Clonidine attenuates morphine withdrawal and subsequent drug sensitization in rhesus monkeys1
Introduction
Drug dependence is a chronic, relapsing disorder in which compulsive drug-seeking and drug-taking behavior persists despite serious negative consequences[1]. Addictive sub-stances, such as opioids, induce pleasant states or relieve distress, effects that contribute to their recreational use. After repeated exposure, adaptive changes occur in the central nervous system that lead to drug dependence[1–3]. Although the intrinsic rewarding properties of addictive drugs such as heroin are important in the acquisition of drug self-administration, compulsive drug-seeking and drug-taking by addicts is not readily explained in terms of simple reward or positive reinforcement processes alone[4]. Abstinence from the drug in dependent subjects induces aversive withdrawal symptoms that are thought to contribute to the compulsive nature of drug self-administration in addiction.
The exact role of withdrawal in heroin addiction is debatable. It has been proposed that a drug addict may self-administer heroin to escape from abstinence symptoms (avoidance theory)[5,6], but also that withdrawal from heroin functions as a motivational state that enhances the incentive value of the drug (incentive-motivational theory)[4,7]. Other integrative reward theories of addiction, such as the incentive salience-sensitization theory[8], propose that drug-addicts are sensitized to some motivational effects of drugs of abuse. Indeed, it has been shown that repeated morphine administration produces hypersensitivity to subsequent doses of morphine (“behavioral sensitization”) and to other drugs of abuse (“cross-sensitization”). Sensitization and cross-sensitization have been extensively studied in rodents[9–11], but there is little evidence for such phenomenon in primates. Since drug priming effectively reinstates extinguished drug self-administration behavior in animals[12–14], it is likely that enhanced reactivity to the effects of drug of abuse may facilitate relapse in drug addicts. The fact that low doses of morphine or cocaine can cause hyperactivity or drug-seeking in drug reinstatement rodent models supports this notion[15,16]. Therefore, there is a great interest in developing medications that may block sensitization processes in order to reduce relapse in humans.
It is well established that noradrenergic pathways are implicated in morphine withdrawal. Activity of central adrenergic neurons is inhibited by opiates[17] and increased firing of the noradrenergic neurons in the locus coeruleus has been clearly demonstrated during opiate withdrawal[18]. Clonidine, an α2 adrenoreceptor agonist, reduces this increased firing in morphine-dependent rats[18], an effect that is thought to mediate the drug’s ability to reduce morphine withdrawal symptoms in animals and humans19,20]. α2 adrenoceptor agonists, such as clonidine and lofexidine, are used to reduce withdrawal syndromes during the initial phase of opioid abstinence in humans[21–23]. Typically, these α2 adrenoreceptor agonists are used to control opioid withdrawal on a tapered dosing schedule for the first week of drug abstinence. Most of the studies conducted in animals have evaluated the effects of clonidine on opiate withdrawal symptoms and little is known about the long-term effects of α2 adrenoceptor agonists after their administration.
Recent evidence suggests that noradrenergic pathways are involved not only in withdrawal states, but also in other aspects of drug dependence such as drug-seeking behavior[24] and behavioral sensitization[25]. The α2 adrenoceptor agonist lofexidine attenuates stress-induced reinstatement of alcohol-seeking and also decreases alcohol self-administration[26]. The α2 adrenoceptor antagonist, yohimbine, induces reinstatement in animal models of abuse of alcohol[26], methamphetamine[27], cocaine[28], heroin[29], and a mixture of cocaine and heroin (speedball)[30]. There is a strong correlation between increases in cortical extracellular norepinephrine levels and the expression of behavioral sensitization to amphetamine[25]; cortical α1-adrenergic receptors are critically involved in locomotor responses to amphetamine and morphine[31,32]. In spite of the widespread use of clonidine during the initial phase of opiate withdrawal in humans, the long-term effects of clonidine on subsequent motivational effects of drugs of abuse have not been explored.
The present study was designed to evaluate the immediate and long-term effects of clonidine administered during the initial phase of morphine withdrawal in rhesus monkeys. First, to induce dependence, rhesus monkeys received an escalading morphine dosage regimen over 90 d. To induce morphine withdrawal, morphine treatment was abruptly stopped. To evaluate the immediate and long-term effects of clonidine on withdrawal signs, the monkeys received clonidine for 1 week and the withdrawal signs were measured daily during a period of 21 d. Finally, the effects of clonidine on the challenge injection of morphine or cocaine were evaluated after prolonged morphine abstinence.
Materials and methods
Drugs Morphine hydrochloride and cocaine phosphate were purchased from Qinghai Pharmaceutical Factory Co Ltd (Xi’ning, China). Solutions of morphine and cocaine were prepared with saline (0.9% sodium chloride) and delivered via sc injection. Clonidine is a commercial agent for human use, given ig.
Animals Laboratory-reared, male rhesus monkeys (Macaca mulatta), weight between 3.5 and 5.5 kg (2–3 years old), were purchased from the Beijing Xierxing Institute of Biological Resources (Beijing, China). The monkeys were housed individually in 80 cm (height)×70 cm (width)×70 cm (length) metal cages. The monkeys were allowed free access to water, and restricted food and fresh fruit access was available at 09:00 h and 15:00 h. The animals were maintained according to the Guide for the Care and Use of Laboratory Animals (National Institute of Health, 1996). The experimental protocol was approved by Institutional Animal Care and Use Committee of National Institute on Drug Dependence, Peking University.
Morphine dependence induction The experiment consisted of 3 phases: morphine dependence induction (90 d), morphine withdrawal (21 d), and drug challenge (7 d). To ensure consistent drug administration and to reduce stress on and increase the cooperativeness of the monkeys, all drug administrations were performed by the same experi-menters, who were blind to the animals’ group assignment.
