Efficacy of sequential treatment of HCT116 colon cancer monolayers and xenografts with docetaxel, flavopiridol, and 5-fluorouracil1
Introduction
Docetaxel, which inhibits microtubule depolymerization, is a chemotherapeutic agent that inhibits tumor reassembly and has been reported to induce apoptosis and inhibit cell proliferation[1]. Presently, docetaxel has been exploited in the treatment of solid tumors, both as a single agent and combined with other chemotherapy agents[1–3]. However as a single agent, docetaxel induces response rates of 16%–24%. When combined with other chemo-drugs, such as 5-FU, it has produced response rates of 33%–56%[4] . Therefore, investigators have examined combining docetaxel with other chemotherapeutic agents to achieve better response and cure rates[5], both in preclinical and clinical models[6–9].
Flavopiridol, the first inhibitor of multiple cyclin-dependent kinases to enter clinical trials[10–12], inhibits cell growth[13] and induces apoptosis in a variety of human cancer cell lines in vitro and in vivo[14–17]. Flavopiridol also induces cell-cycle arrest and tumor regression in the clinical settings[18], and displays sequence-dependent cytotoxic synergy with docetaxel in gastric cancer preclinical models[19,20].
Studies also suggested the feasibility of synergistic interaction between flavopiridol and 5-FU in breast cancer[21,22]. 5-FU induces apoptosis and causes cell injury of several cancer cell lines. It acts by blocking the enzyme thymidylate synthase and inhibiting the synthesis of both RNA and DNA. 5-FU is used for the treatment of cancers, but many tumors are resistant to this chemotherapeutic agent[23]. Efforts to improve response rates have led to the combination of 5-FU with other chemotherapeutic drugs such as CPT-11 and oxaliplatin to improve response rate and prolong survival[24]. Despite these improvements, more than half of patients undergo chemotherapy for advanced colon cancer without any measurable shrinkage of their disease. Thus, further opportunities to enhance the effectiveness of 5-FU in colon cancer are being investigated.
In mammalian models, the sequential combination of docetaxel and 5-FU has shown synergistic cytotoxicity[19], and synergistic interaction between flavopiridol and 5-FU has also been observed[21,22]. Sequential treatment of solid tumors with docetaxel, flavopiridol and 5-FU appears an attractive consideration. The purpose of this study was to define the most effective sequential treatment scheme with docetaxel, flavopiridol and 5-FU in the human colon cancer monolayers and xenografts in the hope that the present findings provide useful information for the establishment of clinical protocols.
Materials and methods
Cell culture and drug treatments for cell line The colon cancer adenocarcinoma cell line HCT116 was obtained from the American Tissue Culture Collection (Manassas, VA, USA). These cells were propagated and maintained in DMEM (Gibco BRL, Rockville, MD, USA) and supplemented with 10% fetal bovine serum (Gibco BRL), 1% penicillin/streptomycin and 1% L-glutamine. The cells were maintained with 5% CO2. Docetaxel and flavopiridol were supplied by Aventis Pharmaceuticals (Bridgewater, NJ, USA) and 5-FU was purchased by INC Pharmaceuticals (Costa-mesa, CA, USA). All chemotherapy agents were diluted in DMEM (Dulbecco’s Modified Eagle Medium) before use.
MTT assay Five thousand HCT116 cells were seeded in a 96-well plate. Twenty-four hours later cells were treated with: (1) no drug as control; (2) docetaxel for 1 h; (3) flavopiridol for 24 h; (4) 5-FU for 24 h; (5) docetaxel for 1 h followed by flavopiridol for 24 h; (6) docetaxel for 1 h followed by 5-FU for 24 h; (7) flavopiridol for 24 h followed by docetaxel for 1 h; (8) 5-FU for 24 h followed by docetaxel for 1 h; (9) flavopiridol for 24 h followed by 5-FU for another 24 h; (10) 5-FU for 24 h followed by flavopiridol for another 24 h; (11) docetaxel for 1 h followed by flavopiridol for 24 h and 5-FU for another 24 h; (12) docetaxel for 1 h followed by 5-FU for 24 h and flavopiridol for an additional 24 h; (13) flavopiridol for 24 h followed by 5-FU for 24 h and docetaxel for 1 more hour; (14) 5-FU for 24 h followed by flavopiridol for 24 h and docetaxel for 1 more hour; and (15) docetaxel, flavopirdol and 5-FU simultaneously for 24 h. After each drug treatment cells were washed twice with drug-free medium. The doses of docetaxel, flavopiridol and 5-FU were 100 nmol/L, 300 nmol/L, and 20 µmol/L, respectively. As soon as drug treatment was completed 10 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT, 5 mg/mL in PBS) was added to each well, the plate was incubated for 4 h at 37 °C and 100 μL of 0.04 mol/L HCl in isopropanol was added. Within an hour the absorbance was measured on an ELISA plate reader with a test wavelength of 570 nm and a reference wavelength of 630 nm. The percent of surviving cells at each concentration relative to the non-treated group was plotted.
