Adeno-associated virus vectors simultaneously encoding VEGF and angiopoietin-1 enhances neovascularization in ischemic rabbit hind-limbs1
Introduction
The treatment of patients suffering from peripheral arterial occlusive disease remains a considerable clinical challenge despite advances in both surgical and percutaneous revascularization techniques. Many patients cannot benefit from these therapies because of the anatomic extent and the distribution of arterial occlusion. In such patients, new therapeutic strategies have been sought to prevent the development of disabling symptoms related to ischemia, such as claudication, resting pain, and loss of tissue integrity in the distal limbs. Therapeutic neovascularization via the processes of angiogenesis (capillary growth) and arteriogenesis (collateral artery growth) is a promising new approach for the treatment of this ischemic disease[1,2].
Among all angiogenic growth factors, the vascular endothelial growth factor (VEGF) is one of the well-studied factors for therapeutic angiogenesis[3]. VEGF exerts its effect by stimulating the proliferation and migration of endothelial cells. However, the development of blood vessels in adult tissues is a complex process in which different growth factors and cytokines act in concert. VEGF-induced vessels are often leaky and do not properly connect to the existing vasculature[4]. Angiopoietin-1 (Ang1), which is responsible for blood vessel maturation, is another promising candidate for therapeutic angiogenesis[5]. It recruits pericyte and smooth muscle cells to stabilize and mature newly-formed blood vessels[6]. Combination gene therapy with several growth factors is a rational approach to creating more stable vessels for functional improvement in ischemic tissues. The combination of VEGF with Ang1 might thus be a more efficient strategy to develop functional and mature vasculature in the treatment of ischemic diseases[7]. The co-administration of plasmid VEGF and plasmid Ang1 led to enhanced arteriogenesis in the ischemic myocardium[8].
For therapeutic angiogenesis, several different strategies have been examined. In some cases, the recombinant protein was tested. In others, gene transfer using naked DNA or adenoviral vectors was performed, but the recombinant protein is limited by the relatively short circulating half-life, as well as by the large quantity needed for therapeutic effects[2]. The transduction efficiency of naked DNA is significantly lower compared with other methods[9], and adenoviral vectors lack sustained expression and antigenicity against viral proteins. Adeno-associated virus (AAV) vectors have a number of attractive features, including non-pathogenicity and the ability to transduce skeletal muscles efficiently, resulting in prolonged gene expression[10,11]. Although most people are seropositive for the virus, the immune response is limited to the secretion of non-neutralizing antibodies[12].
For the combination of VEGF and Ang1 in the treatment of ischemic diseases, we constructed the AAV vectors simultaneously encoding human VEGF165 and Ang1 (AAV-VEGF/Ang1), and performed intramuscular injection of AAV-VEGF/Ang1 to investigate their therapeutic effect in a rabbit hind-limb ischemic model.
Materials and methods
Plasmid construction and vector production The construction of pAAV-VEGF, pAAV-Ang1, and pAAV-VEGF/Ang1 were as previously described[13]. The structures of these plasmids are shown in Figure 1A. Plasmid pAAV-VEGF/Ang1 simultaneously encoded 2 growth factors: human VEGF165 and Ang1, but each of them had its own independent cytomegalovirus (CMV) promoter and human growth hormone polyadenylation signal (hGH polyA).
