Inhibitory effects of coronary vasodilator papaverine on heterologously-expressed HERG currents in Xenopus oocytes
Introduction
Papaverine (6,7-dimethoxy-1-veratrylisoquinoline), a substituted alkaloid from Papaver somniferum, has been known to relax many kinds of smooth muscles. Because of its relaxant effects on smooth muscle, papaverine has been used as a vasodilator agent[1-3] and a therapeutic agent for renal colic[4] and penile impotence[5]. It was also proposed as an “ideal coronary vasodilator” [1]. Additionally, intra-arterial papa-verine infusion has been used for the prevention and treatment of vasospasm following subarachnoid hemorrhage[6,7]. However, under certain clinical settings, such as cases of overdose, papaverine induced cardiac arrhythmias including prolonged QT intervals that can lead to the life-threatening ventricular arrhythmia, torsades de points[8-11].
One means by which drugs can prolong QT intervals is through the inhibition of one or more repolarizing K+ channel currents in the myocardium[12,13]. In humans, the most common channel linked to drug-induced QT interval prolongation is the rapid component of the delayed rectifier K+ channel (IKr)[14]. The human ether-a-go-go-related gene, HERG, encodes the pore-forming subunit of IKr[15,16]. Naturally, many drugs associated with QT interval prolongation have been found to block HERG channels[17-21]. It can be assumed therefore that papaverine may cause LQT by inhibiting HERG/IKr. To examine this possibility, we investigated the effect of papaverine on HERG currents expressed in Xenopus oocytes. We found that papaverine effectively inhibited HERG currents in a state-independent manner in Xenopus oocytes.
Materials and methods
Recording of the action potential in rabbit cardiac myocytes Rabbits (Male, New Zealand white rabbits, weighing 1.8?2.0 kg each) were anesthetized by injections of thiopental sodium (10 mg/kg) into a marginal ear vein. Their hearts were rapidly excised and transferred to Tyrode抯 solution (137 mmol/L NaCl, 5.4 mmol/L KCl, 1.05 mmol/L MgCl2, 1.8 mmol/L CaCl2, 5 mmol/L glucose, and 10 mmol/L HEPES at pH 7.4), and the hearts were oxygenated with 100% O2 at room temperature. The ventricular muscles (1.5?2 mm in width and 2?4 mm in length) were carefully dissected from the left ventricular wall. All tissues were less than 1 mm in depth.
Each specimen of the dissected tissue was mounted horizontally in a recording chamber, and continuously super-fused with oxygenated Tyrode’s solution at a rate of 5 mL/min. One end of each tissue was fixed by an insect pin to the bottom of the chamber coated with Sylgard. The tissue next to the insect pin was pressed against the chamber floor by stimulation electrodes, which were used to elicit the action potentials. The cardiac tissues were stimulated with square pulses (2 Hz, 1 ms duration, 20%-30% above the threshold voltage) using a stimulator with a stimulus isolation unit (WPI, Sarasota, FL, USA). After allowing 2 h for stabiliza-tion, the action potentials were recorded with a 3 mol/L KCl-filled conventional microelectrode (20-30 MΩ) that was connected to an amplifier (KS-700, WPI, USA), and these action potentials were displayed on an oscilloscope (Dual beam storage 5113, Tektronix, Beaverton, OR, USA). After 1 h of stabilization of recording, the drugs were applied.
Oocyte preparation Oocytes were prepared as described previously[22]. Briefly, ovarian lobes excised from the anesthetized Xenopus laevis (Xenopus I, USA) were treated with 0.2% collagenase (type II, Sigma Co, St Louis, MO, USA) for 1-2 h in Ca2+-free Barth's solution. The composition of Ca2+-free Barth's solution is as follows: 88.7 mmol/L NaCl, 1.0 mmol/L KCl, 2.4 mmol/L NaHCO3, 0.8 mmol/L MgSO4 7H2O, and 5 mmol/L HEPES (pH=7.5). cRNA (50 nL, 0.3-1.0 ng/nL) of HERG synthesized from the linearized cDNA using in vitro transcription kit (Ambion Inc, Austin, TX, USA) was injected in stage V or VI oocytes with glass capillaries connected with a microdispensor (VWR Scientific Co, Grove, IL, USA). After the injection, oocytes were cultured at 18 ºC in Barth’s solution containing 88.0 mmol/L NaCl, 1.0 mmol/L KCl, 2.4 mmol/L NaHCO3, 0.8 mmol/L MgSO4 7H2O, 0.3 mmol/L Ca(NO3)2 4H2O, 0.4 mmol/L CaCl2, and 5 mmol/L HEPES (pH=7.5), supplemented with 2 mmol/L pyruvate and 50 g/mL gentamicin sulfate. The culture medium was changed daily. Currents were recorded from 2 to 7 d after injection.