Caspase-3 assays Induction of apoptosis was assessed by detecting caspase activity in cell lysates after drug treatment. The activity of DEVD (Asp-Glu-Val-Asp) specific proteases was measured using an EnzCheck Caspase-3 Assay Kit (Molecular Probes, Eugene, OR) as suggested by the manufacturer. The cells were treated with different combinations of the drugs as follows: (1) no drug; (2) docetaxel for 1 h; (3) flavopiridol for 24 h; (4) 5-FU for 24 h; (5) docetaxel for 1 h followed by flavopiridol for 24 h; (6) docetaxel for 1 h followed by 5-FU for 24 h; (7) flavopiridol for 24 h followed by 5-FU for 24 h; (8) docetaxel for 1 h followed by flavopiridol for 24 h and 5-FU for an additional 24 h; (9)docetaxel, flavopiridol and 5-FU administered at the same time for 24 h. After drug treatment cells were frozen at -80 °C until the time of assay. The frozen cells were resuspended in 50 µL lysis buffer, incubated on ice for 30 min and the cellular debris was pelleted. Lysates (50 µL) were transferred to 96-well plates and incubated at room temperature for 30 min with substrate (Z-DEVD-AMC) in 2× reaction buffer at a 100 µmol/L final concentration. Cell lysates alone with the caspase inhibitor Ac-DEVD-CHO at a 100 µmol/L final concentration was also included. Fluorescence was measured by a fluorometer at an excitation wavelength of 350 nm and a detection wavelength of 450 nm.
Soft agar colony assays The cells were treated with the same drug sequencing schedule as described with the caspase-3 assays. After drug treatment the cells were mixed with tissue culture media containing 0.6% agar to result in a final agar concentration of 0.4%. Cell suspension 1 mL was immediately plated in 6-well plates coated with 1 mL/well of 0.6% agar in tissue culture media. The colonies were counted in triplicate on d 15 after plating and the number of colonies/3×104 cells was calculated.
Murine xenograft model Institutional guidelines and an Animal Research Committee (ARC)-approved protocol were followed for mouse studies. For these studies, 4−6 week old female nudenu/nu mice were obtained from Charles River (Wilmington, MA,) and housed in clean specific pathogen free (SPF) rooms in groups of 5 and in cages containing micro-isolator tops. HCT116 cells were harvested, washed twice in ice-cold serum free DMEM media, and counted for viable cells by trypan blue exclusion. The cells were resuspended in the same medium and 2.5×106 cells in a volume of 0.25 mL were injected subcutaneously into the right flank of each mouse. Drug treatment was started on the seventh day after tumor inoculation by intraperitoneal (ip) injection. Mice received: (1) docetaxel alone (5 mg/kg) (Aventis Pharmaceu-ticals); (2) flavopiridol alone (2.5 mg/kg) (Aventis Pharmaceu-ticals); (3) 5-FU alone (70 mg/kg) (ICN Pharmaceuticals); (4) docetaxel followed by flavopiridol 1 h later; (5) docetaxel followed by 5-FU 1 h later; (6) flavopiridol followed by 5-FU 24 h later; (7) docetaxel followed by flavopiridol 1 h later and 5-FU 24 h later. All drugs were administered ip twice a week every other week until the tumor size exceeded 2 cm. The tumors were measured in three axes from d 7 onwards and the tumor volume was calculated from these measurements.