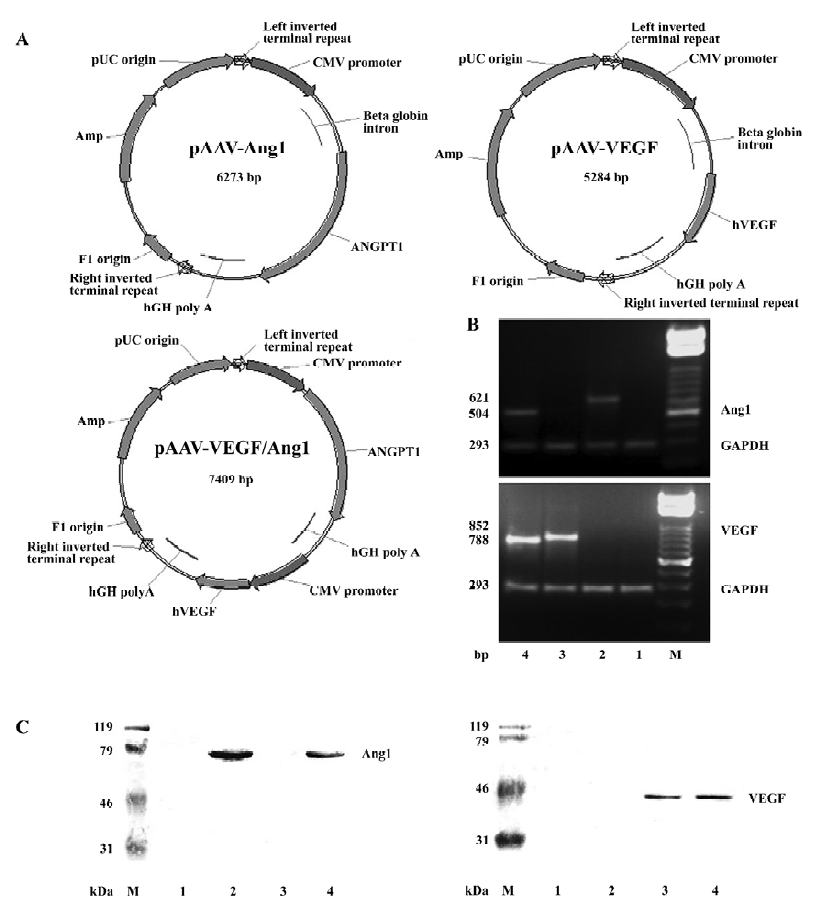
A large quantity of AAV-2 vectors were prepared by using the AAV Helper-Free System (Stratagene, La Jolla, CA, USA). Briefly, the AAV vector plasmid was co-transfected with the pAAV-helper and pAAV-RC into HEK 293 cells by the calcium phosphated method. Three days later, cultures including transfected HEK 293 cells and medium were collected. Ten percent (v/v) chloroform was added to the collected cultures, and vigorously shaken for 1 h[14]. Then solid NaCl was added until the final concentration was 1 mol/L by shaking at room temperature. The supernatant was harvested by centrifugation at 12 000×g for 15 min at 4 °C. An appropriate amount of solid PEG8000 was added to the supernatant to obtain a final concentration of 8% (w/v) by intermittent shaking at room temperature. The supernatant was cooled in ice water for 1 h. The precipitated AAV particles were recovered by centrifugation at 11 000×g for 15 min at 4 °C. The pellets were resuspended in PBS2+ buffer. DNase I (Takara Bio Inc, Otsu, Shiga, Japan) and RNase (Sino-American Bio Co, Beijing, China) were added to the culture and incubated for 30 min. An equal volume of chloroform was added to the suspension which was then shaken vigorously for 2 min. The organic and aqueous phases were separated by centrifugation at 12 000×g for 5 min at 4 °C. The aqueous phases containing the AAV vectors were collected and filtered through 0.2 µm filters (Sartorius AG, Goettingen, Germany). Disposable hollow-fiber tangential-flow filtration devices (8 in, 100 kDa, Amersham Biosciences, Piscataway, NJ, USA) were used to concentrate and diafilter AAV vectors with the buffer (100 mmol/L sodium citrate, 10 mmol/L Tris, pH 8.0, 315 mOsm)[15]. Thus, we obtained concentrated and purified AAV-VEGF, AAV-Ang1 and AAV-VEGF/Ang1. The vector particle titer was determined by quantitative DNA dot-blot hybridization of the DNase I-treated vectors.
Rabbit hind-limb ischemia model and gene transfer Male New Zealand rabbits (weighing 2.0–3.0 kg) were obtained from the Experimental Animal Center of Wuhan University, China and were housed under standard conditions (temperature: 21±1 °C; humidity: 55%–60%) with food and water continuously available. The care of rabbits complied with the Guide for the Care and Use of Laboratory Animals. All animal protocols followed the recommendations and guidelines of the National Institutes of Health and were approved by the Wuhan University Animal Care and Use Committee.
The rabbit hind-limb ischemia model was constructed as previously described[16,17]. The rabbits were pre-anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg), incubated, and mechanically ventilated with a mixture of 1.5% isoflurane and oxygen. The femoral artery was then excised from its proximal origin as a branch of the external iliac artery to the point distally where it bifurcated into the saphenous and popliteal arteries.
Ten days after surgery, the rabbits were randomized to receive an intramuscular injection of AAV–VEGF (n=8), AAV-Ang1 (n=8), AAV-VEGF/Ang1 (n=8) or PBS (n=8) with a 25 gauge needle into 5 different sites (0.5×1013μg/250 µL, 250 µL/site) in the major thigh muscle of the ischemic hind-limbs.
Blood flow measurement Blood flow in the popliteal artery of ischemic and normal hind-limbs was measured at rest with an Aspen Advanced Doppler ultrasound device (Acuson, Siemens Medical Solutions, Mountain View, CA, USA), using a small L10 transducer 8 weeks after the injec-tion, and was valued as a ratio of the contralateral limbs. Three separate measurements were done for each rabbit at every time point, and the results were averaged.