Whole cell current recording in Xenopus oocytes HERG currents were recorded using a 2-electrode voltage-clamp amplifier (OC-725C, Warner Instruments, Hamden, CT, USA) from the oocytes placed in the recording chamber (2.0 mL) superfused with Oocyte-Ringer solution containing 96.0 mmol/L NaCl, 2.0 mmol/L KCl, 1.0 mmol/L MgCl2, 1.8 mmol/L CaCl2 2H2O, and 5.0 mmol/L HEPES (pH=7.5). Stimulation and data acquisition were controlled with Digidata 1200 (Axon Instruments) and pClamp 6.04 (Axon Instruments, USA). Electrodes were fabricated from glass capillaries containing an inner filament (OD 1.5 mm, ID 1.12 mm; WPI, USA). Electrodes filled with 3 mol/L KCl had a resistance of 1?2 for current-passing electrodes and 2?4 for voltage-recording electrodes.
Data analysis The voltage dependence of HERG current activation was determined for each oocyte by fitting peak values of the tail current (Itail) versus test potential (Vt) to a Boltzmann function:
Where Itailmax is the maximum tail current, V1/2 is the voltage at which 50% of the channels are activated, and k is the slope factor. To examine steady-state inactivation, conditioning pulses between -130 and +20 mV in 10 mV increments for 60 ms were applied after a depolarizing pulse to +20 mV for 900 ms, followed by a common test pulse to +20 mV. The peak current amplitudes during the test pulses were plotted as a function of the previous conditioning pulses. Normalized steady-state inactivation as a function of prepulse of test potential was also fitted to a Boltzmann function. The data were expressed as the mean±SEM. Paired Student’s t-test was used to compare the drug effect on HERG currents. ANOVA repeated measures, followed by Tukey’s post-hoc tests were used to study concentration-dependent drug effects.
Drugs All drugs were purchased from Sigma Co (St Louis, USA). Papaverine was dissolved in 10-3 mol/L stock solution and stored at 4 °C until dilution within the perfusion solution immediately before use.
Results
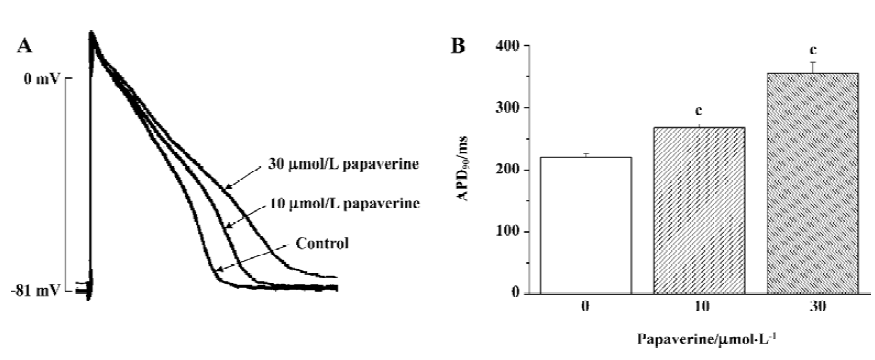
To identify the general electrophysiological effect of papaverine on cardiac action potential, we examined the effects of papaverine on cardiac action potential in isolated rabbit ventricular myocytes stimulated at a frequency of 2 Hz (Figure 1). After 20 min of exposure, the action potential duration at 90% repolarization (APD90) in the ventricular myocytes increased from 219±5 ms to 267±6 ms and 355±18 ms (n=4) by 10 and 30 µmol/L papaverine, respectively. Resting membrane potential was depolarized from -81±3 mV to -77±4 mV (n=3) by 30 µmol/L papaverine, but not by 10µmol/L papaverine (-81± 5 mV, n=4).
