Edaravone (MCI-186), a free radical scavenger, attenuates retinal
Introduction
Ischemic injury to the retina is a major cause of visual loss, and ischemia/reperfusion (I/R)-induced oxidative stress is thought to be the direct cause of retinal injury. Both apoptosis and necrosis have been proven to be involved in I/R-induced neuronal damage[1]. Neuronal apoptosis and necrosis have been indicated to participate in I/R injury in the rat retina at both the inner nuclear layer and outer nuclear layer[2,3]. Superoxide dismutase (SOD) is a major scavenger of reactive oxygen species (ROS), which are known to trigger apoptosis and are massively generated during I/R[4]. Malondialdehyde (MDA), a degraded product of lipid peroxidation, can produce cytotoxicity by reacting with the amino of nucleic acid[5]. Our previous study showed that changes of MDA levels and SOD activity are indicators for lipid peroxidation degrees and therefore reflect the severity of tissue damage[6].
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one, MCI-186) is a free radical scavenger that has been successfully applied for the treatment of acute cerebral ischemia[7,8]. Edaravone is considered to directly scavenge hydroxyl radical and inhibit lipoxygenase activity, which has been reported to play an important role in cardiac I/R injury[9]. A number of studies have shown a protective effect of edaravone against ischemic injury in the brain and liver, even when administered after the onset of ischemia[10,11]. Stull and colleagues found that the action of edaravone involved hydroxyl radical scavenging and the subsequent inhibition of lipid peroxidation chain reaction in the plasma membrane of endothelial cells, neurons, and glial cells[12]. Others have reported that edaravone has antioxidative effects and reduces apoptosis[13,14]. In vitro and in vivo studies have also shown that edaravone could effectively inhibit I/R-induced apoptosis[15,16]. Edaravone was also suggested to have neuroprotective effects on transient forebrain or focal ischemia in rodent models[17,18]. However, up to now, there are not many studies on the effect of edaravone against retinal neuronal damage caused by I/R.
The purpose of the present study was to clarify whether edaravone has protective effects against I/R-induced retinal neuronal damage in a rat retinal I/R model. We established a rat retinal I/R model and determined the MDA and SOD levels of the retina. We also examined the apoptosis of retinal neurons and the functional changes of the retina in an effort to elucidate the mechanism by which edaravone protects retinal neurons. Our findings indicated that edaravone could protect retinal neurons during retinal I/R, and improve I/R-induced retinal dysfunction through inhibiting oxidative stress and apoptosis.
Materials and methods
Animals Male Sprague–Dawley rats, weighing 200–250 g, were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences (Shanghai, China). The animals were housed under controlled temperatures (23–25°C) on a 12 h light/12 h dark cycle (8:00–20:00 hours light, 20:00–8:00 hours dark), with free access to standard food and drinking water. All the animals used in this experiment received humane care, and the study was carried out in compliance with the institutional guidelines for the health and care of experimental animals.
Induction of retinal ischemia Retinal ischemia was induced by the transient elevation of intraocular pressure (IOP), as described in detail elsewhere[19,20]. Briefly, the rats were anesthetized with an intramuscular injection of 75 mg/kg ketamine and 13.6 mg/kg xylazine, and the pupils were dilated with topical phenylephrine (2.5%) and tropicamide (1%). The anterior chamber of the left eye was cannulated with a 27 gauge needle connected to a physiological saline reservoir. IOP was raised to 110 mmHg by elevating the reservoir, and retinal ischemia was confirmed by fundus examination. After 60 min, IOP returned to normal pressure by removing the infusion needle from the anterior chamber.
Group assignment and drug administration The rats were randomly assigned to 3 groups: normal control, I/R with vehicle, and I/R with edaravone. Edaravone (MCI-186: Carbiochem–Novabiochem, USA) was dissolved in 1 mL of 1 mol/L NaOH and adjusted to pH 7.4 by adding 1 mol/L HCl, and then diluted in saline to a concentration of 3 mg/mL. Edaravone was intraperitoneally injected to the rat I/R model at a dose of 3 mg/kg at 30 min before ischemia, and then at 3 mg/kg (ip) twice daily for 1 or 5 d after I/R. The rats in the I/R with vehicle group received an injection of equal volume of normal saline instead. The animals in the normal control group were normally reared without treatment.