RT-PCR analysis The rabbits were sacrificed 8 weeks after the injection. The total cellular RNA of the muscle tissues of the injected limbs and remote tissues (brain, heart, liver, spleen, kidney, and testes) was isolated using Trizol Reagent (GIBCO, Gaithersburg, MD, USA). Extracted RNA was treated with DNase I (Takara Shuzo, Tokyo, Japan) to eliminate DNA contamination. First-strand cDNA was synthesized by random hexamer (Invitrogen Corp, Carlsbad, CA, USA). The PCR amplifications were performed using human VEGF165 and Ang1 specific forward primers, and specific reverse primer for hGH polyA. GAPDH was detected by RT-PCR as an internal control. All primers were described as follows: human VEGF165 specific forward primer 5'-GACCCT-GGTGGACATCTTC-3'; human Ang1 specific forward primer 5'-TAACAGGAGGATGGTGGTTTGATGCTTG-3'; hGH polyA specific reverse primer 5'-ATGCCTGGAATCCCAA-CAACT-3'; GAPDH primers: forward 5'-TCACCATCTTCC-AGGAGCGA-3'; reverse 5'-CACAATGCCGAAGTGGTCGT-3'.
Western blot analysis Eight weeks after the injection, muscle samples from the thigh muscle of the ischemic limbs were harvested and resuspended in lysis buffer (1% Nonidit P-40, 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 200 U/mL aprotinin, 1 mmol/L phenylmethanesulfonyl fluoride). The tissue lysates (50 mg of protein) were separated by 12% polyacrylamide gel electrophoresis and blotted onto poly-vinylidene difluoride membranes. Immunoblotting was performed with antibodies against human VEGF165 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) or human Ang1(Sigma-Aldrich Corp, St Louis, MO, USA).
Histological assessment Eight weeks after the injection, tissue sections from the thigh muscle of ischemic limbs were harvested. Muscle samples were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek USA, Inc, Torrance, CA, USA), snap-frozen in liquid nitrogen, and cut into 5 μm thick sections. To detect the expression of VEGF and Ang1, some tissue sections were immunostained with antibodies against human VEGF165 or Ang1.
Some sections were stained with hematoxylin. To detect capillary endothelial cells, tissue sections were stained for alkaline phosphatase with an indoxyltetrazolium method as previously described[18]. Capillaries stained dark brown were scored as positive. A total of 20 different fields were randomly selected, and the number of capillaries was counted (mean number of capillaries per square millimeter).
To identify arterioles and differentiate them from capillaries and veins, tissue sections were immunostained for smooth muscle α-actin (α-SMA). The monoclonal antibody against α-SMA (DAKO, Kyoto, Japan) was applied at a 1:500 dilution after blocking with 1% normal horse serum. Subsequent incubation with biotinylated horse anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA) and an ABC Elite kit (Vector Laboratories, USA) was performed. The number of α-SMA-positive vessels was counted in a similar way to that for capillary density.
Serum levels of human VEGF165 and Ang1 To assess the systemic levels of human VEGF165 and Ang1, blood samples were carried out 8 weeks after the injection. Serum concentrations of VEGF165 and Ang1 were measured by ELISA using a VEGF165 ELISA kit and an Ang1 ELISA kit (R&D Systems, Minneapolis, MN, USA).
Evans Blue permeability assay Eight weeks after the injection, a modified Miles permeability assay was performed as described[19]. The rabbits were anesthetized and Evans Blue-PBS (30 mg/kg) was injected (iv). The rabbits were killed 30 min after the injection of Evans Blue and were perfusion-fixed with 1.5 L of 1% paraformaldehyde in 0.05 mol/L citrate buffer (pH 3.5) via the left ventricle. After the rabbits were killed, the muscles were dissected, washed, weighed, and extracted in formamide at 55 °C overnight. The Evans Blue absorbance of the formamide was calculated in a spectrophotometer set at 610 nm and the ratio between transduced and intact muscle samples was calculated.
Statistical analysis Data were expressed as mean±SEM. Statistical comparisons were performed using ANOVA followed by Fisher’s test. Values of P<0.05 were considered to be statistically significant.