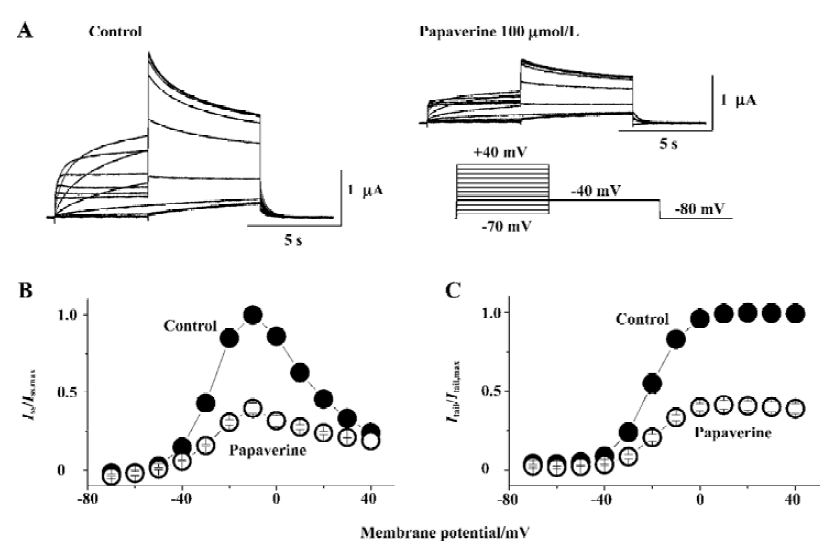
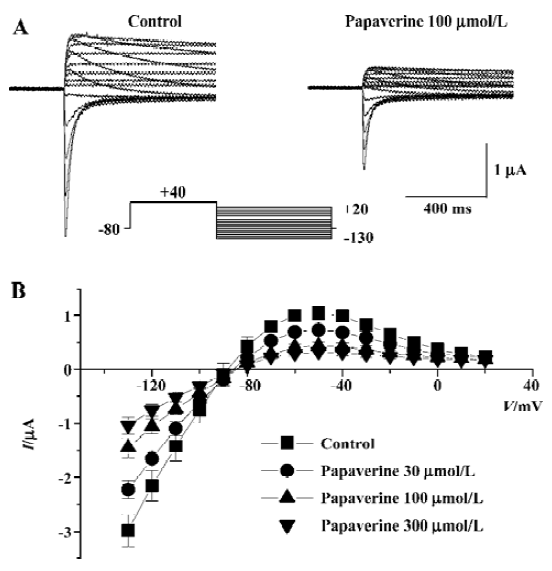
The effect of papaverine on HERG currents was studied in Xenopus oocytes injected with HERG mRNA. Figure 2A shows voltage-clamp data obtained 10 min after application of 100 µmol/L of papaverine. Families of current traces from 1 cell are shown for control conditions and after exposure to papaverine with the voltage protocol shown in Figure 2A. Cells were clamped at a holding potential of -80 mV. Depolarizing steps were applied for 4 s to voltages between -70 and +40 mV in 10 mV increments. Papaverine (100 μmol/L) suppressed both the outward and tail currents (Figure 2A), which was only partially (72%±8% of the control, n=4) recovered in the 30 min drug wash-out. Current-voltage plots of outward currents present at the end of the depolarizing step and peak tail current are depicted in Figure 2B and 2C, respectively. Current amplitudes were normalized to peak values obtained from each cell. For control conditions, the threshold for activating HERG currents was close to -40 mV, and full activation was obtained at a voltage close to +10 mV. In the presence of 100 μmol/L of papaverine, outward currents at the depolarizing step and tail current amplitude were reduced, compared with control condition, to ~40% at all tested potentials.