During the 90 d period of morphine dependence induction, the monkeys were housed in an environment similar to that in which they were reared. Eighteen monkeys were randomly divided into 3 groups of 6 monkeys per group (3 groups: Sal-Sal, Mor-Sal, and Mor-Clo). Table 1 summarizes the details of the experimental procedure. Morphine dependence was induced by repeated administration of morphine at increasing dosages for 90 d. Every day, the monkeys received sc injections in their back legs (08:00 h, 13:00 h, and 20:00 h). The Sal-Sal group was given 0.5 mL/kg of saline. The Mor-Sal and Mor-Clo groups were given morphine on a dose schedule of 3 mg/kg (d 1–7), 6 mg/kg (d 8–14), 9 mg/kg (d 15–21), 12 mg/kg (d 22–28), 15 mg/kg (d 29–90). Each monkey in the Mor-Clo and Mor-Sal groups received a total of 3420 mg/kg morphine over 90 d.
Full table
Morphine withdrawal and clonidine treatment During the 21 d period of morphine withdrawal, none of the groups were given morphine. In the first week of withdrawal, the monkeys in the Mor-Clo group received 0.02 mg/kg (ig) clonidine twice per day just prior to feeding (09:00 h, 15:00 h), while the monkeys in the Sal-Sal and Mor-Sal groups received an equal volume of saline (ig). In the second and third weeks of withdrawal, all the monkeys were given saline injections twice per day just prior to feeding. Withdrawal signs, including holding the abdomen, tremor, spasm, grimacing, face flush, eye closing, dysphoric facial expres-sions, and provoked screams, were assessed immediately after each injection.
Drug challenge During the 7 d period of drug challenge, all the monkeys received a saline injection daily in the first 3 d, a challenge injection of 5 mg/kg morphine (sc) on the fourth day, a saline injection on the fifth and sixth days, and a challenge injection of 5 mg/kg cocaine (sc) on the seventh day. The monkeys’ response activity, including locomotor activity, irritability, vocalization and grooming, were assessed immediately after the challenge injection.
Physiological recording and behavioral scoring On the last day of the 90 d morphine induction period, the body temperature, breath rate, heart rate, and body weight of each monkey were recorded just before each feeding (09:00 h, 15: 00 h), and the average of the 2 values of each parameter was recorded as the pretest value. This measure was repeated on withdrawal day 1–21. While the measurements were taken, the monkeys were limited to a small corner of the cage by an apparatus designed by the experimenters. The monkeys were allowed to rest for at least 10 min prior to the recording. Withdrawal signs were observed from 08:30–09:00 and 14:30–13:00. If symptoms of withdrawal appeared in the 30 min observation, the monkey was given a score of 1, and observation periods in which symptoms were not shown were given 0. The average score (0, 0.5, or 1) of the 2 daily observations of each monkey was taken as their current day score value. On the challenge days (challenge d 4 and 7), the monkeys’ behavioral changes were scored according to the following scale: -3 (apparently reduced), 0 (no change), 3 (few observed), 6 (many observed), and 10 (extremely changed). The observation span was 6 h after the challenge injection (09:00 h). All the recording of physiological signs and behavioral scoring were carried out by the same experimenters who were kept blind to the group assignment in order to ensure consistency.
Statistical analysis The data are expressed as mean± SEM. and were analyzed with SPSS 13 software (SPSS Inc, Chicago, Illinois, USA). Physiological signs and withdrawal score were analyzed using a repeated measure ANOVA followed by a mean comparison with the Bonferroni test. Behavioral scores from the challenge tests were analyzed with repeated measure ANOVA followed by a mean comparison with the Bonferroni test.
Results
After the 90 d morphine treatment, the monkeys exposed to morphine (Mor-Sal and Mor-Clo groups) showed body weight loss (3.88±0.14 kg before morphine treatment vs 3.61±0.11 kg thereafter, n=11) compared with the morphine-naive monkeys (Sal-Sal group, 3.87±0.23 kg before vs 4.08±0.20 kg after, n=6, P<0.05). During the chronic morphine treatment, 1 monkey in the Mor-Clo group died at d 69 after receiving a total dosage of 2430 mg/kg morphine.
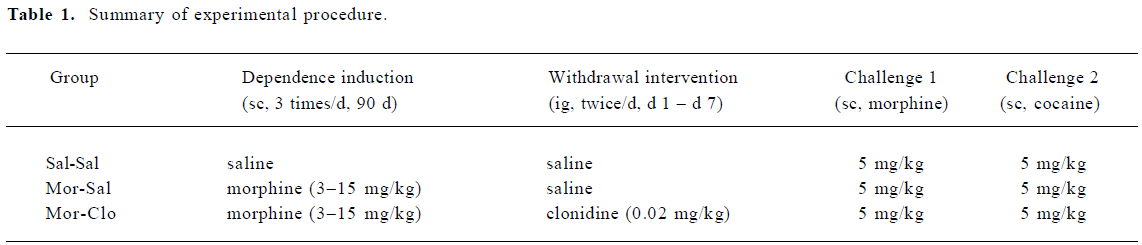
Effects of clonidine on morphine withdrawal signs During the 21 d withdrawal period, different weight loss trends was observed in the 3 groups (F[2,11]=3.341 P=0.074; Figure 1A). Most withdrawal signs were observed in the first week after cessation of morphine treatment; thus, the effects of clonidine on body weight were analyzed in the first, second, and third week separately. A significant difference was observed in the weight loss in the first (F[2,11]=12.146, P<0.01), but not the second or the third withdrawal weeks. However, clonidine had no effect on the body weight in the first week of morphine withdrawal (P>0.05).