Results
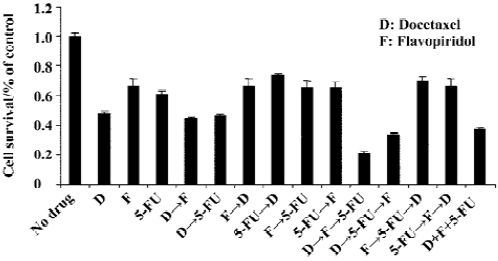
HCT116 phase growth inhibition is enhanced by sequential treatment with docetaxel, flavopiridol and 5-FU In order to explore the optimal sequencing of docetaxel, flavopiridol and 5-FU, we performed MTT assay with the HCT 116 cell line using the following concentrations: docetaxel 100 nmol/L, flavopiridol 300 nmol/L, and 5-FU 20 µmol/L. The cells were exposed to docetaxel, flavopiridol and 5-FU either sequentially or simultaneously as described. Sequential treatment of HCT116 colon cancer cells with docetaxel for 1 h followed by flavopiridol for 24 h and 5-FU for an additional 24 h resulted in 87% of phase growth inhibition. However, simultaneous addition of the three drugs to the cells for 24 h was less effective with 60% of phase growth inhibition. Phase growth inhibition was only 30%–40% when cells were treated with other sequential administrative schedules (Figure 1). These results indicated that the optimal sequencing of docetaxel, flavopiridol and 5-FU enhanced cell killing.
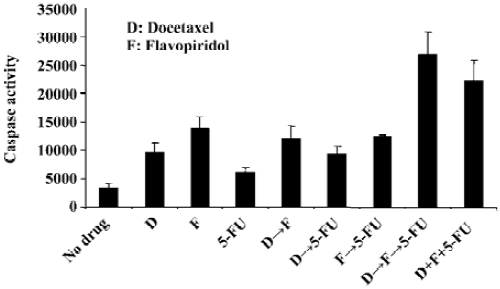
Sequencing of docetaxel, flavopiridol and 5-FU alters the ability of these drugs to induce apoptosis To evaluate the effect on apoptosis of three drug sequencing with docetaxel, flavopiridol and 5-FU, the activity of caspase-3 and related DEVD-specific proteases was determined. The assay involved the fluorescent measurement of the proteolytic cleavage of Z-DEVD-AMC to the fluorescent molecule AMC in both the absence and presence of the caspase-3 inhibitor Ac-DEVD-CHO. Sequential treatment of HCT116 cells with docetaxel for 1 h followed by flavopiridol for 24 h and 5-FU for an additional 24 h resulted in an 8-fold increase in caspase activity as compared to no drug treatment. When the cells were exposed simultaneously to docetaxel, flavopiridol, and 5-FU the caspase activity increased by 6-fold, while single or double drug treatment resulted in less than 4-fold increase (Figure 2).
Inhibition of colony formation in soft agar is dependent on the sequencing of docetaxel, flavopiridol and 5-FU We next determined the colony formation of HCT116 cells in soft agar by sequential treatment of the cells with docetaxel, flavopiridol and 5-FU. As compared to no drug treatment cells exposed to 100 nmol/L docetaxel, 300 nmol/L flavopiridol or 20 µmol/L 5-FU resulted in less than 5-fold inhibition on colony formation. Sequential treatment of the cells with any two drugs or simultaneous treatment of the cells with three drugs inhibited colony formation less than 9-fold. Colony formation was inhibited over 34-fold when cells were exposed to the three drugs sequentially, demonstrating that the combination of docetaxel, flavopiridol, and 5-FU reduced tumor cell growth in soft agar (Figure 3).