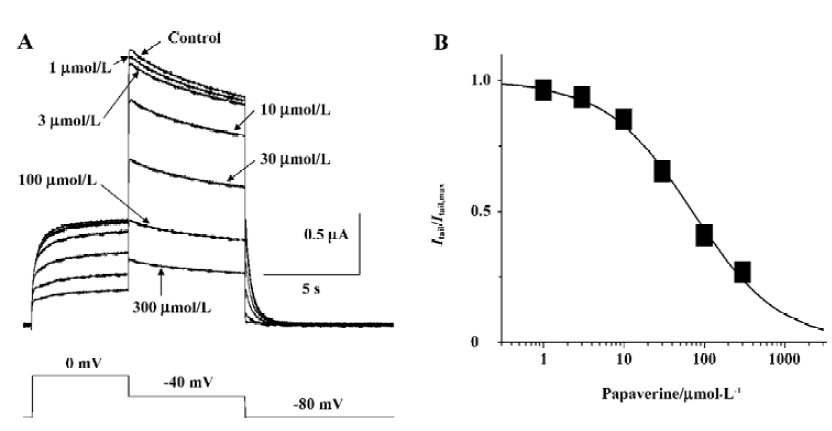
Papaverine blocked HERG currents in a concentration-dependent manner Steady-state block was obtained by applying depolarizing steps from -80 to 0 mV for the 10 s period every 30 s, and peak tail current was measured after the repolarizing steps to -40 mV for 6 s at different drug concentrations. Analysis of the data with the Hill equation gave a half-maximal inhibitory concentration (IC50) value of 71.0 μmol/L and Hill coefficient of 0.81 (Figure 3).
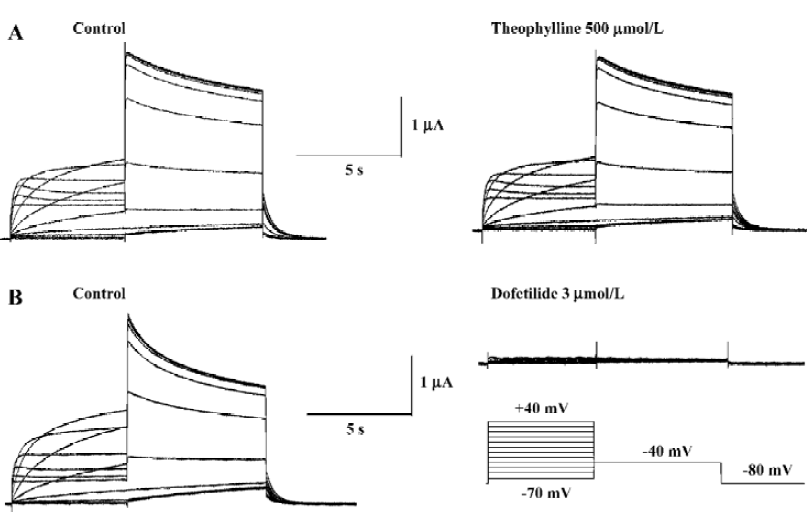
Papaverine is known as a phosphodiesterase inhibitor. To determine whether the effect of papaverine on HERG was related to phosphodiesterase inhibition, the effects of theophylline, another phosphodiesterase inhibitor, on HERG currents was investigated. Theophylline (500 μmol/L) did not affect HERG currents (Figure 4A), which were completely blocked by dofetilide (Figure 4B), known to be a selective IKr blocker, suggesting that the papaverine block on HERG is independent from the phosphodiesterase inhibition.
Drugs that block ion channels often alter the voltage dependence of channel kinetics. Therefore, we also analyzed the voltage dependence of the activation of the peak amplitude of the decaying tail currents in the absence or presence of papaverine (100 μmol/L). The voltage dependence of the HERG activation was determined using 4 s test pulses to potentials ranging from -70 to +40 mV. Peak amplitudes of tail currents were measured at -40 mV, plotted as a function of test potential, and were fitted with a Boltzmann function (Figure 2C). In the control experiment, the activation curve had a midpoint of -16.4?1.1 mV and a slope factor of 6.8?0.4 mV (n=6). There was no significant change in voltage dependence of the activation curve (V1/2 -16.5?2.4 mV and a slope factor of 5.8?1.5 mV, n=6, P>0.05 vs the control in both) by papaverine.
We also investigated the effects of papaverine at a concentration of 100 µmol/L on the inactivation of HERG currents. Steady-state inactivation currents were studied before and after the application of papaverine using a dual-pulse protocol as described in Materials and methods. The steady-state inactivation curve was shifted to a negative potential (V1/2 -68.7±3.2 mV in the control and -77.4±1.6 mV after papaverine, P<0.05, n=5; Figure 5). The slope factor of steady-state inactivation decreased slightly from 24.3±2.9 mV in the control to 17.2-1.3 mV in the presence of papaverine.