During the 21 d withdrawal period, significant differences were found in the body temperatures (F[2,11]=16.381, P<0.01; Figure 1B) of the 3 groups. As compared with the Sal-Sal group, morphine withdrawal (Mor-Sal) produced a significant decrease in body temperature (P<0.01), but clonidine treatment had no effect on these withdrawal-induced body temperature decreases. Significant differences were found between the 3 groups for body temperature during the first (F[2,11]=20.6, P<0.01), second (F[2,11]=11.8, P<0.005), and third (F[2,11]=4.8, P<0.05) weeks of the withdrawal period.
During the 21 d withdrawal period, a significant difference in heart rate (F[2,11]=4.722, P<0.05; Figure 1C) was observed in the 3 groups. Clonidine was found to increase the monkeys’ heart rate in the first withdrawal week. No significant difference was found for breath rate (Figure 1D) in the 3 groups.
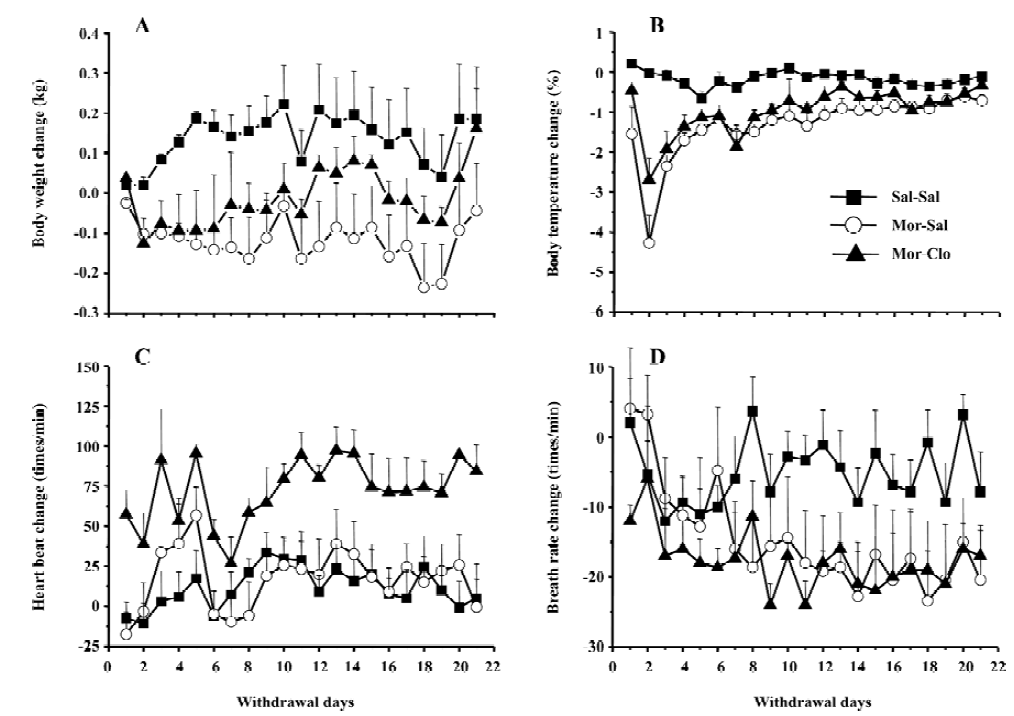
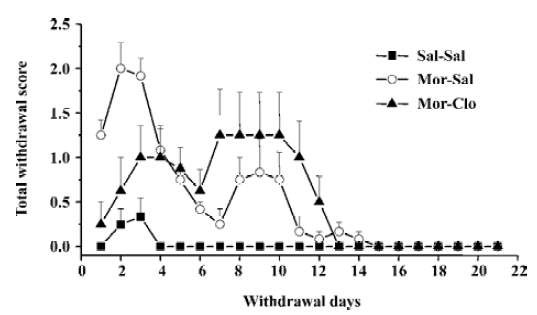
Cessation of morphine treatment produced obvious withdrawal signs, including holding the abdomen, tremor, spasm, grimacing, face flush, eye closing, dysphoric facial expres-sions, and provoked screams. During the first week of withdrawal, 1 monkey in the Mor-Sal group died at withdrawal d 7. A significant difference was only observed for the overall withdrawal score during the first 14 d withdrawal period (F[2,13]=19.9, P<0.01); most withdrawal signs disappeared after the fourteenth day (Figure 2). In the 14 d obser-vation, clonidine reduced the symptoms of withdrawal only during the first 4 withdrawal days (P<0.01, Mor-Sal vs Mal-Clo). Clonidine had no further effects on withdrawal symptoms after 4 d (P>0.05). The main behaviors that contributed to the total withdrawal score were holding the abdomen, tremor, spasm, grimacing, face flush, eye closing, dysphoric facial expressions, and provoked screams (Figure 3). Clonidine proved effective in controlling the withdrawal signs of abdomen holding, tremor, eye closing, and provoked screams in the first 7 withdrawal days, but not all the signs of morphine withdrawal were eliminated by clonidine.