Preparation of retinal tissue One day after I/R injury, the rats were killed by an anesthesia overdose. The eyes were enucleated, fixed in 4% paraformaldehyde for 24 h, washed with phosphate-buffered saline (PBS), and embedded in paraffin. Five micrometer-thick sections that included the optic disk were collected. The sections were processed for terminal deoxynucleotidyl transferase-mediated digoxigenin-dUTP nick-end labeling (TUNEL). In addition, the enucleated eyes were put on to ice slices immediately after harvesting. Coronal dissection through the pars plana was made in the enucleated eyes, the vitreous were removed, and the retinal tissue was harvested under an operating microscope. The specimens were stored at –80 °C for the biochemical assay.
Determination of MDA level and SOD activity The retina samples were prepared as 10% homogenate in 0.9% saline by a homogenizer on ice according to their respective weight. Then the homogenate was centrifuged, and the supernatant was collected and diluted. The assay of the MDA and SOD levels was according to the manufacturer’s instructions of the MDA and SOD detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
TUNEL staining The DeadEnd fluorometric TUNEL system was provided by Promega (Madison, WI, USA). The paraffin sections were prepared as described by the manufacturer and subjected to TUNEL analysis after incubation with proteinase K, followed by propidium iodide counterstaining. A laser scanning confocal microscope (Zeiss 510, Jena, Germany) was used for the observation, and Zeiss 4.6 version software was used for image collection. The apoptotic photoreceptors within the retina showed a yellow fluorescence.
Electroretinography On d 5 after I/R injury, electroretinography (ERG) of the left eye was recorded in the rats of each group. Scotopic ERG were recorded with a ganzfeld bowl. The animals were initially adapted to dark overnight before the recording of their flash ERG. The pupils were dilated with 1% tropicamide and 2.5% phenylephrine. Stainless steel wire (0.1 mm diameter) loops were placed on the center of the cornea. A reference electrode was placed in the middle of the forehead, and a grounding electrode was placed near the tail. All procedures were performed in dim red light, and the rats were kept warm during and after the procedure. The responses to a light flash (3.0 cd·s/m2) from a photic stimulator were amplified, and the preamplifier bandwidth was set at 0.2–300 Hz. The amplitude of the a-wave was measured from the baseline to the maximum a-wave trough, and the b-wave was measured from the maximum a-wave trough to the maximum b-wave peak.
Statistical analysis All the data were expressed as mean±SD, and ANOVA and Student–Newman–Keuls test was applied. The analysis was done with SAS 6.12 software (Cary, NY, USA). P<0.05 was considered significant.
Results
Administration of edaravone reduced MDA production and enhanced SOD activity in I/R-induced retinal tissues To estimate the antioxidative effect of edaravone, the MDA levels and SOD activity of the retina tissue were measured on d 1 after I/R. MDA is a marker of lipid peroxidation. In this study, MDA content in the retinal tissues of the vehicle group was significantly higher than that of the normal control group (7.96±0.84 nmol/mg protein vs 5.21±0.23 nmol/mg protein, P<0.01) after I/R injury. Edaravone significantly inhibited MDA production in the retina tissue after I/R compared with the vehicle group (5.45±0.57 nmol/mg protein vs 7.96±0.84nmol/mg protein, P<0.01). Data are shown in Figure 1A.
