Lentiviral vector-mediated siRNA knockdown of SR-PSOX inhibits foam cell formation in vitro1
Introduction
Atherosclerosis is an inflammatory condition characterized by the accumulation of lipids and foam cell formation of monocyte-derived macrophages and smooth muscle cells (SMC)[1,2]. Oxidatively–modified, low-density lipoprotein (Ox-LDL) and other substances associated with atherogenesis may participate in the activation of monocytes into macrophages. The monocyte-derived macrophage uptake of Ox-LDL is mediated by a relatively large family of scavenger receptors, including scavenger receptor class A (SR-A), CD36[3], CD68[4], the lectin-like Ox-LDL receptor[5] and scavenger receptor that binds phosphatidylserine and oxidized lipoprotein (SR-PSOX)[6]. SR-PSOX was later found to be identical to CXC chemokine ligand 16 (CXCL16)[7].
SR-PSOX/CXCL16 (henceforth referred to as SR-PSOX) exists in membrane-bound and soluble forms. When binding to the specific chemokine receptor, CXC receptor 6 (CXCR6), SR-PSOX promotes the firm adhesion of cells[8] and SMC proliferation[9]. The proteolytic cleavage of membrane-bound SR-PSOX results in the release of soluble SR-PSOX[8], which acts as a chemoattractant for CXCR6+ cells[10]. SR-PSOX has been shown to stimulate SMC proliferation and cell–cell adhesion[11]. In atherosclerotic lesions, SR-PSOX has been found in lipid-laden intimal macrophages, although its expression has not been detected in normal aortas[12,13]. These results indicate that SR-PSOX may play a role in foam cell formation and is associated with atherosclerosis[14]. Nevertheless, there has been no efficient and simple approach for studying the effect of SR-PSOX in vitro.
In this study, we successfully constructed a lentiviral expression vector that contained green fluorescence protein (GFP) and SR-PSOX small interfering RNA (siRNA; Lenti-SR-PSOXsi) to investigate the expression of SR-PSOX induced by Ox-LDL and the effect of SR-PSOX in the process of foam cell formation.
Materials and methods
Reagents RPMI-1640 medium, Dulbecco’s modified Eagle’s medium (DMEM), and HEPES were purchased from Gibco. Fetal bovine serum (FBS) was purchased from Bio International. Low-density lipoprotein (LDL) was from Sigma (St Louis, MO, USA). Lipofectamine 2000 and Trizol were from Invitrogen.
HpaI, XhoI, and T4 DNA ligase were purchased from New England Biolabs. MMLV and the RT–PCR kit were purchased from Promega. The SYBR green PCR kit was purchased from TAKARA. The goat antihuman CXCL16 antibody was from R&D. Polybrene and Oil red O were purchased from Sigma (St Louis, MO,USA). The ViraPower packaging mix (GenScript siRNA expression vector pGCL-GFP, pHelper 1.0, and pHelper 2.0;) and ENi.S (enhanced infection solution) were purchased from Genechem, and oligonucleotide primers were synthesized by Genechem). The ECL Western blotting detection reagent was purchased from Amersham Biosciences.
Cell culture The human monocyte-derived THP-1 cell line and 293T cell line under passage 20 (Shanghai Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, China) were grown in RPMI-1640 medium or DMEM containing 10% fetal bovine serum (v/v), 2 mmol/L L-glutamine, 0.45% glucose (w/v), 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, 1×10–5 mol/L β-mercaptoethanol, 100 units/mL penicillin, and 100 μg/mL streptomycin in a humidified 5% CO2 atmosphere at 37 °C. The medium was replaced every 3 d during the time of culture. Experimental cells were incubated with Ox-LDL (10, 20, and 40 mg/L) for different times.
Preparation of Ox-LDL LDL was oxidized at a concentration of 3 mg protein/mL by exposure to 7.5 µmol/L CuSO4 for 20 h at 37 °C[15]. Oxidation was monitored by measuring the amount of thiobarbituric acid-reactive substances (10.7 nmol/mg protein) produced, and their greater mobilities on agarose gel electrophoresis due to increased negative charge were compared with LDL.