Results
Expression of VEGF and Ang1 To confirm human VEGF165 and Ang1 gene expression in transduced rabbit ischemic limb muscles, RT-PCR, Western blot, and immunohistochemical analysis were performed 8 weeks after injection. As shown in Figure 1, the size of the PCR products for VEGF165 in groups AAV-VEGF and AAV-VEGF/Ang1, Ang1 in groups AAV-Ang1 and AAV–VEGF/Ang1, and GAPDH were 852, 788, 621, 504, and 293 bp, respectively. The molecular weights of human VEGF165 and Ang1 were 42 and 70 kDa, respectively. Skeletal muscle cells positive for human VEGF or human Ang1 was stained brown, as shown in Figure 2. All these data demonstrated that human VEGF165 was expressed in groups AAV-VEGF and AAV-VEGF/Ang1, but not in groups AAV-Ang1 and PBS, and that human Ang1 was expressed in groups AAV-Ang1 and AAV-VEGF/Ang1, but not in groups AAV-VEGF and PBS.
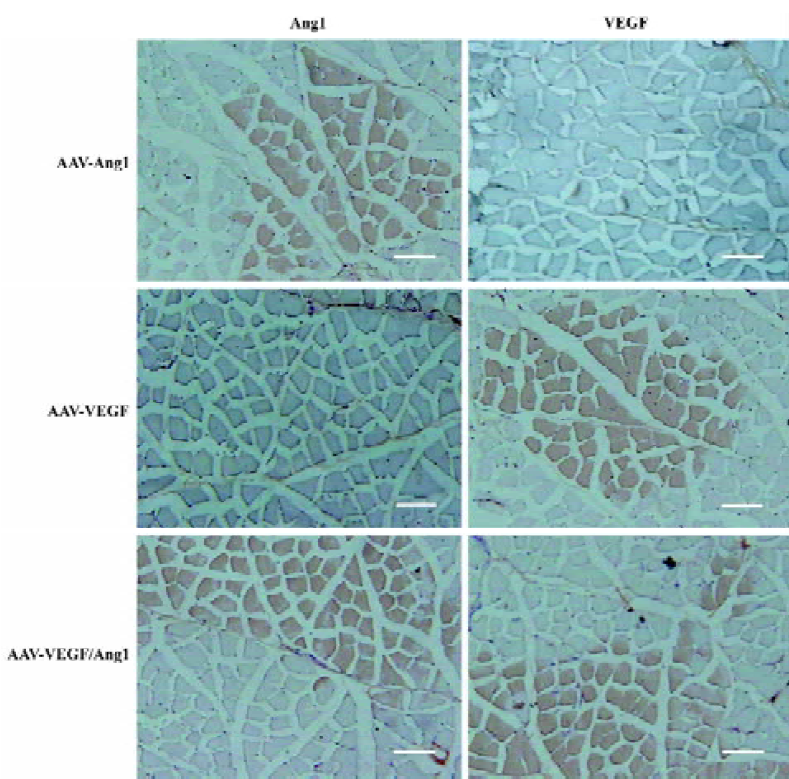
Blood samples were also obtained from the peripheral veins of rabbits 8 weeks after injection and analyzed with the VEGF ELISA kit specific for human VEGF165 and the Ang1 ELISA kit specific for human Ang1. Non-human VEGF165 and Ang1 were detected in plasma, and no human VEGF165 and Ang1 mRNA could be detected by RT-PCR from remote organs (brain, heart, liver, spleen, kidney, and testes) in the AAV-treated rabbits 8 weeks after injection. All these findings demonstrated that 2 proteins could be simultaneously encoded in 1 AAV vector and co-expressed in transduced tissues without ectopic expression.
Improvement of blood flow Eight weeks after the injection, we assessed the blood flow in the popliteal artery of ischemic and normal limbs with the Doppler ultrasound device. As shown in Figure 3B, the ratio of mean ischemic/normal blood flow in group AAV-VEGF/Ang1 (0.78±0.04) was the highest compared with groups AAV-VEGF (0.67±0.04), AAV-Ang1 (0.49±0.04), and PBS (0.47±0.04). Intramuscular administration of AAV-VEGF/Ang1 could obviously improve the blood flow of ischemic hind-limbs.