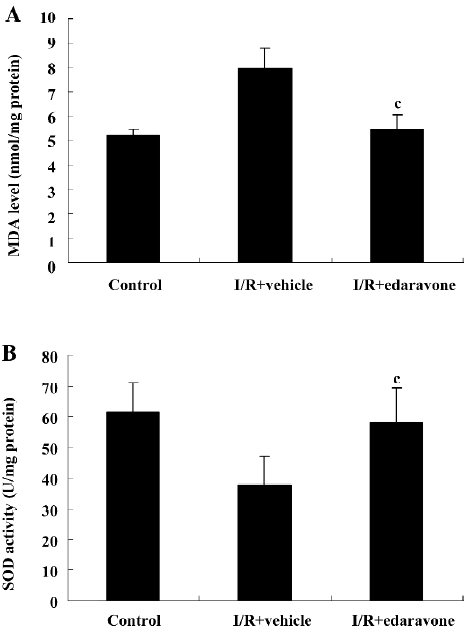
SOD is a pivotal enzyme scavenging ROS in vivo. SOD activity in the retinal tissue was significantly reduced in the vehicle group than in the normal control group after I/R injury (61.46±9.71 U/mg protein vs 37.52±9.43 U/mg protein, P<0.01). Edaravone significantly prevented the decrease of SOD activity compared with the vehicle group (37.52±9.43 U/mg protein vs 58.14±11.37 U/mg protein, P<0.01). Data are shown in Figure 1B.
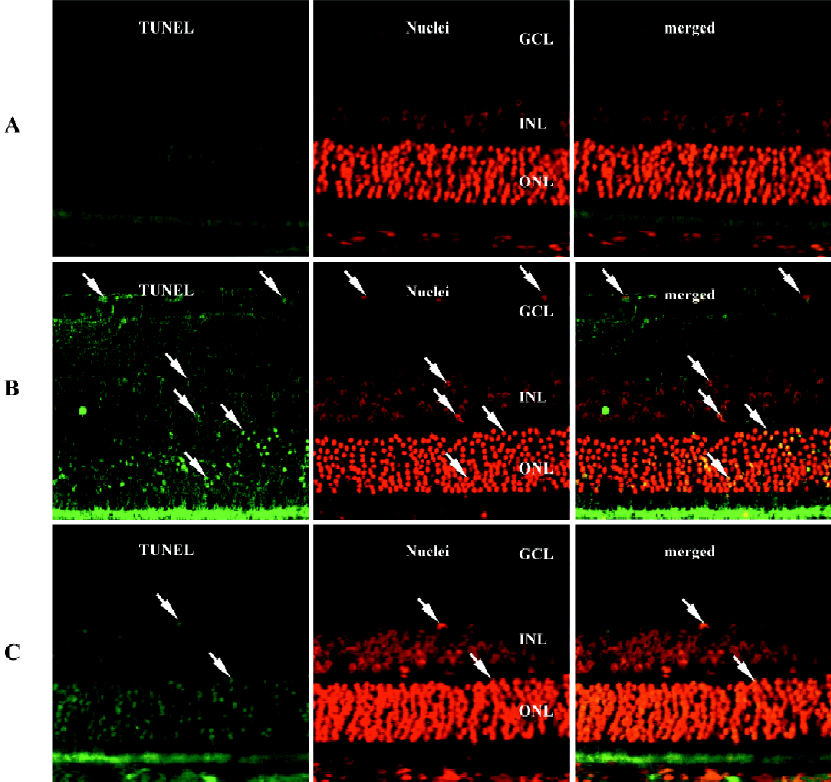
Edaravone treatment attenuated I/R-induced retinal neurons apoptosis The fluorescence detection of retinal neuronal apoptosis was examined on d 1 after I/R. Data are shown in Figure 2. The retina cell nuclei were negative for TUNEL staining in the rats of the normal control group (Figure 2A) with no yellow fluorescence. Abundant yellow fluorescent retinal neuronal nuclei were found within the inner nuclear, ganglion cell, and outer nuclear layers in the vehicle group (Figure 2B). However, in the rats treated with edaravone (Figure 2C), only sparse yellow fluorescent nuclei were noticed in the TUNEL staining within the retina.