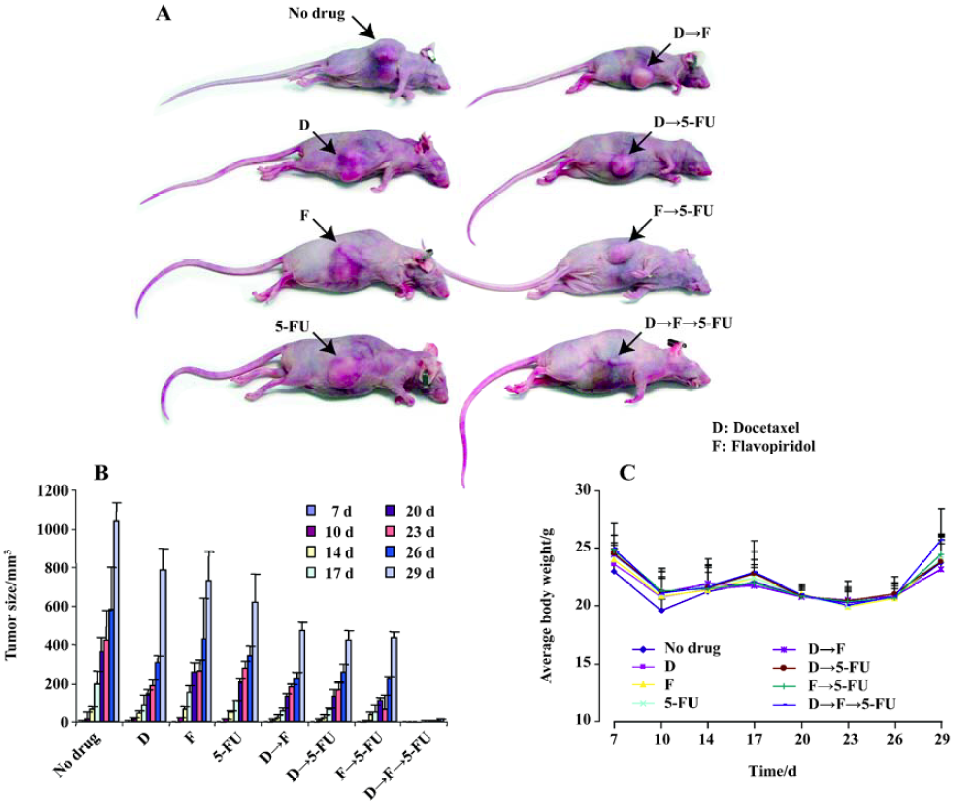
Combination of docetaxel, flavopiridol and 5-FU reduces in vivo tumor growth As sequential treatment with docetaxel, flavopiridol and 5-FU resulted in enhanced antiproliferative and apoptotic effects on HCT116 cells in vitro, we next tested this sequence in HCT116 xenograft models. For this experiment, 6 to 8-week-old female nudenu/nu mice were inoculated with 2.5×106 HCT116 cells subcutaneously in the right flank. One week after tumor cell implantation, mice were randomized into eight groups of 5 mice each and injected with either, 5 mg/kg docetaxel, 2.5 mg/kg flavopiridol or 70 mg/kg 5-FU alone or in different sequences as described previously. The treatment was given twice weekly every other week by ip injection. Tumor size was analyzed over a 29 d time period. At d 29, single drug treatment resulted in a 50% reduction in tumor size as compared to no drug treatment. The reduction of tumor size was 70% with two drug combination (Figure 4). However, with the three drugs sequentially, tumor growth was significantly impaired with a decrease in tumor volume by 95% as compared to control (Figure 4). These studies suggested that in vivo administration of docetaxel, flavopiridol and 5-FU sequentially provided significant tumor growth inhibition of HCT116 xenograft.
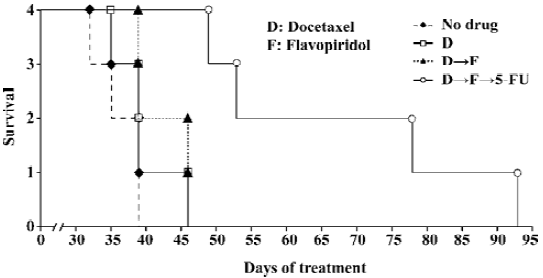
Sequential treatment of nude mice with docetaxel, flavopiridol and 5-FU resulted in prolonged survival A survival experiment was performed by using four groups of 6 to 8-week-old nude mice. Seven days after HCT116 cells were inoculated subcutaneously in the right flank mice received: (1) no drug treatment; (2) 5 mg/kg of docetaxel alone; (3) 5 mg/kg of docetaxel followed by 2.5 mg/kg of flavopiridol 1 h later; and (4) 5 mg/kg of docetaxel followed by 2.5 mg/kg of flavopiridol 1 h later and 70 mg/kg of 5-FU 24 h later. Mice were injected with drugs twice weekly by ip every other week until killed with a tumor size of over 2 cm at any one of the three dimensions. The tumor volumes were measured in three axes starting on d 7 after inoculation. The first two mice treated with three drugs were killed around d 53, another two were killed at d 78 and d 93. The mice treated by docetaxel only or docetaxel followed by flavopiridol were killed between d 36 to d 46. The tumor size of no-drug-treatment mice was over 2 cm in one dimension between d 33 to d 39. Treatment of xenografts with three agents sequentially doubled the survival of mice as compared to two drug combination and control (Figure 5). Thus, sequencing of the chemotherapy resulted in significant prolongation of survival in established HCT116 animal model.