SR-PSOX siRNA design and plasmid construction The human SR-PSOX cDNA sequence (Genebank accession number:
Lentivirus production and transduction Lenti-SR-PSOXsi and Lenti-NC virus were produced by plasmid cotransfection of 293T cells, as described previously[16]. 293T cells were transfected with ViraPower packaging mix using 100 μL Lipofectamine 2000 reagent according to the manufacturer’s instructions. The viral supernatant was harvested 48 h after transfection, passed through 0.45 μm filters and concentrated, and the viral titer was determined. The viral supernatant was added into target THP-1 cells at multiplicity of infection (10, 100, 200) with ENi.S and 5 μg/mL polybrene to obtain stably-transfected THP-1KD and THP-1NC cells.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay for cell viability Upon exposure of the cells to Ox-LDL, Lenti-SR-PSOXsi, or Lenti-NC for 48 h, cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay[17]. In brief, the cells were incubated with 1 mg/mL MTT for 2 h at 37 °C. MTT/formazan was extracted by overnight incubation at 37 °C with 100 μL extraction buffer (20% SDS and 50% formamide adjusted to pH 4.7 with 0.02% acetic acid and 0.025 mol/L HCl). Optical densities at 570 nm were measured using extraction buffer as a blank.
Flow cytometry analysis Flow cytometry was performed using Becton Dickinson FACSCalibur instrument. GFP was detected in cells suspended in fluorescence-activated cell sorting buffer comprising 0.5% bovine serum albumin in phosphate-buffered saline (PBS) supplemented with 0.05% sodium azide. The sorting of live GFP positive cells was calculated.
Real-time quantitative RT–PCR analysis RNA was collected by using Trizol reagent, and cDNA was reverse transcribed using MMLV. The expression level of SR-PSOX mRNA was analyzed by real-time quantitative reverse transcriptase PCR using a LightCycler instrument. cDNA was used for real-time quantitative RT–PCR using the SYBR green PCR master mix. The PCR conditions consisted of 35 cycles, with 10 s denaturation at 94 ºC, 30 s annealing at 62 ºC, and 30 s primer extension at 72 ºC. The primers were as follows: SR-PSOX (forward: 5'-CAGGAATTGATGAGCTGTCTTG-3'; reverse: 5'-CCGCAGTGTGAATGGTGG-3') and human actin (SR-PSOX values were normalized against human actin; forward: 5'-GTGGACATCCGCAAAGAC-3'; reverse: 5'-AAAGGGTGTAACGCAACTA-3'). All real-time RT–PCR results were expressed as fold changes in mRNA expression with respect to the control cells. Target gene expression was normalized to the expression of the housekeeping gene 18S for each sample. Data were analyzed using the 2-ΔΔCT method[18]. All reactions were performed in triplicate. The efficacy of the 4r target sequences was evaluated, and the best was chosen for subsequent experiments.
Western blotting analysis After the transfection of THP-1 cells, the cells were rinsed with PBS and lysed with SDS–PAGE protein loading buffer containing 5% 2-mercaptoethanol. Cell lysates with equal amounts of total protein were then separated on 10% SDS–polyacrylamide gels with a 12% polyacrylamide gel, and transferred onto nitrocellulose paper. The resulting Western blots were incubated with 5% non-fat milk for 1 h and then incubated overnight at 4 °C with a 1 μg/mL of goat antihuman CXCL16 antibody. The blots were then washed 3 times with 0.05% Tween-20 in TBS and incubated with 1:5000 dilution of peroxidase-conjugated secondary antigoat immunoglobulin for 2 h at an ambient temperature. After further washing, the chemiluminescent substrate stable peroxidase and substrate luminol/enhancer solutions were added. The blots were developed using ECL Western blotting detection reagent. The blots were then applied to Kodak X-Omat film, and immunoreactive proteins were visualized on the developed film.