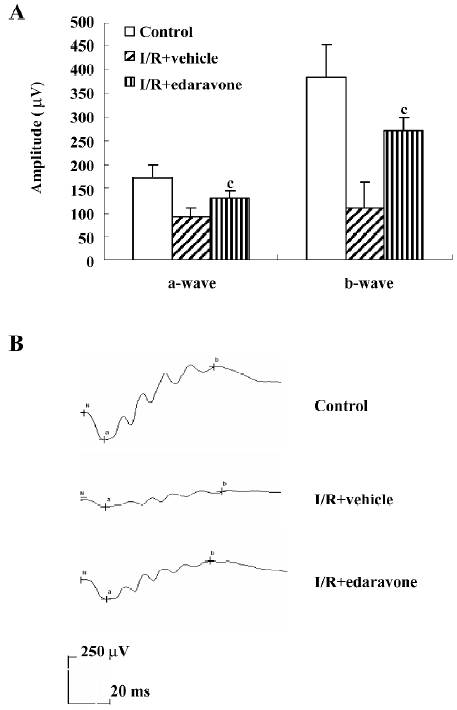
Edaravone treatment prevented I/R-induced reduction of a- and b-wave amplitudes To evaluate the functional changes induced by I/R injury, ERG were recorded on d 5 after I/R injury. Data are shown in Figure 3A. Compared with the normal control group, I/R-induced injury caused a 48% and 72% reduction in the a- and b- wave amplitudes, respectively. However, in the edaravone-treated group, the reduction in the a- and b-wave amplitudes was approximately 25% and 29%, respectively. The a- and b-wave amplitudes were significantly enhanced in the edaravone-treated group compared with the vehicle group (P<0.01). Figure 3B shows the typical ERG records of 3 groups at d 5 after I/R. The a- and b-waves amplitudes were significantly lower in the vehicle group than in the normal control group. In contrast, the a- and b-wave amplitudes of ERG were clearly enhanced in edaravone-treated I/R rats.
Discussion
In the present study, we evaluated the effect of edaravone on the retinal damage caused by I/R in a rat retinal I/R model. We found that edaravone improved the oxidative parameters of the retinal tissue in the I/R model and prevented retinal neurons from apoptosis, indicating that edaravone has protective effects on retinal cells after retinal I/R injury.
Oxygen is critical for life and for the maintenance of metabolic processes; however, reactive metabolites of oxygen can be toxic to cells. In particular, the cellular damage that occurs secondary to ischemia may be exacerbated by the sudden reintroduction of oxygen into tissues during reperfusion, triggering free radical cascades that overwhelm endogenous free radical scavengers.
Edaravone has potent hydroxy radical scavenging activity[7]. It can eliminate hydrogen oxide radicals that trigger lipid peroxidation and subsequently inhibit the initiation and progression of lipid peroxidation induced by hydrogen peroxide radicals[21]. Edaravone can interact with both peroxyl and hydroxyl radicals, producing a stable oxidation product (OPB, 2-oxo-3-[phenylhydrazono]-butanoic acid)[22]. Edaravone has previously been reported to protect organs, such as the brain[17,18], heart[23,24], kidney[25], and liver[26,27] from free radical-mediated injury. However, the direct scavenging property of edaravone in retinal I/R injury in rats remains to be explored.
As a major cause of tissue damage, ROS mainly consist of superoxide anions produced by the mitochondrion, hydrogen peroxide produced by superoxide anions in presence of SOD, and peroxynitrite produced by superoxide anions in presence of nitrogen monoxidum[28]. Previous studies have proven that SOD administration can protect the retina from I/R injury[29]. Compared with other production of lipid peroxidation, MDA has a longer life span and is more favorable for the evaluation of the severity of tissue damage[30]. In the present study, we found that the retinal antioxidants in the I/R with vehicle group were excessively consumed after I/R injury, and the tissue lipids were severely damaged. Our data revealed that edaravone significantly reduced MDA levels and enhanced SOD activity of the retinal tissue. This suggests that edaravone can remove free radicals and therefore inhibit lipid peroxidation. Dilsiz et al used vitamin E as a reference drug to treat retinal I/R injury in a similar study[31] and found that vitamin E decreased MDA levels in the retinal tissue, reduced consumption of GSH, and inhibited the activation of caspase-3. In our future study we may also use vitamin E as a positive control.