Discussion
Single agent chemotherapy has clearly had an inferior clinical tumor response rate and cure rate when compared to multi-agent therapy. However, most of the planning and use of multi-agent chemotherapy has focused on the use of agents with some degree of efficacy for a given cancer where the differing toxicities of the drugs allow them to be included in the treatment plan. One might intuitively consider a sequence of drug therapy that is based on logical cellular or molecular sites of action, or bioavailability of the drugs, or ease of administration[21,25].
Flavopiridol has been reported to enhance the cytotoxic effects of various chemotherapeutic drugs. In several preclinical studies, sequence-dependent synergistic cytotoxic effects were observed when flavopiridol was administered after a variety of chemotherapeutic agents[26–28]. It has also been reported that flavopiridol enhanced docetaxel-induced apoptosis in gastric cancer cells. This effect was highly sequence dependent, such that docetaxel needed to precede flavopiridol treatment to achieve this effect[20]. Our cell proliferation assay data also clearly showed that sequencing the agents with docetaxel preceding flavopiridol decreased tumor volumes as compared to the reverse sequence. The addition of 5-FU to docetaxel and flavopiridol-treated cells increased cell death further in a sequence-dependent manner. Therefore, choosing the optimum administrative schedule may lead to a more effective chemotherapy regimen with the combinations of docetaxel, flavopiridol and 5-FU. When this administrative schedule was followed, there was an 8-fold increase in caspase activity as compared to no drug treat-ment, while other administrative schedules resulted a less than 4-fold increase in caspase activity. This sequential treatment also induced the highest percentage of cell death, apoptosis and resulted in the greatest reduction of HCT116 colony formation in soft agar.
The projected goal in this study is to aim for maximum therapeutic efficacy without increasing or compounding toxicity. When we extended in vitro observations to an in vivo system, tumor regression was increased and survival was prolonged with the sequential treatment of docetaxel, flavopiridol and 5-FU as compared to single drug treatment. Thus the sequence of administrating the chemotherapy agents affects the degree of cytotoxicity and our data showed that the sequential treatment of colon cancer cells with docetaxel followed by sequential flavopiridol and 5-FU had a significant inhibitory effect on tumor proliferation both in vitro and in vivo.
Ever increasing evidence continues to define cancer chemotherapy efficacy in terms of the ability to achieve significant effects on checkpoints of cell- cycle control with resultant induction of programmed cell death. Although, for many chemotherapy drugs, one can preclude or identify a reasonably specific molecular site of action, the clear identification and delineation of the actual events have been difficult to prove. For many drugs the absolute and singular site of action is often difficult to recognize in vivo and the relationship to the microenvironment and potential release of correlate cytokines or related regulatory proteins further compounds precision. In our current studies we have attempted to overcome these obstacles by developing the data in a series of in vitro studies and then examining the findings in the in vivo circumstances. These results strongly suggest that this type of sequential administrative schedule may have important therapeutic implications and a defined construct now exists for translation to early phase clinical trials.
Acknowledgements
We thank Richard GAYNOR for his helpful comments on preparation of the experiments. We thank Janice BOX for preparation of the manuscript and Alejandra HERRERA for the graphic help.
References
- Nishizaki M, Meyn RE, Levy LB, Atkinson EN, White RA, Roth JA, et al. Synergistic inhibition of human lung cancer cell growth by adenovirus-mediated wild-type p53 gene transfer in combination with docetaxel and radiation therapeutics in vitro and in vivo. Clin Cancer Res 2001;7:2887-97.
- Hennequin C, Giocanti N, Favaudon V. Interaction of ionizing radiation with paclitaxel (Taxol) and docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res 1996;56:1842-50.
- Mason KA, Hunter NR, Milas M, Abbruzzese JL, Milas L. Docetaxel enhances tumor radioresponse in vivo. Clin Cancer Res 1997;3:2431-8.