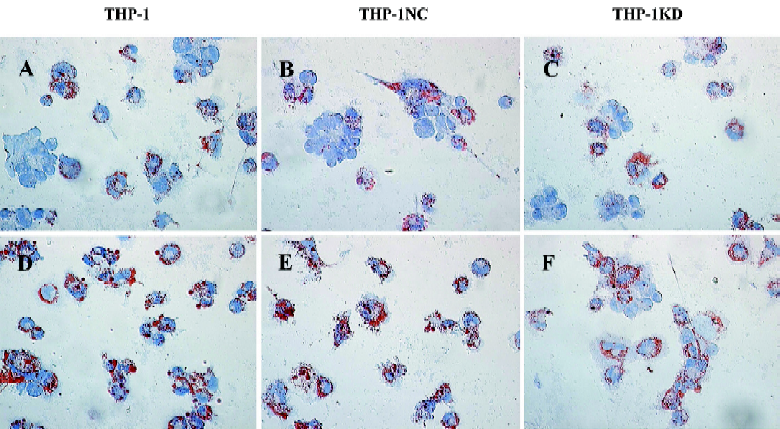
Oil red O-stain analysis For the Ox-LDL uptake experiments, THP-1KD cells were differentiated into adherent macrophages by treatment with 160 nmol/L phorbol ester for 24 h[10]. The foam cell model derived from macrophages was established 48 h after the addition of 40 mg/L Ox-LDL into a RPMI-1640 medium of macrophages. At the same time, the normal THP-1 cells were incubated with or without Ox-LDL as the control. At the designated time points after the Ox-LDL uptake experiments, THP-1 or THP-1KD cells were fixed in PBS–buffered 2% paraformaldehyde solution for 15 min, and 0.5% Oil red O (in 60% isopropanol) staining was done for 20 min as described[10]. Cell nuclei were then stained in hematoxylin for a few seconds. All procedures were performed at room temperature. Lipid droplet accumulation in the foam cells was evaluated under a microscope (Leica, Germany). Foam cells were defined as macrophages in which the entire cytoplasm was filled with Oil Red O-stainable lipid droplets.
Statistical analysis Each experiment was performed at least 3 times, and data are shown as the mean±SD where applicable. Statistically significant differences between groups in each assay were determined by ANOVA, followed by post-hoc Dunnett’s t-tests. The probability of <0.05 was considered to be statistically significant.
Results
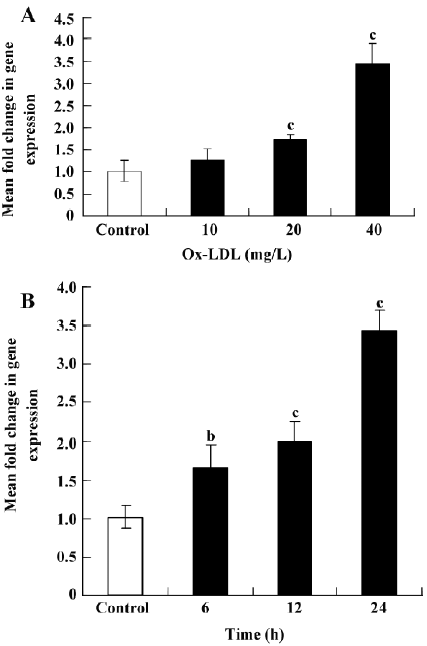
Ox-LDL induces SR-PSOX expression in THP-1 cells The expression of SR-PSOX mRNA in THP-1 cells with or without Ox-LDL (0, 10, 20, and 40 mg/L) for 24 h or Ox-LDL (40 mg/L) for different hours (0, 6, 12, and 24 h) was investigated to determine whether they could regulate the SR-PSOX expression (Figure 1). The SR-PSOX mRNA expression was increased significantly in a concentration-dependent manner 24 h after Ox-LDL treatment compared to the untreated THP-1 cells. SR-PSOX mRNA induction was increased by 3.43-fold after 40 mg/L Ox-LDL treatment.