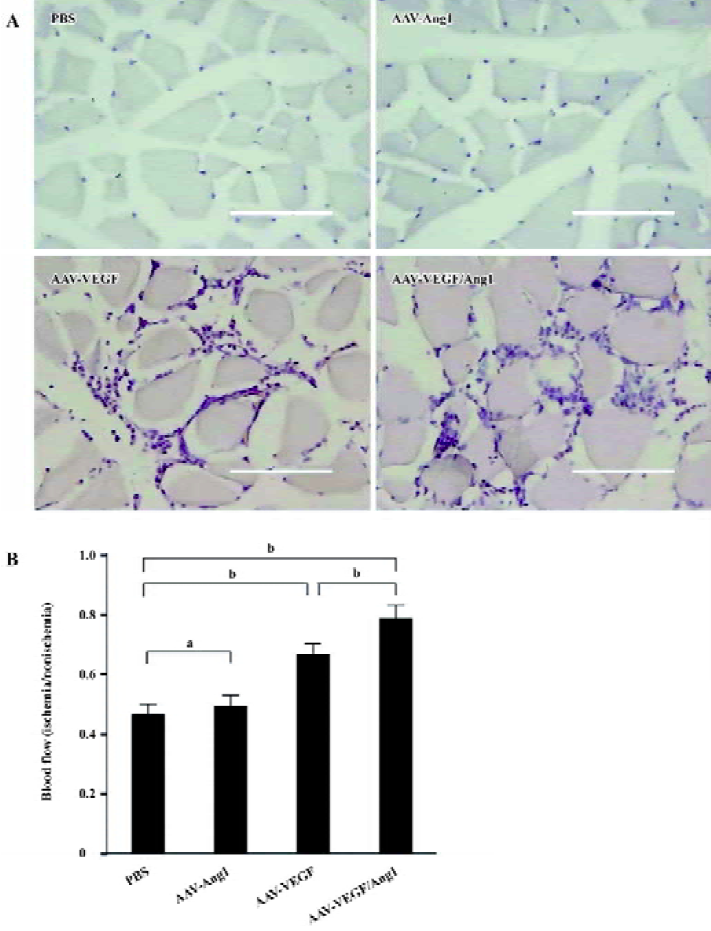
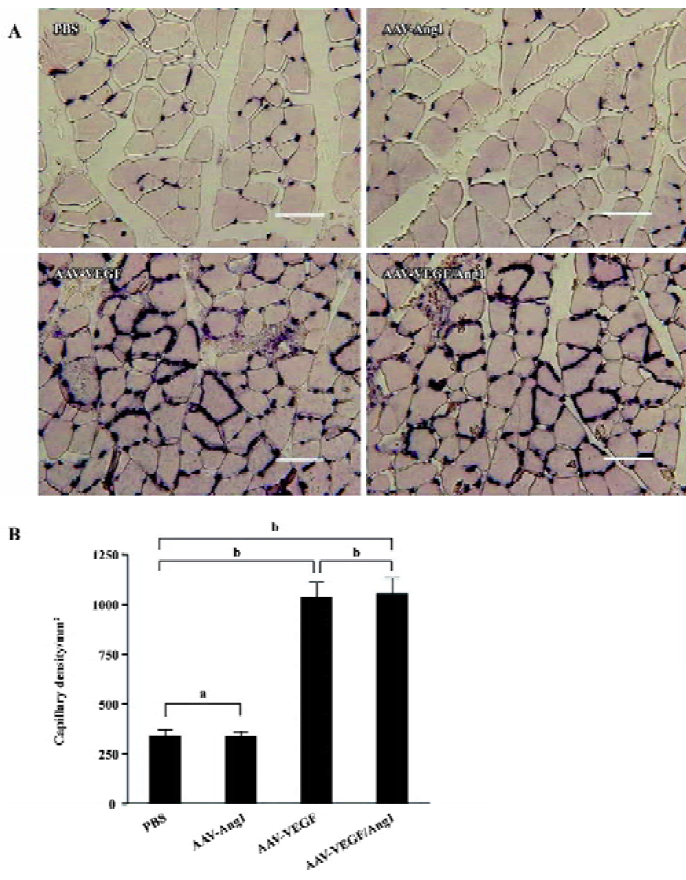
Promotion of neovascularization The muscle samples of rabbit ischemic hind-limbs were examined histologically 8 weeks after the gene transfer. When histological sections were stained with hematoxylin, we found that the muscles of group AAV-VEGF or AAV-VEGF/Ang1 showed remarkably increased cellularity as compared to that of group PBS or AAV-Ang1 (Figure 3A). To detect capillary endothelial cells, tissue sections were stained for alkaline phosphatase. As shown in Figure 4B, capillary density in transduced muscles of group AAV-VEGF (1032±80/mm2) or AAV-VEGF/Ang1 (1054±76/mm2) was significantly higher than that of group AAV-Ang1 (336±26/mm2) or PBS (340±29/mm2). To identify arterioles positive for α-SMA, we performed immunohistochemistry with an antibody against α-SMA. The α-SMA-positive vessel density was significantly increased when VEGF was co-expressed together with Ang1 in group AAV-VEGF/Ang1 (Figure 5B). At the same time, we never observed the formation of angioma-like structures in group AAV-VEGF or AAV-VEGF/Ang1. Intramuscular administration of AAV-VEGF/Ang1 could significantly enhance neova-scularization of ischemic tissue.
