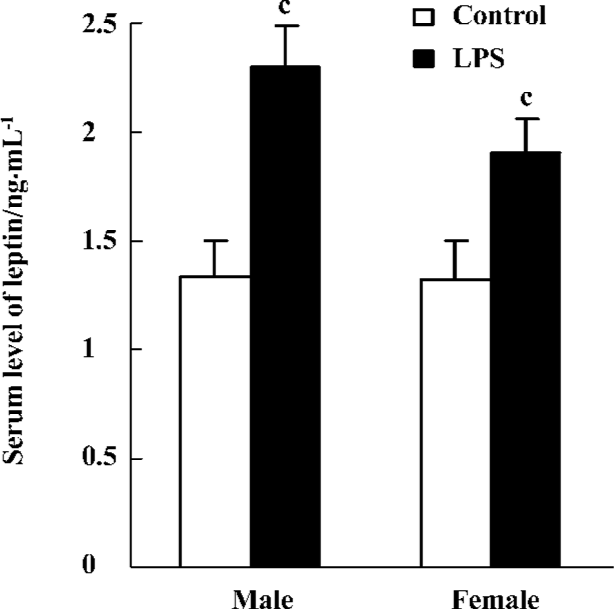
Serum level of leptin The serum level of leptin was significantly higher in offspring with prenatal exposure to LPS than that of the controls (P<0.01) both in males (2.30±0.19 ng/mL vs 1.34±0.16 ng/mL, respectively) and females (1.91±0.15 ng/mL vs 1.32±0.16 ng/mL, respectively; Figure 4).
Discussion
Hypertension is a major risk factor for cardiovascular disease, so effective control of hypertension is an important goal of cardiovascular therapies. Current evidence supports a central role for inflammation in all phases of atherosclerosis[15]. The recognition that atherosclerosis is a special case in the general category of inflammation provides a tool to unify and simplify the understanding of complex processes. The possibility that hypertension, at least in part, is a product of arterial pathology similar to that of atherosclerosis is intriguing. It was thus suggested that there might be a correlation between inflammation and hypertension. Some researchers reported that prenatal exposure to LPS could result in obesity and insulin resistance in adult male rat offspring[16]. As we know, obesity and insulin resistance are risk factors for hypertension. We noticed that LPS could alter maternal immune systems and induce maternal inflammation[17]. Maternal LPS exposure can also result in the existence of cytokines in the amniotic fluid and corticotrophin-releasing hormone in the fetal rat brain[18]. Thus, maternal inflammation may participate in the pathogenesis of hypertension in adult rat offspring. In order to test such a hypothesis, we chose LPS, which acted as a non-specific immunostimulant and intraperitoneally injected it to pregnant rats. The dosage of LPS we chose could induce systemic inflammation, resulting in a low percentage of fetal anomalies, but not abortion[19]. In addition, the rationale for choosing time phages on gestational d 8, 10, and 12 was that this period was in the second trimester, a period of early fetal brain development[20].
The present study found that prenatal exposure to LPS led to increases in blood pressure that might potentially develop into hypertension in male and female offspring. This phenomenon has not been reported elsewhere until now. The mechanisms of increases in blood pressure should be related to maternal inflammation induced by LPS. Maternal systemic inflammation may serve as a stressful event for fetus development and can induce high blood pressure in adult offspring.
We also found that the offspring (regardless of sex) with prenatal exposure to LPS showed hyperphagia and increases in body weights and abdominal fats weight when they grew up. All of the chosen pups were redistributed within the same treatment group of dams when they were born. Each group consisted of 3 males and 3 females per lactating mother. Therefore, the offspring in either the LPS group or the control group had the same pattern of lactation. However, the offspring in the LPS group still showed heavier body weight since the age of 6 weeks in males and at the age of 14 weeks in females, and hyperphagia in the 15th week compared with those in the control group. This hyperphagia and increases in body weight in the offspring might have been induced by maternal inflammation as well as increases in blood pressure. The male offspring became fat earlier than the female ones, which might be due to the difference in sex. Meanwhile, the basic blood pressure in the LPS offspring was higher than that of the control offspring since 6 weeks of age. However, body weight showed no change in LPS female offspring as compared with the control offspring. In the male offspring with prenatal exposure to LPS, the blood pressure had little change compared with that of the control group. However, the body weight in the LPS offspring increased more than that of the control group. Thus, increases in blood pressure were body weight-independent in offspring with prenatal exposure to LPS. Increases in body weight induced by maternal inflammation was perhaps only a risk factor in the development of hypertension[21].