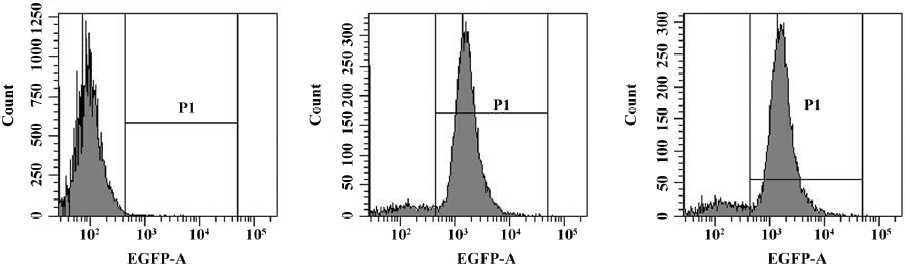
Lentiviral stably-transfected THP-1 cells The lentiviral vector expression cassette allowed for the permanent expression of GFP and SR-PSOX siRNA in transduced cells. GFP expression and the percentage of GFP expression cells were determined by flow cytometry analysis. Figure 2 indicates the efficient transduction of Lenti-NC (88.5%) and THP-1KD cells (85.9%) after 4 d transfection.
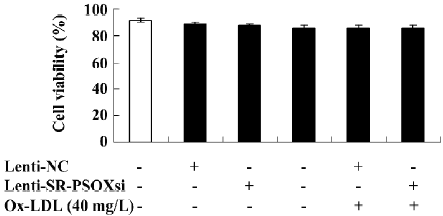
Cell viability Upon exposure of the cells to Ox-LDL, Lenti-SR-PSOXsi or Lenti-NC for 48 h, cell viability was measured by MTT assay (Figure 3). There was no difference in cell viability between all groups.
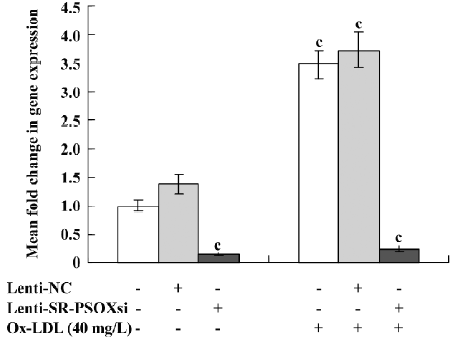
Effect of Lenti-SR-PSOXsi on mRNA expression Lenti-SR-PSOXsi to reduce the level of SR-PSOX mRNA in THP-1 cells pretreated with 40 mg/L Ox-LDL was tested. Four days after the transduction of Lenti-SR-PSOXsi, SR-PSOX mRNA expression was significantly decreased in THP-1KD cells with or without Ox-LDL, and the SR-PSOX mRNA inhibition rate was 84.6% and 75.3%, respectively, compared with the untreated THP-1 cells (Figure 4).
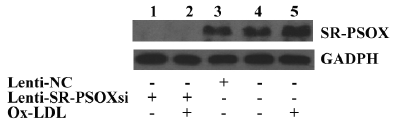
Effect of Lenti-SR-PSOXsi on protein expression The exposure of THP-1 cells to 40 mg/L Ox-LDL for 48 h resulted in a significant increase in the SR-PSOX protein expression (Figure 5). Four days after transfection with Lensi-SR-PSOXsi, the expression of the SR-PSOX protein in the THP-1KD cells was significantly decreased. The expression of the SR-PSOX protein was not detectable even in THP-1KD cells incubated with Ox-LDL.
Effect of Lenti-SR-PSOXsi on foam cell formation Normal THP-1-derived macrophages contained few neutral lipids and were weakly stained with Oil red O, a dye specific for neutral lipids. At 48 h after the Ox-LDL uptake experiment, a number of THP-1 and THP-1NC-derived foam cells were seen filled with large cytoplasmic lipid droplets (Figure 6). However, THP-1KD-derived foam cells led to a marked decrease of the number of macrophages transforming into foam cells.
Discussion
Ox-LDL is generally accepted as a pathogenic factor in atherosclerosis. Macrophages express several scavenger receptors[1-6] that are capable of taking up Ox-LDL and mediate massive accumulation of cholesterols characteristic of macrophage foam cells[1,2]. SR-PSOX is a unique chemokine and a scavenger receptor for Ox-LDL in macrophages. SR-PSOX was previously found in lipid-laden intimal macrophages in atherosclerotic lesions of human carotid and coronary arteries[6]. This supports the notion that SR-PSOX could be involved in Ox-LDL uptake by lesion macrophages. It was found in our experiment that the SR-PSOX expression was significantly increased in THP-1 cells induced by Ox-LDL in a time- and dose-dependent manner. These results suggested that the SR-PSOX expression in THP-1 cells was regulated by Ox-LDL.