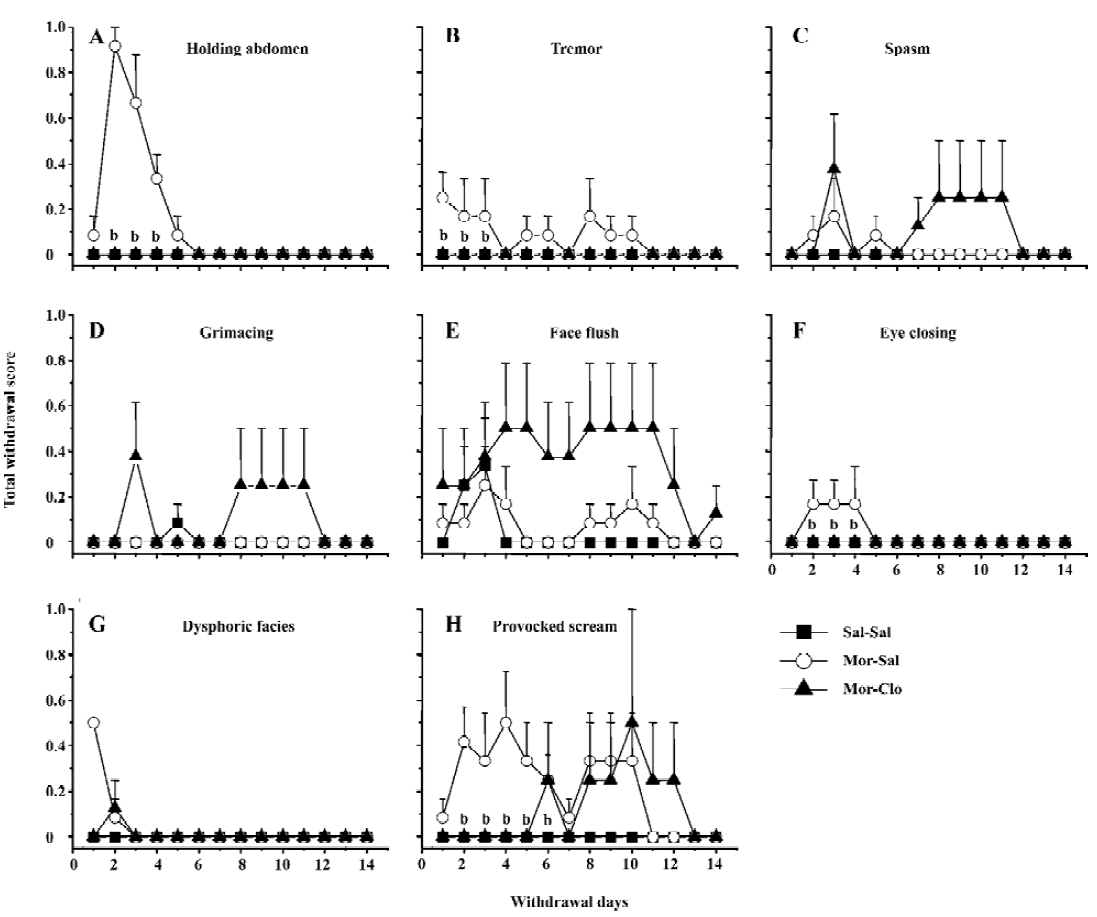
Effects of clonidine on the effect of cocaine and morphine challenge injection Body weight, body temperature, and heart and breath rate were also recorded 5 min before and 1 and 5 h after the challenge injection of morphine or cocaine. No apparent change was observed in the monkeys receiving morphine priming. The response activities to morphine or cocaine, including locomotor activity, vocalization, grooming, and irritability were reliably observed and used to discern the sensitization of the monkeys to the challenge of morphine and cocaine.
An injection of 5 mg/kg morphine caused a significant increase in locomotor activity (F[2,11]=29.9, P<0.01) and irritability (F[2,11]=24.8, P<0.01), but not in vocalization (F[2,11]=1.445, P>0.05) and grooming (F[2,11]=1.445, P>0.05; Figure 4A). The monkeys naive to morphine (Sal-Sal) showed deceased locomotor activity and irritability, which may reflect the sedative effects of morphine[33–35]. Compared with the Sal-Sal group, the monkeys with a history of morphine treatment demonstrated increased locomotor activity and irritability (P<0.01, Sal-Sal vs Mor-Sal), and these behavioral responses were greatly attenuated in the monkeys that had previously received clonidine during the first week of morphine withdrawal (P<0.01, Mor-Clo vs Mor-Sal).
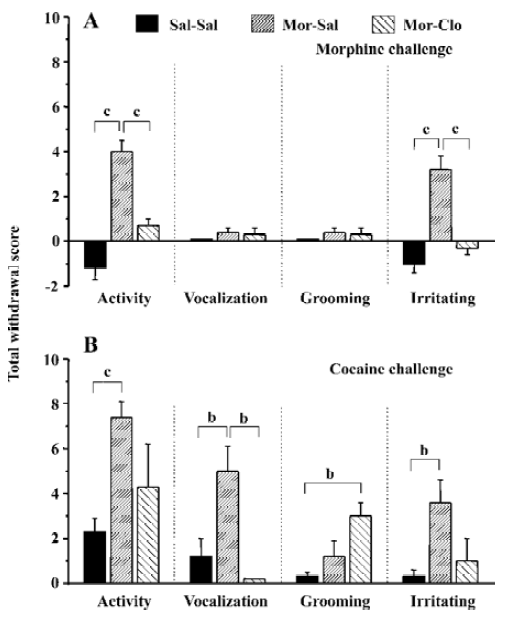
An injection of 5 mg/kg cocaine increased all the observed behaviors in the Mor-Sal group (Figure 4B): locomotor activity (F[2,11]=8.941, P<0.01), vocalization (F[2,11]=7.364, P<0.01), grooming (F[2,11]=5.535, P<0.05), and irritability (F[2,11]=5.914, P<0.05). The monkeys that received clonidine treatment during the first week of morphine withdrawal showed significantly decreased vocalization compared with no clonidine treatment after morphine withdrawal (P<0.05, Mor-Clo vs Mor-Sal), and enhanced grooming performance compared with saline control (P<0.05, Sal-Clo vs Sal-Sal). One monkey in the Mor-Sal group demonstrated a dramatic increase in locomotor activity (scored 10), irritability (scored 8), and vocalization (scored 6) 1 h after the cocaine injection. After 4 h, all the above behaviors declined, and 1 monkey died 13 h after the cocaine injection in the Mor-Sal group.