Meanwhile, the serum level of leptin in both males and females was elevated in the LPS group than in the control group. Leptin was produced by adipose tissue and its receptors were localized in the hypothalamic area in the brain[22]. The increased food intake, despite higher levels of leptin in serum, showed that the regulation of food intake by leptin was inefficient after maternal immune challenge. Leptin, also considered a pro-inflammatory cytokine that belongs to a family of long-chain helical cytokines and has structural similarity with IL-6, prolactin (a growth hormone), plays an important role in inflammatory processes and immune responses. The increase in leptin production that occurred during infection and inflammation strongly suggests that leptin is a part of the cytokine network that governs the inflammatory-immune response[23]. Thus, in the LPS offspring, the higher levels of leptin implied that the offspring might have been in an inflammatory and immune-stimulated state.
In conclusion, our results have demonstrated that prenatal exposure to LPS results in increases in blood pressure that might develop into hypertension in adult rats. This discovery may serve to modify our current strategy and medical interventions for the prevention or treatment of cardiovascular disease. The exact mechanisms underlying the development of increases in blood pressure and body weight in the LPS offspring remain unclear, so further studies should be conducted to determine the mechanisms of how maternal systemic inflammation results in increases in blood pressure and body weight in offspring.
References
- Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm 2006;12:4229-45.
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340:115-26.
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, and coronary heart disease: isinterleukin-6 the link? Atherosclerosis 2000;148:209-14.
- Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990;323:236-41.
- Rodriguez-Reyna TS, Arrieta O, Castillo-Martinez L, Orea-Tejeda A, Guevara P, Rebollar V, et al. Tumour necrosis factor alpha and troponin T as predictors of poor prognosis in patients with stable heart failure. Clin Invest Med 2005;28:23-9.
- Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA 2003;290:2945-51.
- Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J Hum Hypertens 2005;19:149-54.
- Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension 2001;38:399-403.
- Samuelsson AM, Ohrn I, Dahlqren J, Eriksson D, Angelin B, Folkow B, et al. Prenatal exposure to interleukin-6 results in hypertension and increased hypothalamic-pituitary-adrenal axis activity in adult rats. Endocrinology 2004;145:4897-911.
- Harden LM, du-Plessis I, Poole S, Laburn HP. Interleukin-6 and leptin mediate lipopolysaccharide-induced fever and sickness behavior. Physiol Behav 2006;89:146-55.
- Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB J 2004;18:1439-41.
- Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res 2003;9:3-24.
- Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, Russell JA. Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. J Neurosci 2005;25:5117-26.
- Górska D, Andrzejczak D. Influence of mianserin on the activity of some hypotensive drugs in spontaneously hypertensive rats. Pol J Pharmacol 2003;55:409-17.
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135-43.
- Nilsson C, Larsson BM, Jennische E, Eriksson E, Bjorntorp P, York DA, et al. Prenatal endotoxemia results in obesity and insulin resistance in adult male offspring. Endocrinology 2001;142:2622-30.
- Urakubo A, Jarkoq LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res 2001;47:27-36.
- Gayle DA, Beloosesky R, Desai M, Amidi F, Nunez SE, Ross MG. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am J Physiol Regul Intergr Comp Physiol 2004;286:R1024-9.
- Ornoy A, Altshuler G. Maternal endotoxemia, fetal anomalies and central nervous system damage: a rat model of a human problem. Am J Obstet Gynecol 1976;124:196-204.
- Paxinos G, Tork I, Tecott LH, Valentino KL. Atlas of the developing rat brain. London: Academic Press; 1991.
- Graundy SM. Inflammation, hypertension and the metabolic syndrome. JAMA 2003;290:3000-2.
- Lord GM. Leptin as a proinflammatory cytokine. Contrib Nephrol 2006;151:151-64.
- Otero M, Lago R, Lago F, Casanueva FF, Diequez C, Gomez-Reino JJ, et al. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett 2005;579:295-301.