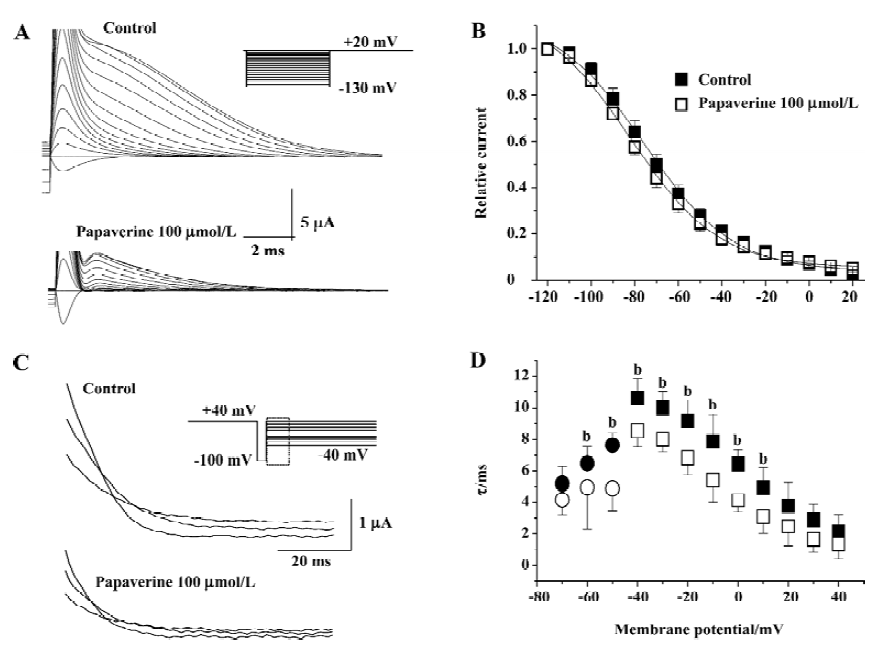
To directly test the effect of papaverine on HERG inactivation, we examined the time course of inactivation in the absence and presence of papaverine. The inactivation time course of expressed currents was analyzed by applying brief hyperpolarizing pulses to allow the HERG channel to recover from inactivation after an initial long depolarizing pulse. Depolarizing test pulses were then applied to record inactivating currents (Figure 5C). The time course of fast, inactivating currents could be fitted by a single-exponential function. In the presence of papaverine, the time constants for inactivation significantly decreased at all tested potentials (Figure 5D). Recovery from inactivation was also measured using the same pulse protocol shown in Figure 4. Tail currents could be fitted by a double-exponential function, and the fast component was defined as the time constant of recovery from inactivation. In the presence of papaverine, the time constants for inactivation and recovery from inactivation significantly decreased at all potentials (Figure 5D).
To further delineate the underlying mechanism for papaverine inhibition of HERG currents, we examined the fully activated I-V relationships by applying various test potentials after a depolarizing conditioning pulse (Figure 6). The slope conductance measured as a slope of the current-voltage relationship curve between -130 and -110 mV decreased from 78.03±4.25 µS of the control to 56.84?5.33, 36.06?6.53, and 27.09±5.50 µS (n=6) in the presence of 30, 100, and 300 µmol/L papaverine, respectively (P<0.05 vs the control at each concentration).