Discussion
In the present study, withdrawal symptoms were found to persist for 14 d after cessation of the 90 d morphine administration in the rhesus monkeys. Clonidine administration reduced morphine withdrawal symptoms. However, the effects of clonidine were not persistent and disappeared when clonidine administration was stopped. After prolonged abstinence from morphine, the morphine-dependent monkeys displayed an enhanced response to a challenge injection of morphine (“behavioral sensitization”) and cocaine (“cross-sensitization”). Clonidine administration during the initial week of the withdrawal phase produced attenuation of subsequent behavioral sensitization and cross-sensitization.
After a period of chronic opiate administration, failure to continue periodic administration of the drug to an animal resulted in severe physiological and behavioral disturbances several hours after the last dose of the drug. This complex of signs and symptoms, termed opiate abstinence syndrome, indicates that the organism has become physically dependent on the opiate[36]. Here, the withdrawal symptoms were measured daily for 21 d to determine precisely the time-course of appearance and disappearance of the symptoms in rhesus monkeys as compared with the symptoms previously documented[37,38]. Few reports are available on the time-course of the various opiate withdrawal symptoms following spontaneous abstinence in rhesus monkeys. Here we found the peak of withdrawal signs at day 2 and 3 following the cessation of the morphine injections (Figure 2). Morphine withdrawal was associated with significant weight loss and decrease of body temperature (Figure 1A, 1B), but there was no significant change in heart rate. Although breath rates decreased in morphine-dependent monkeys compared to control group, this difference was not significant (Figure 1D).
The various signs of morphine withdrawal that were scored during the withdrawal phase were highly specific, since control monkeys did not exhibit these signs (see however Figure 3 the presence of face flush in some control monkeys). After 14 d of withdrawal, nearly all of the observ-ed withdrawal signs disappeared (Figures 2, 3). It should be noted that withdrawal in this study was not induced by injections of opiate antagonist, but with the cessation of morphine treatment, a situation that mimics that of human addicts that stop taking drugs. In contrast, many previous investigators have used opioids antagonists, such as naloxone or nalorphine, to induce morphine withdrawal syndrome in monkeys[39] and rodents[40–46] (see ref 47 for a comparison of studies). The symptoms of withdrawal are often more pronounced when provoked by an injection of an opioid antagonist than provoked by cessation of morphine injec-tions. Evidence suggests that the motivational signs of withdrawal appear first in a situation of mild withdrawal, whereas physical signs are seen in a situation of severe withdrawal[46–48]. However, some physical signs of withdrawal were also relatively severe during the spontaneous withdrawal. This present finding was strengthened by the fact that 1 monkey died during the period of morphine withdrawal (see Results). Interestingly, the withdrawal syndrome created upon the cessation of access to opioids in rats previously trained to self-administer morphine caused many of the signs we reported here in monkeys, such as weight loss, tremor, hypersensitivity, agitation, soft stools, and increased respiration[49].
Administration of clonidine, an α2 adrenoceptor agonist, significantly decreased morphine withdrawal symptoms during the first week of withdrawal. This finding is in agreement with previous reports on rhesus monkeys[38], rodents[47,50–58], and humans[21,22,59] that clearly demonstrate that clonidine effectively attenuates some opiate withdrawal signs and symptoms. Here, clonidine was able to reduce some, but not all, symptoms of morphine withdrawal (Figure 3). Notably, clonidine significantly reduced the overall withdrawal signs during the first week of withdrawal (Figure 2). Some signs, such as the provoked screams and holding of the abdomen in the monkeys, were totally abolished by clonidine administration. In contrast, clonidine had no or limited effects on other symptoms such as face flush or grimacing. Previous reports had shown that clonidine only affected a subset of symptoms in monkeys[38], rats[47], and humans[21,22,59]. The effects of clonidine on withdrawal symptoms were short-lasting, since the intensity of the morphine withdrawal signs were even higher at the cessation of the clonidine treatment (at week 2), suggesting a rebound phenomenon (Figure 2). It should be noted, that clonidine produced a significant effect on weight loss induced by withdrawal that was noted at the third week of morphine withdrawal. However, most of the withdrawal symptoms disappeared at d 14, regardless of the presence or absence of clonidine treatment during the first week of withdrawal, indicating that clonidine did not dramatically alter the time-course of the abstinence symptoms and is only able to attenuate its acute manifestations. Hypotension or sedation are 2 frequent side-effects induced by clonidine treatment in opiate addicts[60,61]. The increase of heart rate found in the group of monkeys receiving clonidine treatment may reflect a compensatory mechanism to a hypotensive effect of clonidine. No particular sedation was noticed in the monkeys receiving clonidine treatment.
We have also investigated the effects of priming injections of morphine and cocaine after prolonged abstinence of morphine. The monkeys with a history of morphine treatment displayed enhanced locomotor responses to 5 mg/kg cocaine and to 5 mg/kg morphine, as compared with naive control monkeys (Figure 4). These enhanced responses likely reflect “behavioral sensitization” to morphine and “cross-sensitization” to cocaine. It is also possible that this behavioral sensitization to morphine may have been facilitated by the development of tolerance to the sedative or depressing effect of morphine, since morphine administration decreased locomotor activity in naive monkeys. However, since these experiments have been performed following prolonged withdrawal from morphine and it is well known that extended abstinence strongly decreases the tolerance to the effects of opiate, it is likely that the increased response to morphine in the monkey with a history of morphine administration reflects the development of behavioral sensitization.