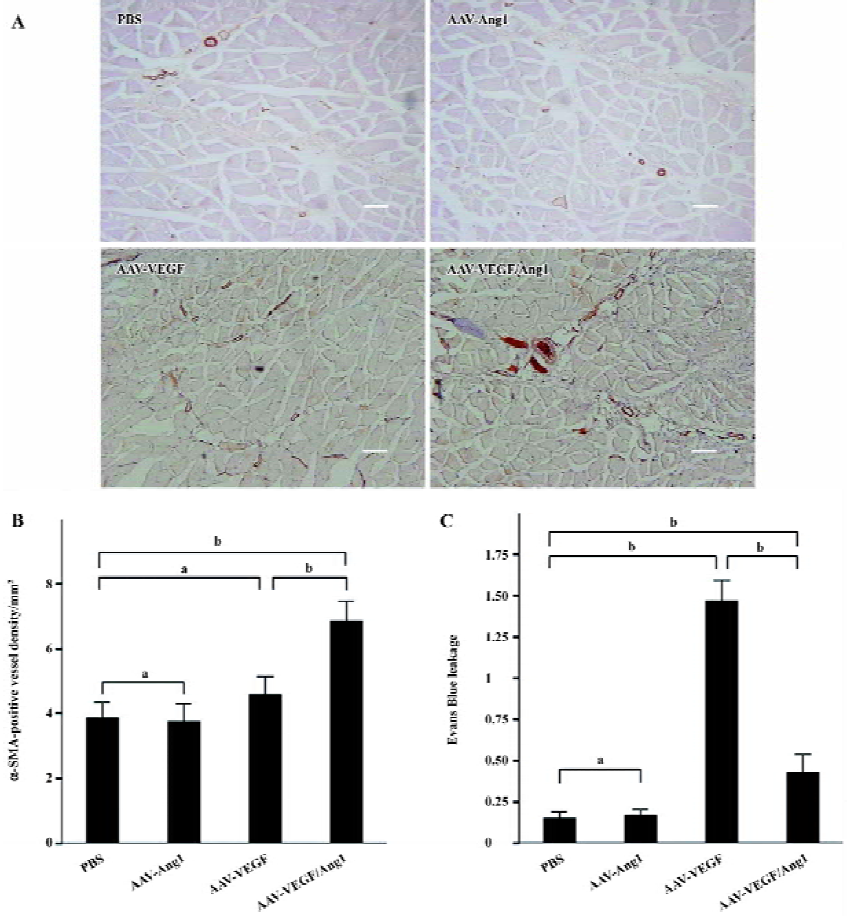
Prevention of capillary leakage To test the function of the observed increase in capillary density, we measured vascular leakage with Evans Blue dye as previously described. As shown in Figure 5C, the vascular permeability in group AAV-VEGF (1.46±0.13) was significantly higher than that in group PBS (0.15±0.04) or AAV-Ang1 (0.17±0.03) induced by VEGF, but the permeability was obviously reduced when VEGF was co-expressed together with Ang1 in group AAV-VEGF/Ang1 (0.43±0.11). Intramuscular administration of AAV-VEGF/Ang1 could obviously prevent capillary leakage.
Discussion
For combination gene therapy with VEGF and Ang1 in the treatment of ischemic diseases, we constructed the AAV–VEGF/Ang1 viral vectors simultaneously encoding 2 angiogenic growth factors, VEGF and Ang1. These 2 genes had their own independent CMV promoter and hGH polyA. To prove the feasibility of this approach, we performed an intramuscular injection of AAV–VEGF/Ang1. Eight weeks after the injection, we could detect the co-expression of VEGF and Ang1 in transduced muscles with RT-PCR and Western blotting methods, and we also found their synergistic effects of forming mature vasculature. This finding demonstrated that 2 proteins could be simultaneously encoded in one AAV vector and co-expressed in transduced tissues. This finding had an important application value for other combination gene therapies.
The most striking change we observed was the increase in the number of blood vessels present in the AAV-VEGF and AAV-VEGF/Ang1 injected areas. This event was somehow expected as far as capillaries were concerned, given the very well-known properties of VEGF on endothelial cell proliferation and migration[20] and according to studies that analyzed capillary formation after intramuscular injection of adenoviral vectors expressing VEGF[21,22] or of skeletal myoblasts engineered to secrete this factor[23,24]. However, the vessels in the AAV-VEGF injected areas were leaky. When VEGF co-expressed with Ang1 in group AAV-VEGF/Ang1, the permeability of vessels was remarkably reduced. Consistent with that VEGF is a strong inducer of vascular permeability[25], Ang1 has been shown to be essential for the maturation of blood vessels during embryonic development[26] and for the mitigation of vascular leakage promoted by inflammation and VEGF in the adult vasculature[27].