In the present study, TUNEL-positive staining, particularly in the inner nuclear, ganglion cell and outer nuclear layers, was evident on d 1 after I/R, which is consistent with results from previous studies[2,3]. The TUNEL analysis revealed abundant positive yellow fluorescent cells in the inner nuclear, ganglion cell, and outer nuclear layers in the I/R with vehicle group, but only sparse positive cells in the edaravone-treated group, which once again indicates that apoptosis produces retinal neuronal death after I/R injury, and edaravone reduces cell apoptosis by scavenging free radicals. Similar results were also obtained by other authors[32,33].
IOP was elevated above the systolic blood pressure for 60 min, and during this period, the blood supply to the retina is drastically reduced, as indicated by a whitening of the fundus. This insult caused partial irreversible damage to the retina demonstrated by the ERG data where the a- and b-wave amplitudes are significantly reduced on d 5 after I/R. The a-wave provides information about the photoreceptors, while the b-wave provides information on the physiology of the ON-bipolar and Müller cells[34,35]. Therefore, it is clear that the outer retina was physiologically affected following I/R. More importantly, in the present study, the reduction in the a- and b-wave amplitudes caused by I/R was significantly improved in animals treated with edaravone. A previous study showed that edaravone could decrease calcium overloading, increase mitochondrial potential, and reduce neuronal apoptosis through inhibiting lipid peroxidation in the rat hippocampal I/R model[15]. Wen et al reported that edaravone could relieve oxidative stress through inhibiting the JNK–c-Jun pathway in the senile rat cerebral I/R model and therefore protect cerebral neurons[14]. The present study has indicated the involvement of antioxidative and anti-apoptotic effects of edaravone on photoreceptor cells in the rat retinal I/R model, but whether these 2 pathways also apply to the retinal I/R injury model warrants further study.
In conclusion, edaravone can protect retinal neurons during retinal I/R and improve I/R-induced retinal dysfunction through inhibiting oxidative stress and apoptosis. Further study is warranted to determine the clinical use of edaravone in the treatment of I/R-related eye disorders.
Acknowledgement
The authors wish to thank Dang-hui YU of the Second Military Medical University Press for his assistance with the manuscript.
References
- Katai N, Yoshimura N. Apoptotic retinal neuronal death by ischemia reperfusion is executed by two distinct caspase family proteases. Free Radic Biol Med 2004;15:2697-705.
- Zhang C, Rosenbaum DM, Shaikh AR, Li Q, Rosenbaum PS, Pelham DJ, et al. Ischemic preconditioning attenuates apoptotic cell death in the rat retina. Invest Ophthalmol Vis Sci 2002;43:3059-66.
- Lam TT, Abler AS, Tso MO. Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Invest Ophthalmol Vis Sci 1999;40:967-75.
- Banin E, Berenshtein E, Kitrossky N, Pe’Er J, Chevion M. Gallium-desferrioxamine protects the cat retina against injury after ischemia and reperfusion. Free Rad Biol Med 2000;28:315-23.
- Anderson RE, Maude MB, Neilsen JC. Effect of lipid peroxidation on rhodopsin regeneration. Exp Eye Res 1985;4:65-71.
- Xie Z, Wu X, Gong Y, Song Y, Qiu Q, Li C. Intraperitoneal injection of Ginkgo biloba extract enhances antioxidation ability of retina and protects photoreceptors after light-induced retinal damage in rats. Curr Eye Res 2007;32:471-9.
- Watanabe K, Hayase T. Radical scavenging mechanisms of MCI-186. Jpn Pharmacol Ther 1999;40:729-36.
- Watanabe T, Egawa M. Effects of an antistroke agent MCI-186 on cerebral arachidonate cascade. J Pharmacol Exp Ther 1994;271:1624-9.