Although many investigators have demonstrated that repeated administration of morphine can produce long-lasting behavioral sensitization in rodents[62], very limited evidence has been published so far that this phenomenon is also observed in humans or monkeys. Therefore, this is (to our knowledge) the first evidence that monkeys with history of morphine treatment displayed both sensitized responses to morphine and cross-sensitization to psychostimulants. These findings were in agreement with previous experiments performed on rodents which showed that heroin exposure facilitates subsequent locomotor and drug-seeking responses to cocaine in rats[10,63], and enhanced locomotion to ethanol in mice[64]. In addition, animals with a history of heroin self-administration displayed locomotor sensitization to amphetamine[15].
It may seem surprising that a sensitized response to cocaine administration has been observed on the locomotor activity, but not grooming behavior following cocaine admini-stration, since grooming is also a behavior mediated by dopamine transmission and notably by the stimulation of the dopamine D1 receptor in rodents[65–68]. Since locomotor activity is mediated by both the D1 and D2 receptor stimula-tion, this dissociation may reflect a preferential activation of the D1 receptor. However, the fact that morphine administration does not produce vocalization or grooming behavior, that are known to reflect stress in monkeys[28], strongly suggests that cocaine may have produced some aversive effects in these monkeys that morphine dose not produce. It is well known that cocaine, like other drugs of abuse[69], produces both positive and aversive effects[70,71]. It appears that opiates produce less aversive effects compared to cocaine[70,71], which may explain the absence of grooming and vocalization induced by the morphine challenge in these monkeys. This hypothesis can be tested in subsequent studies using doses of hormones that approximate the level of stress in these monkeys following drug administration.
Interestingly, some responses to a challenge injection of cocaine and morphine were not affected by the previous exposure to morphine (see Results). Notably, the cardiovascular response to 5 mg/kg cocaine and 5 mg/kg morphine was identical in all groups. Unfortunately, despite a considerable research effort in the last 2 decades, the causes of cardiovascular response to cocaine are still poorly understood[72]. Many investigators are convinced that the cardiovascular changes are mediated by changes in catecholamines levels at the periphery[72], but this interpretation is debatable. Opiates are mainly known to reduce heart rate and blood pressure[73]. However, an initial cardiovascular stimulating effect of opiate has been noted in cats[74], dogs[75], and humans[76]. These effects may also involve catecholamine and histamine release[73]. Here, we extended these findings to rhesus monkeys, since we had found significant cardiovascular activation 1 h following morphine and cocaine administration. It was noteworthy that the monkeys were sensitized only to response mediated by the dopaminergic system, whereas other responses that were likely to reflect peripheral effects of drugs of abuse were not affected.
Early clonidine intervention during the first week of withdrawal reduced sensitized behavior responses. This effect was significant for locomotor activity and the irritation score for morphine and for the vocalization score for cocaine. A trend downward (a non-significant decrease) was also noted for the locomotor activity and irritation behavior in the monkeys. These findings suggest that clonidine treatment during the first week of withdrawal serves not only to reduce the acute withdrawal symptoms, but also to affect the subsequent effects of drug challenge. Drug priming is able to reinstate drug-seeking behavior in animals in a reinstatement paradigm and can produce relapses in humans. It is possible that clonidine administration has blocked some neurobiological adaptations that are involved in behavioral sensitization processes (“incentive salience-sensitization theory”) or that the reduction of the intensity of the withdrawal symptoms accounts for the subsequent behavioral response to a drug priming injection (“incentive-motivational theory”). Further experiments are needed to delineate which of these hypotheses underlie the effects of clonidine. Since the noradrenergic structure mediates the expression of opioid abstinence, one possibility is that clonidine normalizes the firing of locus coeruleus neurons during withdrawal to produce its effects[53,77]. However, the recent and surprising finding that total neurochemical lesion of noradrenergic neurons of the locus coeruleus does not alter either naloxone-precipitated or spontaneous opiate withdrawal, nor influence ability of clonidine to reverse opiate withdrawal[78] suggests that other brain sites may be involved. It is noteworthy that a noradrenaline-rich subdivision has been recently identified in the human nucleus accumbens[79] and that behavioral sensitization processes to psychostimulants and opiates may involve sensitized responses of noradrenaline in frontal areas that project to the nucleus accumbens[25]. Recent evidence suggests that the blockade of noradrenaline release induced by clonidine during opiate withdrawal[57] may involve brain areas other than the locus coeruleus, and may implicate brain areas involved in the motivational control of drug-seeking behavior.
In conclusion, the present study demonstrates that morphine-dependent monkeys will not only present typical morphine withdrawal symptoms at the cessation of morphine administration, but will also display enhanced behavioral responses to challenge injections of both morphine and cocaine, providing evidence for both “behavioral sensitization” and “cross-sensitization” processes in non-human primates. In agreement with previous findings in humans and laboratory animals, clonidine served to decrease morphine withdrawal symptoms. However, these effects were short-lasting. Clonidine produces long-lasting effects on subsequent morphine and cocaine challenge after prolonged abstinence. While these experiments do not allow us to determine if the effects of clonidine are mediated by an interaction with behavioral sensitization processes or with the possible link between morphine withdrawal and subsequent effects of drugs, these experiments indicate that active intervention in the first stage of withdrawal using an α2 adrenoceptor agonist has a positive effect on reducing drug relapse in the overall therapeutic strategy.
Acknowledgements
We would like to thank Dr Yavin SHAHAM (National Institute on Drug Abuse, NIH, USA) for helpful comments on an early version of this manuscript and Dr Ying WANG (School of Medicine, Xi’An Jiaotong University, China) for assistance in data analysis.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed. Washington DC: American Psychiatric Association; 2000.