- Kaye SB. Docetaxel (Taxotere) in the treatment of solid tumors other than breast and lung cancer. Semin Oncol 1995;22:30-3.
- Shibuya Y, Tanimoto H, Umeda M, Yokoo S, Komori T. Induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil for Tongue cancer. Kobe J Med Sci 2004;50:1-7.
- Ghaemmaghami M, Jett JR. New agents in the treatment of small cell lung cancer. Chest 1998;113:86S-91S.
- Miller V. Docetaxel (Taxotere) in combination with vinorelbine in non-small cell lung cancer. Semin Oncol 1999;26:12-4.
- Nabholtz JM, Smylie M, Mackey J, Au HJ, Tonkin K, Au R, et al. Docetaxel and anthracycline polychemotherapy in the treatment of breast cancer. Semin Oncol 1999;26:47-52.
- Rizvi NA. Docetaxel (Taxotere) and gemcitabine in combination therapy. Semin Oncol 1999;26:19-22.
- Shapiro GI. Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol. Clin Cancer Res 2004;10:4270s-75s.
- Dai Y, Grant S. Small molecule inhibitors targeting cyclin-dependent kinases as anticancer agents. Curr Oncol Rep 2004;6:123-30.
- Hunter T, Pines J. Cyclins and cancer II: cyclin D and CDK inhibitors come of age. Cell 1994;79:573-82.
- Drees M, Dengler WA, Roth T, Labonte H, Mayo J, Malspeis L, et al. Flavopiridol (L86-8275): selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin Cancer Res 1997;3:273-9.
- Achenbach TV, Muller R, Slater EP. Bcl-2 independence of flavopiridol-induced apoptosis. Mitochondrial depolarization in the absence of cytochrome c release. J Biol Chem 2000;275:32089-97.
- Li Y, Bhuiyan M, Alhasan S, Senderowicz AM, Sarkar FH. Induction of apoptosis and inhibition of c-erbB-2 in breast cancer cells by flavopiridol. Clin Cancer Res 2000;6:223-9.
- Matranga CB, Shapiro GI. Selective sensitization of transformed cells to flavopiridol-induced apoptosis following recruitment to S-phase. Cancer Res 2002;62:1707-17.
- Semenov I, Akyuz C, Roginskaya V, Chauhan D, Corey SJ. Growth inhibition and apoptosis of myeloma cells by the CDK inhibitor flavopiridol. Leuk Res 2002;26:271-80.
- Senderowicz AM, Headlee D, Stinson SF, Lush RM, Kalil N, Villalba L, et al. Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol 1998;16:2986-99.
- Bissery MC, Guenard D, Gueritte-Voegelein F, Lavelle F. Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res 1991;51:4845-52.
- Motwani M, Rizzo C, Sirotnak F, She Y, Schwartz GK. Flavopiridol enhances the effect of docetaxel in vitro and in vivo in human gastric cancer cells. Mol Cancer Ther 2003;2:549-55.
- Bible KC, Kaufmann SH. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: the importance of sequence of administration. Cancer Res 1997;57:3375-80.
- Burris HA 3rd. Docetaxel in combination with fluorouracil for advanced solid tumors. Oncology (Huntingt) 1997;11:50-2.
- Johnston PG, Kaye S. Capecitabine: a novel agent for the treatment of solid tumors. Anticancer Drugs 2001;12:639-46.
- Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2000;18:136-47.
- Motwani M, Delohery TM, Schwartz GK. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res 1999;5:1876-83.
- Motwani M, Jung C, Sirotnak FM, She Y, Shah MA, Gonen M, et al. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in Hct116 colon cancer monolayers and xenografts. Clin Cancer Res 2001;7:4209-19.
- Schwartz GK, Farsi K, Maslak P, Kelsen DP, Spriggs D. Potentiation of apoptosis by flavopiridol in mitomycin-C-treated gastric and breast cancer cells. Clin Cancer Res 1997;3:1467-72.
- Wittmann S, Bali P, Donapaty S, Nimmanapalli R, Guo F, Yamaguchi H, et al. Flavopiridol down-regulates antiapoptotic proteins and sensitizes human breast cancer cells to epothilone B-induced apoptosis. Cancer Res 2003;63:93-9.