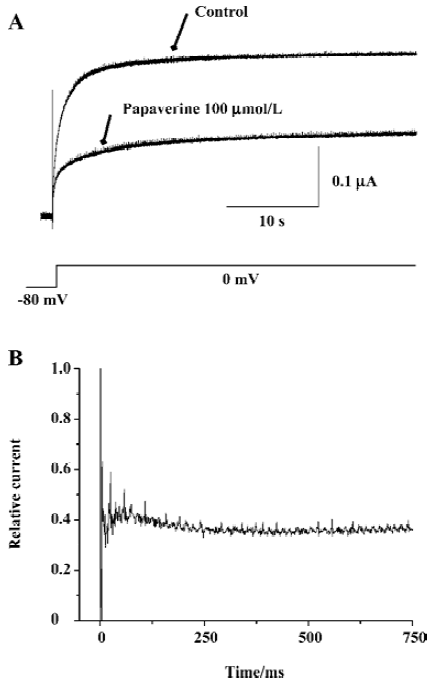
On the basis of single-channel studies, a 5-state model has been suggested for HERG channels summarizing 1 open, 1 inactivated, and 3 closed states[23]. Therefore, we investigated whether papaverine block is state dependent (Figure 7). After the control currents were measured, the membrane potential was held at -80 mV to keep the channel in the closed conformation during wash-in of 100 µmol/L of papaverine. The first depolarization to 0 mV after 10 min at -80 mV yielded most of the blocking effect. The initial current inhibition (61.0%±4.6%, n=4) reflects that papaverine bound HERG without channel opening and/or activation, whereas the additional time-dependent inhibition (4.8%±2.2%, n=4) observed after channel activation may represent additional papaverine inhibition on HERG channels during the open state.
Discussion
In the present study, we show that a clinical vasodilator, papaverine, increases cardiac action potential duration and inhibits cardiac K+ channel HERG expressed in Xenopus oocytes. Papaverine has been shown to prolong QT intervals and cause ventricular arrhythmia[11]. Many commonly used drugs, including antiarrhythmic, antihistamine, antipsychotic, and antibiotic agents are associated with drug-induced LQTS. Most of these drugs either block HERG-dependent K current (IKr) in ventricular myocytes or inhibit liver enzymes that are important for metabolic degradation of other drugs that block IKr. It is well known that heterologously-expressed HERG currents share pharmacological and biophysical properties with IKr[15,16]. The characteristics of the currents recorded in the present study correspond to HERG currents: slow current activation at negative potentials, large long-lasting tail currents on repolarization, strong inward rectification, and sensitivity to class III antiarrhythmic methanesulfonanilide such as dofetilide (Figure 4). Considering all of these, papaverine may cause LQT through the inhibition of HERG/IKr in cardiac myocytes. The present study is the first to characterize the interaction between papaverine and HERG showing an ionic mechanism underlying papaverine-induced ventricular arrhythmia.
The major finding of the present study is that papaverine blocks HERG channels in a concentration-dependent, but voltage- and state-independent manner in Xenopus oocytes. Taken into account that in vitro data is influenced by several artificial factors such as expression system properties, temperature, and lack of subunits, the degree of HERG blocked by papaverine seems appropriate. The affinity of ion channel blockers is reduced in Xenopus oocytes due to follicular tissues and the yolk[24]. Consistent with this, papaverine inhibited HERG currents with an IC50 of ~70 µmol/L in Xenopus oocytes (Figure 3), and significantly increased action potential duration in rabbit myocytes at 10 µmol/L of papaverine (Figure 1). This suggests the different pharmacokinetics of papaverine in the 2 different cells. However, we cannot exclude the possibility that papaverine may affect other ionic currents[25,26] in addition to the blockage of IKr, for it depolarized resting membrane potential in ventricular myocytes. Further evaluation of IC50 of papaverine on IKr in ventricular myocytes will be helpful to address it.
The state-dependence of HERG channel block has been described for numerous substances, such as bertosamil[27], fluvoxamine[28], and KCB-328[29] with the majority of those binding to the channel in the open state. Choe et al[25] provide evidence for open channel blockade by papaverine in their studies of Kv1.5 stably expressed in Ltk- cells by demonstrating the accelerated decline of the current during depolarization, as well as steeper blockade in the voltage range of the channel opening. The Kv1.5 current is a rapidly activating and relatively slow inactivating delayed rectifier current, and this differs significantly from the HERG channel. In the present study, papaverine did not modify the time course of channel activation. If present, a tail current “crossover”, due to transient channel unblocking during repolarization[30], provides evidence for open channel block. This was not seen in the present study. Depolarization of membrane potential did not increase HERG current inhibition by papaverine at which most channels would be in the inactivated state. Consistent with the tonic block achieved without channel opening or activation (Figure 7), these results suggest that papaverine mainly bind closed HERG channels.