In the present study, we also found that the α-SMA positive vessel density was significantly increased when VEGF was co-expressed together with Ang1 in group AAV-VEGF/Ang1. So this demonstrated that VEGF co-expressed with Ang1 could enhance arteriogenesis processes in ischemic tissues. Ang1 could recruit and stimulate the formation of pericyte and smooth muscle cells leading to maturation of blood vessels. However, in addition to its property of being a strong endothelial cell mitogen via its Flk-1 receptor, VEGF also seemed to stimulate smooth muscle cell generation or migration[28,29]. Consistent with that, co-administration of plasmid VEGF and plasmid Ang1 led to enhanced arterio-genesis in the ischemic myocardium[8].
Another interesting finding of our study was the observation that muscles injected with AAV-VEGF or AAV-VEGF/Ang1 showed remarkably increased cellularity as compared to PBS or the AAV-Ang1 injected groups. The same findings were also reported by Arsic et al[30] who found that these cells were positive for CD31 and VEGFR-2, markers of hematopoietic/endothelial cell precursors, and also positive for the c-kit/CD117 marker, an antigen that commonly defines pluripotent bone marrow stem cells. Different investigators have recently reported that c-kit-positive bone marrow cells are capable of infiltrating the infracted myocardium (in which expression of VEGF is probably high) where they underwent alternate differentiation routes, including cardiomyocytes, endothelial, and smooth muscle cells[31–33]. In addition, circulating endothelial progenitor cells of bone marrow origin that had been isolated were incorporated in sites of myocardial neovascularization[34]. Altogether, these observations suggest that circulating stem cells might sense ischemic, damaged tissue, or VEGF and migrate to these areas where they promote tissue formation by a mechanism that is probably different from angiogenic sprouting and might resemble embryonic vasculogenesis.
A potential problem of our study was that the prolonged and high-level expression of VEGF may result in the formation of hemangioma. Hemangioma has been seen in the heart injected with plasmid[35] and retroviral vector-mediated VEGF gene transfer[24,36]. Retroviruses could mediate transgene expression at high levels in transduced tissue[24], and high concentrations of VEGF in local areas could induce exaggerated angiogenesis and angioma formation. The injection of a large dose of VEGF plasmid (500 mg of plasmid DNA) to the ischemic rat heart induced angioma formation. No angioma was seen in a rat model when a smaller dose (125 mg of plasmid DNA) was used[35]. Springer et al[24] reported that high levels of serum VEGF (200 mg/mL) caused hemangiomas in adult skeletal muscles, and that low serum levels (30 mg/mL) did not cause vascular malformations, but were sufficient to induce angiogenesis in ischemic muscles. Hence, it was important to control the level of VEGF expres-sion. In the present study, AAV-VEGF/Ang1 induced angiogenesis in the local ischemic environment, but no angioma was observed and no hVEGF165 was detected in rabbit serum. These differences were most likely related to AAV gene transfer biology, in which the onset of growth factor expression was progressive and the peak levels of the produced factor were lower than those of other vector systems[11]. This suggested that AAV-mediated VEGF expression was not as high as those mediated by adenoviral and retroviral vectors, and yet it was enough to induce new vascular formation in the ischemic myocardium. Previous studies have shown that transduction with AAV vectors could result in gene expression lasting >1 year[37]. Although the dose of AAV-VEGF/Ang1 used in this experiment did not induce angioma formation, it was possible that long-term expression of VEGF could eventually lead to angioma formation. A method of circumventing this possibility was to add the hypoxia response elements found in erythropoietin or VEGF genes to control the expression of VEGF which could be activated under hypoxia and turned off once hypoxic conditions had been resolved[38,39]. Thus, AAV vectors combined with an inducible promoter could provide a safe delivery system for AAV-VEGF/Ang1 in clinical use.
In summary, AAV vectors can simultaneously encode 2 proteins which can be efficiently and stably co-expressed in transduced tissues without ectopic expression. AAV-mediated VEGF and Ang1 genes transfer enhances angiogenesis and arteriogenesis, prevents capillary leakage, and improves blood flow in a rabbit hind-limb ischemic model. Intramuscular administration of AAV-VEGF/Ang1 may be a useful stra-tegy for the treatment of ischemic diseases.