RNA interference (RNAi) is a powerful tool for studying protein function[19-22]. The effective delivery of siRNA molecules into target cells or tissues is critical for successful RNAi application. Lentiviral vectors can effectively transduce exogenous genes with the long-term expression of transgenes[16] and have a promising future in clinical applications[20,22]. To study the role of SR-PSOX, we described the use of HIV-1-based lentiviral vectors for the delivery of short hairpin RNA, a precursor of SR-PSOX siRNA, into THP-1 cells to suppress the SR-PSOX gene expression.
Four days after transfection with Lenti-SR-PSOXsi or Lenti-NC, fluorescence microscope photographs and flow cytometry analysis demonstrated the efficient transduction of THP-1 cells with lentiviral vectors. The cells could be transfected with prepared Lenti-SR-PSOXsi up to 82.4% for 2 months (data not shown). We successfully used lentiviral vectors for the delivery of the SR-PSOX RNAi-induced stable knockdown of SR-PSOX gene expression in THP-1 cells. mRNA and protein expressions of SR-PSOX in THP-1KD cells 4 d after transduction were significantly decreased compared with THP-1 and THP-1NC cells. THP-1KD-stable cells could be used as a powerful tool for studying the effect of the SR-PSOX gene in atherosclerosis.
The accumulation of Ox-LDL resulted in foam cell formation, a hallmark of early atherosclerotic lesions[23]. In foam cell models derived from SMC incubated with Ox-LDL, SR-PSOX/CXCL16 and TNF-α were increased during foam cell formation; the stable transfection of SMC with recombinant human SR-PSOX/CXCL16 could promote foam cell formation and upregulate the expression of TNF-α[6]. In our study investigating the involvement of SR-PSOX in macrophage-derived foam cell formation, we found that SR-PSOX siRNA could reduce the uptake of Ox-LDL and decrease the development of macrophage-derived foam cells by inhibiting the expression of SR-PSOX. This finding strongly suggests that the high expression of macrophage SR-PSOX increased foam cell formation in vitro. A recent finding[24] showed that internalized macrophages from CXCL16–/– mice significantly lessened Ox-LDL compared to wild-type macrophages. This result is similar to our study in vitro, indicating that CXCL16 mediates a significant portion of Ox-LDL uptake in vivo and in vitro. This result closely approximates the values obtained in SR-A-deficient macrophages[25], suggesting that a significant portion of SR-A-independent accumulation of Ox-LDL is mediated by SR-PSOX. We therefore conclude that the effects of SR-PSOX on monocyte differentiation, foam cell formation, and the progress of atherosclerosis are complex and need more studies in vivo.
In this study, we demonstrated that the lentiviral expression vector is a powerful and effective tool for investigating the effect of SR-PSOX in vitro, and SR-PSOX deficiency could decrease Ox-LDL uptake by monocyte-derived macrophages and the formation of foam cells, which may be a key factor involving the progression of atherosclerosis. The precise mechanisms require further clarification.
Acknowledgements
We thank Dr Shun-xing ZHANG, Dr Jing ZHANG and Dr Xiao-jian ZHANG for preparing this manuscript.
References
- Gleissner CA, Leitinger N, Ley K. Effects of native and modified low-density lipoproteins on monocyte recruitment in atherosclerosis. Hypertension 2007;50:276-83.
- Evans CE, Mylchreest S, Charlton-Menys V, Durrington P. The role of hydrostatic pressure in foam cell formation upon exposure of macrophages to LDL and oxidized LDL. Atherosclerosis 2008;197:596-601.
- Sun B, Boyanovsky BB, Connelly MA, Shridas P, van der Westhuyzen DR, Webb NR. Distinct mechanisms for OxLDL uptake and cellular trafficking by class B scavenger receptors CD36 and SR-BI. J Lipid Res 2007;48:2560-70.
- Eguchi A, Murakami A, Ohigashi H. Nobiletin, a citrus flavonoid, suppresses phorbol ester-induced expression of multiple scavenger receptor genes in THP-1 human monocytic cells. FEBS Lett 2006;580:3321-8.
- Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res 2007;100:1634-42.
- Quan Z, Yang H, Yang Y, Yan B, Cao R, Wen G, et al. Construction and functional analysis of a lentiviral expression vector containing a scavenger receptor (SR-PSOX) that binds uniquely phosphatidylserine and oxidized lipoprotein. Acta Biochim Biophys Sin (Shanghai) 2007;39:208-16.
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol 2000;1:298-304.
- Hundhausen C, Schulte A, Schulz B, Andrzejewski MG, Schwarz N, von Hundelshausen P, et al. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J Immunol 2007;178:8064-72.
- Hofnagel O, Luechtenborg B, Plenz G, Robenek H. Expression of the novel scavenger receptor SR-PSOX in cultured aortic smooth muscle cells and umbilical endothelial cells. Arterioscler Thromb Vasc Biol 2002;22:710-1.
- Jiang X, Shimaoka T, Kojo S, Harada M, Watarai H, Wakao H, et al. Cutting edge: critical role of CXCL16/CXCR6 in NKT cell trafficking in allograft tolerance. J Immunol 2005;175:2051-5.
- Chandrasekar B, Bysani S, Mummidi S. CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa B kinase, and nuclear factor-kappa B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem 2004;279:3188-96.
- Minami M, Kume N, Shimaoka T, Kataoka H, Hayashida K, Akiyama Y, et al. Expression of SR-PSOX, a novel cell-surface scavenger receptor for phosphatidylserine and oxidized LDL in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2001;21:1796-800.
- Wuttge DM, Zhou X, Sheikine Y, Wagsater D, Stemme V, Hedin U, et al. CXCL16/SR-PSOX is an interferon-gamma-regulated chemokine and scavenger receptor expressed in atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2004;24:750-5.
- Sheikine Y, Sirsjo A. CXCL16/SR-PSOX-A friend or a foe in atherosclerosis? Atherosclerosis 2008;197:487-95.
- Yang PY, Rui YC, Jin YX, Li TJ, Qiu Y, Zhang L, et al. Antisense oligodeoxynucleotide inhibits vascular endothelial growth factor expression in U937 foam cells. Acta Pharmacol Sin 2003;24:610-4.
- Fish RJ, Kruithof EK. Short-term cytotoxic effects and long-term instability of RNAi delivered using lentiviral vectors. BMC Mol Biol 2004;5:9.
- Jiang G, Li T, Qiu Y, Rui Y, Chen W, Lou Y. RNA interference for HIF-1alpha inhibits foam cells formation in vitro. Eur J Pharmacol 2007;562:183-90.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402-8.
- Tang Y, Ge YZ, Yin JQ. Exploring in vitro roles of siRNA in cardiovascular disease. Acta Pharmacol Sin 2007;28:1-9.
- Wang H, Tan SS, Wang XY, Liu DH, Yu CS, Bai ZL, et al. Silencing livin gene by siRNA leads to apoptosis induction, cell cycle arrest, and proliferation inhibition in malignant melanoma LiBr cells. Acta Pharmacol Sin 2007;28:1968-74.
- Gao LF, Wen LJ, Yu H, Zhang L, Meng Y, Shao YT, et al. Knockdown of Stat3 expression using RNAi inhibits growth of laryngeal tumors in vivo. Acta Pharmacol Sin 2006;27:347-52.
- Thomas M, Greil J, Heidenreich O. Targeting leukemic fusion proteins with small interfering RNAs: recent advances and therapeutic potentials. Acta Pharmacol Sin 2006;27:273-81.
- Yin R, Dong YG, Li HL. PPARgamma phosphorylation mediated by JNK MAPK: a potential role in macrophage-derived foam cell formation. Acta Pharmacol Sin 2006;27:1146-52.
- Aslanian AM, Charo IF. Targeted disruption of the scavenger receptor and chemokine CXCL16 accelerates atherosclerosis. Circulation 2006;114:583-90.
- Lougheed M, Lum CM, Ling W, Suzuki H, Kodama T, Steinbrecher U. High affinity saturable uptake of oxidized low density lipoprotein by macrophages from mice lacking the scavenger receptor class A type I/II. J Biol Chem 1997;272:12938-44.