Various drugs inhibit HERG currents binding to the inactivated HERG channel[32]. Sites in the outer pore region such as G628, G631, S620, A614, and V630 have been known to involve HERG channel inactivation, which also postulated as a binding site(s) of the drug acting with the inactivated HERG channel[33]. H587 of the S5-P loop[34] and M651 of the S6 sites[35] also involved HERG inactivation kinetics. Papaverine markedly accelerated inactivation at most potentials, consistent with the observed 9 mV hyperpolarizing shift in the voltage-dependence of HERG channel inactivation, which implies a decrease in channel availability[31]. Papaverine also markedly increased the rate of recovery from inactivation (Figure 5D). These data imply that the drug acts on inactivated HERG. Therefore, we may speculate that these sites involved in HERG inactivation at least partly contribute to HERG inhibition by papaverine.
There is no consensus about confirming the threshold for the predication of clinically important HERG channel blockade based on an in vitro model. One hypotheses is that drugs will be free of liability for the ventricular arrhythmia torsades de pointes if they fail to produce a 20% blockade at the highest achievable free plasma concentration[36]. Another hypothesis is that drugs for which the IC50 is at least 30-fold greater than the highest achievable free or total plasma concentration will be free of liability for torsades de pointes[18,36]. Although limited data are available with regard to expected maximum plasma concentrations after administration of papaverine, it reached to 49.4–56.6 µmol/L with various carriers in rabbits[37]. The values are similar to papaverine IC50 on HERG block even in Xenopus oocytes. Although maximum Cmax values would be useful in estimating a worst-case clinical relevance of HERG channel blockade, it is notable that papaverine did cause arrhythmia in humans[8-11].
In summary, this report is the first to detail the effects of papaverine on HERG, explaining cellular mechanism for QT prolongation. We found that papaverine blocked HERG currents via binding closed, open, and inactivated channels. These blocking properties may contribute to the arrhythmo-genic properties of papaverine.
References
- Wilson RF, White CW. Intracoronary papaverine: an ideal coronary vasodilator for studies of the coronary circulation in conscious humans. Circulation 1986;73:444-51.
- Franz N, Modersohn D, Romaniuk P, Linss G. Coronary reserve determination by intracoronary papaverine: effects of various doses on coronary circulation and hemodynamics. Z Gesamte Inn Med 1991;46:15-7.
- Newell DW, Elliott JP, Eskridge JM, Winn HR. Endovascular therapy for aneurysmal vasospasm. Crit Care Clin 1999;15:685-99.
- Jonsson PE, Olsson AM, Petersson BA, Johansson K. Intravenous indomethacin and oxycone-papaverine in the treatment of acute renal colic. A double-blind study. Br J Urol 1987;59:396-400.
- Handelsman H. Diagnosis and treatment of impotence. Health Technol Assess Rep 1990;2:1-23.
- Flemming KD, Brown RD Jr, Wiebers DO. Subarachnoid hemorrhage. Curr Treat Options Neurol 1999;1:97-112.
- Tsurushima H, Hyodo A, Yoshii Y. Papaverine and vasospasm. J Neurosurg 2000;92:509-11.
- Jain A, Jenkins MG. Intracoronary electrocardiogram during torsade des pointes secondary to intracoronary papaverine. Cathet Cardiovasc Diagn 1989;18:255-7.
- Kern MJ, Deligonul U, Serota H, Gudipati C, Buckingham T. Ventricular arrhythmia due to intracoronary papaverine: analysis of QT intervals and coronary vasodilatory reserve. Cathet Cardiovasc Diagn 1990;19:229-36.
- Vrolix M, Piessens J, De Geest H. Torsades de pointes after intracoronary papaverine. Eur Heart J 1991;12:273-6.
- Inoue T, Asahi S, Takayanagi K, Morooka S, Takabatake Y. QT prolongation and possibility of ventricular arrhythmias after intracoronary papaverine. Cardiology 1994;84:9-13.
- Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 alpha subunit. Cir Res 1998;83:560-7.
- Jeron A, Mitchell GF, Zhou J, Murata M, London B, Buckett P, et al. Inducible polymorphic ventricular tachyarrhythmias in a transgenic mouse model with a long Q-T phenotype. Am J Physiol 2000;278:H1891-8.
- Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA 2000; 97: 12 329-33.
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 1995;81:299-307.
- Wang S, Liu S, Morales MJ, Strauss HC, Rasmusson RL. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. The J Physiol 1997;502:45-60.