References
- Rubanyi GM. The future of human gene therapy. Mol Aspects Med 2001;22:113-42.
- Rissanen TT, Vajanto I, Yla-Herttuala S. Gene therapy for therapeutic angiogenesis in critically ischaemic lower limb - on the way to the clinic. Eur J Clin Invest 2001;31:651-66.
- Koransky ML, Robbins RC, Blau HM. VEGF gene delivery for treatment of ischemic cardiovascular disease. Trends Cardiovasc Med 2002;12:108-14.
- Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med 2000;6:1102-3.
- Jain RK, Munn LL. Leaky vessels? Call Ang1! Nat Med 2000;6:131-2.
- Loughna S, Sato TN. Angiopoietin and Tie signaling pathways in vascular development. Matrix Biol 2001;20:319-25.
- Chae JK, Kim I, Lim ST, Chung MJ, Kim WH, Kim HG, et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol 2000;20:2573-8.
- Siddiqui AJ, Blomberg P, Wardell E, Hellgren I, Eskandarpour M, Islam KB, et al. Combination of angiopoietin-1 and vascular endothelial growth factor gene therapy enhances arteriogenesis in the ischemic myocardium. Biochem Biophys Res Commun 2003;310:1002-9.
- Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, et al. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation 1996;94:3281-90.
- Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, . Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA 1996; 93: 14 082–7.
- Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med 1997;3:306-12.
- Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther 1999;6:1574-83.
- Chen DJ, Tan Z, Xie YL, Liu F. Construction of adeno-associated virus coexpression system for human angiopoietin-1 and VEGF gene. Chin Med J (Engl) 2004;117:562-5.
- Wu XB, Dong XY, Wu ZJ, Cao H, Niu DB, Qu JG, et al. A novel method for purification of recombinant adeno-associated virus vectors on a large scale. Chin Sci Bull 2001;46:485-9.
- Wright JF, Le T, Prado J, Bahr-Davidson J, Smith PH, Zhen Z, et al. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol Ther 2005;12:171-8.
- Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest 1994;93:662-70.
- Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 1998;101:2567-78.
- Ziada AM, Hudlicka O, Tyler KR, Wright AJ. The effect of long-term vasodilatation on capillary growth and performance in rabbit heart and skeletal muscle. Cardiovasc Res 1984;18:724-32.
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999;286:2511-4.
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999;13:9-22.
- Gowdak LH, Poliakova L, Wang X, Kovesdi I, Fishbein KW, Zacheo A, et al. Adenovirus-mediated VEGF(121) gene transfer stimulates angiogenesis in normoperfused skeletal muscle and preserves tissue perfusion after induction of ischemia. Circulation 2000;102:565-71.
- Vajanto I, Rissanen TT, Rutanen J, Hiltunen MO, Tuomisto TT, Arve K, et al. Evaluation of angiogenesis and side effects in ischemic rabbit hindlimbs after intramuscular injection of adenoviral vectors encoding VEGF and LacZ. J Gene Med 2002;4:371-80.
- Lu Y, Shansky J, Del Tatto M, Ferland P, Wang X, Vandenburgh H. Recombinant vascular endothelial growth factor secreted from tissue-engineered bioartificial muscle promotes localized angio-genesis. Circulation 2001;104:594-9.
- Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell 1998;2:549-58.
- Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol 1999;237:97-132.
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996;87:1171-80.
- Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000;6:460-3.
- Dor Y, Djonov V, Abramovitch R, Itin A, Fishman GI, Carmeliet P, et al. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J 2002;21:1939-47.
- Ishida A, Murray J, Saito Y, Kanthou C, Benzakour O, Shibuya M, et al. Expression of vascular endothelial growth factor receptors in smooth muscle cells. J Cell Physiol 2001;188:359-68.
- Arsic N, Zentilin L, Zacchigna S, Santoro D, Stanta G, Salvi A, et al. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol Ther 2003;7:450-9.
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430-6.
- Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001;107:1395-402.
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701-5.
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999;85:221-8.
- Schwarz ER, Speakman MT, Patterson M, Hale SS, Isner JM, Kedes LH, et al. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat — angiogenesis and angioma formation. J Am Coll Cardiol 2000;35:1323-30.
- Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation 2000;102:898-901.
- Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol 1996;70:8098-108.
- Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 1996; 271: 17 771–8.
- Su H, Arakawa-Hoyt J, Kan YW. Adeno-associated viral vector-mediated hypoxia response element-regulated gene expression in mouse ischemic heart model. Proc Natl Acad Sci USA 2002;99:9480-5.