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science 1988;242:715-23.
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2001;2:119-28.
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci 2001;4:943-7.
- Wikler A, Pescor FT. Classical conditioning of a morphine abstinence phenomenon, reinforcement of opioid-drinking behavior and “relapse” in morphine addicted rats. Psychopharma-cologia 1967;20:255-84.
- Koob GF. Drug addiction: the yin and yang of hedonic homeo-stasis. Neuron 1996;16:893-6.
- Wikler A, Pescor FF, Miller D, Morrel H. Persistent potency of a secondary (conditioned) reinforcer following withdrawal of morphine from physically dependent rats. Psychopharmacologia 1971;20:103-17.
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev 1993;18:247-91.
- Wise RA, Leeb K. Psychomotor-stimulant sensitization: a unitary phenomenon? Behav Pharmacol 1993;4:339-49.
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction 2003;98:7-22.
- Mcdaid J, Dallimore JE, Mackie AR, Mickiewicz AL, Napier TC. Cross-sensitization to morphine in cocaine-sensitized rats: behavioral assessments correlate with enhanced responding of ventral pallidal neurons to morphine and glutamate, with diminished effects of GABA. J Pharmacol Exp Ther 2005;313:1182-93.
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 2002;168:3-20.
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology 2003;168:31-41.
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 2003;168:21-30.
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci 1998;10:3565-71.
- Lu L, Xu NJ, Ge X, Yue W, Su WJ, Pei G, et al. Reactivation of morphine conditioned place preference by drug priming: role of environmental cues and sensitization. Psychopharmacology 2002;159:125-32.
- Korf J, Bunney BS, Aghajanian GK. Noradrenergic neurons: morphine inhibition of spontaneous activity. Eur J Pharmacol 1974;25:165-9.
- Aghajanian GK. Tolerance of locus coeruleus neurons to morphine and suppression of withdrawal response by clonidine. Nature 1978;276:186-7.
- Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature 1980;285:476-8.
- Gold MS, Pottash AC, Extein IL, Kleber HD. Neuroanatomical sites of action of clonidine in opiate withdrawal: the locus coeruleus connection. Prog Clin Biol Res 1981;71:285-98.
- Gossop M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug Alcohol Depend 1988;21:253-9.
- Gerra G, Marcato A, Caccavari R, Fontanesi B, Delsignore R, Fertonani G, et al. Clonidine and opiate receptor antagonists in the treatment of heroin addiction. J Subst Abuse Treat 1995;12:35-41.
- Gowing LR, Farrell M, Ali RL, White JM. α2-adrenergic agonists in opioid withdrawal. Addiction 2002;97:49-58.
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology 2004;47 Suppl 1:167-79.
- Salomon L, Lanteri C, Glowinski J, Tassin JP. Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci USA 2006;103:7476-81.
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology 2005;179:366-73.
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 2004;55:1082-9.
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of α2-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsycho-pharmacology 2004;29:686-93.
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 2000;12:292-302.
- Highfield D, Yap J, Grimm J, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology 2001;25:320-31.
- Drouin C, Blanc G, Trovero F, Glowinski J, Tassin JP. Cortical α1-adrenergic regulation of acute and sensitized morphine locomotor effects. Neuroreport 2001;12:3483-6.
- Drouin C, Blanc G, Villegier AS, Glowinski J, Tassin JP. Critical role of α1-adrenergic receptors in acute and sensitized locomotor effects of D-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteri-stics. Synapse 2002;43:51-61.
- Lineberry CG, Kulics AT. Morphine effects on escape in the rhesus monkey. Neuropharmacology 1980;19:107-10.
- Marcais H, Bonnet JJ, Costentin J. Evidence for sedative effects of low doses of morphine in mice involving receptors insensitive to naloxone. Life Sci 1981;28:2737-42.
- Bartoletti M, Gaiardi M, Gubellini G, Bacchi A, Babbini M. Long-term sensitization to the excitatory effects of morphine. A motility study in post-dependent rats. Neuropharmacology 1983;22:1193-6.
- Tatum SL, Seevers MH, Collins KH. Morphine addiction and its physiological interpretation based on experimental evidences. J Pharmacol Exp Ther 1929;36:447-75.
- Katz JL, Valentino RJ. The opiate quasiwithdrawal syndrome in rhesus monkeys: comparison of naloxone-precipitated withdrawal to effects of cholinergic agents. Psychopharmacology (Berl) 1984;84:12-5.
- Katz JL. Effects of clonidine and morphine on opioid withdrawal in rhesus monkeys. Psychopharmacology (Berl) 1986;88:392-7.
- Goldberg SR, Schuster CR. Conditioned suppression by a stimulus associated with nalorphine in morphine-dependent monkeys. J Exp Anal Behav 1967;10:235-42.
- Martin WR, Wikler A, Eades CG, Pescor FT. Tolerance to and physical dependence on morphine in rats. Psychopharmacologia 1963;65:247-60.
- Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia 1973;33:19-38.
- Collier HO. Cellular site of opiate dependence. Nature 1980;283:625-9.
- Cador M, Taylor JR, Robbins TW. Potentiation of the effects of reward-related stimuli by dopaminergic-dependent mechanisms in the nucleus accumbens. Psychopharmacology 1991;104:377-85.
- Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther 1992;261:669-77.
- Aghajanian GK, Kogan JJ, Moghaddam B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res 1994;636:126-30.
- Higgins GA, Sellers EM. Antagonist-precipitated opioid withdrawal in rats: evidence for dissociations between physical and motivational signs. Pharmacol Biochem Behav 1994;48:1-8.