- Rampe D, Murawsky MK, Grau J, Lewis EW. The antipsychotic agent sertindole is a high affinity antagonist of the human cardiac potassium channel HERG. J Pharmacol Exp Ther 1998;286:788-93.
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res 2003;58:32-45.
- Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 2000;102:1178-85.
- Suessbrich H, Schonherr R, Heinemann SH, Lang F, Busch AE. Specific block of cloned Herg channels by clofilium and its tertiary analog LY97241. FEBS Lett 1997;414:43-8.
- Li BX, Yang BF, Zhou J, Xu CQ, Li YR. Inhibitory effects of berberine on IK1, IK, and HERG channels of cardiac myocytes. Acta Pharmacol Sin 2001;22:125-31.
- Kim CS, Son SJ, Kim HS, Kim YD, Lee KS, Jeon BH, et al. Modulating effect of ginseng saponins on heterologously expressed HERG currents in Xenopus oocytes. Acta Pharmacol Sin 2005;26:551-8.
- Kiehn J, Lacerda AE, Brown AM. Pathways of HERG inactiva-tion. Am J Physiol 1999;277:H199-210.
- Madeja M, Musshoff U, Speckmann EJ. Follicular tissues reduce drug effects on ion channels in oocytes of Xenopus laevis. Eur J Neurosci 1997;9:599-604.
- Choe H, Lee YK, Lee YT, Choe H, Ko SH, Joo CU, et al. Papaverine blocks hKv1.5 channel current and human atrial ultrarapid delayed rectifier K+ currents. J Pharmacol Exp Ther 2003;304:706-12.
- Mubagwa K, Shirayama T, Moreau M, Pappano AJ. Effects of PDE inhibitors and carbachol on the L-type Ca current in guinea pig ventricular myocytes. Am J Physiol 1993;265:H1353-63.
- Zitron E, Karle CA, Wendt-Nordahl G, Kathofer S, Zhang W, Thomas D, et al. Bertosamil blocks HERG potassium channels in their open and inactivated states. Br J Pharmacol 2002;137:221-8.
- Milnes JT, Crociani O, Arcangeli A, Hancox JC, Witchel HJ. Blockade of HERG potassium currents by fluvoxamine: incomplete attenuation by S6 mutations at F656 or Y652. Br J Pharmacol 2003;139:887-98.
- Park JB, Choe H, Lee YK, Ha KC, Rhee KS, Ko JK, et al. Open channel block by KCB-328 [1-(2-amino-4-methanesulfonamido-phenoxy)-2-[N-(3,4-dimethoxyphenethyl)-N-methylamino]ethane hydrochloride] of the heterologously expressed human ether-a-go-go-related gene K+ channels. J Pharmacol Exp Ther 2002;302:314-9.
- Snyders J, Knoth KM, Roberds SL, Tamkun MM. Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac potassium channel. Mol Pharmacol 1992;41:322-30.
- Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature 1996;379:833-6.
- Yang BF, Xu DH, Xu CQ, Li Z, Du ZM, Wang HZ, et al. Inactivation gating determines drug potency: a common mechanism for drug blockade of HERG channels. Acta Pharmacol Sin 2004;25:554-60.
- Walker BD, Valenzuela SM, Singleton CB, Tie H, Bursill JA, Wyse KR, et al. Inhibition of HERG channels stably expressed in a mammalian cell line by the antianginal agent perhexiline maleate. Br J Pharmacol 1999;127:243-51.
- Dun W, Jiang M, Tseng GN. Allosteric effects of mutations in the extracellular S5-P loop on the gating and ion permeation properties of the hERG potassium channel. Pflugers Arch 1999;439:141-9.
- Lees-Miller JP, Duan Y, Teng GQ, Duff HJ. Molecular determinant of high-affinity dofetilide binding to HERG1 expressed in Xenopus oocytes: involvement of S6 sites. Mol Pharmacol 2000;57:367-74.
- Webster R, Leishman D, Walker D. Towards a drug concentration effect relationship for QT prolongation and torsades de pointes. Curr Opin Drug Discov Devel 2002;5:116-26.
- Czarnecki W, Wielgus S. Effect of carriers and hydrophilic agents on papaverine spheres bioavailability. Acta Pol Pharm 1996;53:337-40.