- Pinelli A, Trivulzio S. Quantitative evaluation of opioid withdrawal signs in rats repeatedly treated with morphine and injected with naloxone, in the absence or presence of the antiabstinence agent clonidine. J Pharmacol Toxicol Methods 1997;38:117-31.
- Frenois F, Cador M, Caille S, Stinus L, Le Moine C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur J Neurosci 2002;16:1377-89.
- Weeks JR. Experimental morphine addiction: Method for automatic intravenous injections in unrestrained rats. Science 1962;143:143-4.
- Olds J, Milner PM. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 1954;47:419-27.
- Tseng LF, Loh HH, Wei EE. Effects of clonidine on morphine withdrawal signs in the rat. Eur J Pharmacol 1975;30:93-9.
- Vetulani J, Bednarczyk B. Depression by clonidine of shaking behaviour elicited by nalorphine in morphine-dependent rats. J Pharm Pharmacol 1977;29:567-9.
- Esposito E, Kruszewska A, Ossowska G, Samanin R. Noradrenergic and behavioural effects of naloxone injected in the locus coeruleus of morphine-dependent rats and their control by clonidine. Psychopharmacology 1987;93:393-6.
- Buccafusco JJ. Participation of different brain regions in the anti-narcotic withdrawal action of clonidine in the dependent rat. Brain Res 1990;513:8-14.
- Coupar IM. Effect of α2-adrenoceptor agonists on the expression of morphine-withdrawal in rats. Naunyn Schmiedebergs Arch Pharmacol 1992;345:553-7.
- Garzon J, Sanchez-Blazquez P. A N-acetyl human β-endorphin-(1-31) alleviates the morphine withdrawal syndrome in rodents: a comparative study with clonidine. Life Sci 1992;50:2099-109.
- Silverstone PH, Done C, Sharp T. Clonidine but not nifedipine prevents the release of noradrenaline during naloxone-precipitated opiate withdrawal: an in vivo microdialysis study in the rat. Psychopharmacology 1992;109:235-8.
- Vaupel DB, Kimes AS, London ED. Comparison of 7-nitro-indazole with other nitric oxide synthase inhibitors as attenuators of opioid withdrawal. Psychopharmacology (Berl) 1995;118:361-8.
- Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl) 1997;130:41-58.
- Kleber HD, Riordan CE, Rounsaville B, Kosten T, Charney D, Gaspari J, et al. Clonidine in outpatient detoxification from methadone maintenance. Arch Gen Psychiatry 1985;42:391-4.
- Preston KL, Bigelow GE, Liebson IA. Self-administration of clonidine, oxazepam, and hydromorphone by patients undergoing methadone detoxification. Clin Pharmacol Ther 1985;38:219-27.
- Vanderschuren LJ, Tjon GH, Nestby P, Mulder AH, Schoffelmeer AN, De Vries TJ. Morphine-induced long-term sensitization to the locomotor effects of morphine and amphetamine depends on the temporal pattern of the pretreatment regimen. Psychopharmacology 1997;131:115-22.
- He S, Grasing K. Chronic opiate treatment enhances both cocaine-reinforced and cocaine-seeking behaviors following opiate withdrawal. Drug Alcohol Depend 2004;75:215-21.
- Lessov CN, Phillips TJ. Cross-sensitization between the locomotor stimulant effects of ethanol and those of morphine and cocaine in mice. Alcohol Clin Exp Res 2003;27:616-27.
- Beninger RJ, Mazurski EJ, Hoffman DC. Receptor subtype-specific dopaminergic agents and unconditioned behavior. Pol J Pharmacol Pharm 1991;43:507-28.
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, et al. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell 1994;79:729-42.
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther 2005;312:875-83.
- Sokoloff P, Leriche L, Le Foll B. Dopamine receptors: structure, function and implication in psychiatric disorders (in press).
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology (Berl) 2006;184:367-81.
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav 1993;44:191-8.
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev 2004;27:721-8.
- Knuepfer MM. Cardiovascular disorders associated with cocaine use: myths and truths. Pharmacol Ther 2003;97:181-222.
- Schug SA, Zech D, Grond S. Adverse effects of systemic opioid analgesics. Drug Saf 1992;7:200-13.
- Kayaalp SO, Kaymakcalan S. A comparative study of the effects of morphine in unanaesthetized and anaesthetized cats. Br J Pharmacol Chemother 1966;26:196-204.
- Vatner SF, Marsh JD, Swain JA. Effects of morphine on coronary and left ventricular dynamics in conscious dogs. J Clin Invest 1975;55:207-17.
- Mildh LH, Tuomisto LM, Scheinin M, Kirvela OA. Morphine-induced cardiovascular stimulation: the effects of two doses on healthy subjects. Anesth Analg 2000;91:51-7.
- Valverde O, Smadja C, Roques BP, Maldonado R. The attenuation of morphine-conditioned place preference following chronic mild stress is reversed by a CCKB receptor antagonist. Psychopharmacology (Berl) 1997;131:79-85.
- Caille S, Espejo EF, Reneric JJ, Cador M, Koob GF, Stinus L. Total neurochemical lesion of noradrenergic neurons of the locus ceruleus does not alter either naloxone-precipitated or spontaneous opiate withdrawal nor does it influence ability of clonidine to reverse opiate withdrawal. J Pharmacol Exp Ther 1999;290:881-92.
- Andree TH, Mikuni M, Tong CY, Koenig JI, Meltzer HY. Differential effect of subchronic treatment with various neuroleptic agents on serotonin 2 receptors in rat cerebral cortex. J Neuro-chem 1986;46:191-7.