- Kuzuya T, Hoshida S, Kim Y, Oe H, Hori M, Kamada T, et al. Free radical generation coupled with arachidonate lipoxygenase reaction relates to reoxygenation induced myocardial cell injury. Cardiovasc Res 1993;27:1056-60.
- Okatani Y, Wakatsuki A, Enzan H, Miyahara Y. Edaravone protects against ischemia/reperfusion-induced oxidative damage to mitochondria in rat liver. Eur J Pharmacol 2003;465:163-70.
- Vanden Hoek T, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res 2000;86:541-8.
- Stull ND, Polan DP, Iacovitti L. Antioxidant compounds protect dopamine neurons from death due to oxidative stress in vitro. Brain Res 2002;931:181-5.
- Kokura S, Yoshida N, Sakamoto N, Ishikawa T, Takagi T, Higashihara H, et al. The radical scavenger edaravone enhances the anti-tumor effects of CPT-11 in murine colon cancer by increasing apoptosis via inhibition of NF-κB. Cancer Lett 2005;229:223-33.
- Suzuki K, Kazui T, Terada H, Umemura K, Ikeda Y, Bashar AH, et al. Experimental study on the protective effects of edaravone against ischemic spinal cord injury. J Thorac Cardiovasc Surg 2005;130:1586-92.
- Wu T, Ding XS, Wang W, Wu J. MCI-186 (3-methyl-1-phenyl-2-pyrazolin-5-one) attenuated simulated ischemia/reperfusion injury in cultured rat hippocampal cells. Biol Pharm Bull 2006;29:1613-7.
- Wen J, Watanabe K, Ma M, Yamaguchi K, Tachikawa H, Kodama M, et al. Edaravone inhibits JNK-c-Jun pathway and restores anti-oxidative defense after ischemia-reperfusion injury in aged rats. Biol Pharm Bull 2006;29:713-8.
- Mizuno A, Umemura K, Nakashima M. Inhibitory effect of MCI-186, a free radical scavenger, on cerebral ischemia following rat middle cerebral artery occlusion. Gen Pharmacol 1998;30:575-8.
- Xiao B, Bi FF, Hu YQ, Tian FF, Wu ZG, Mujlli HM, et al. Edaravone neuroprotection effected by suppressing the gene expression of the Fas signal pathway following transient focal ischemia in rats. Neurotox Res 2007;12:155-62.
- Stefansson E, Wilson CA, Schoen T, Kuwabara T. Experimental ischemia induces cell mitosis in the adult rat retina. Invest Ophthalmol Vis Sci 1988;29:105-5.
- Shibuki H, Katai N, Yodoi J, Uchida K, Yoshimura N. Lipid peroxidation and peroxynitrite in retinal ischemia–reperfusion injury. Invest Ophthalmol Vis Sci 2000;41:3607-14.
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990;186:421-31.
- Watanabe K, Morinaka Y, Iseki K, Watanabe T, Yuki S, Nishi H. Structure-activity relationship of 3-methyl-1-phenyl-2-pyrazolin-5-one (edaravone). Redox Rep 2003;8:151-5.
- Wu TW, Zeng LH, Wu J, Fung KP. Myocardial protection of MCI-186 in rabbit ischemia-reperfusion. Life Sci 2002;71:2249-55.
- Yagi H, Horinaka S, Matsuoka H. Edaravone prevented deteriorated cardiac function after myocardial ischemia-reperfusion via inhibiting lipid peroxidation in rat. J Cardiovasc Pharmacol 2005;46:46-51.
- Matsuyama M, Hayama T, Funao K, Tsuchida K, Takemoto Y, Sugimura K, et al. Treatment with edaravone improves the survival rate in renal warm ischemia-reperfusion injury using rat model. Transplant Proc 2006;38:2199-200.
- Taniguchi M, Uchinami M, Doi K, Yoshida M, Sasaki H, Tamagawa K, et al. Edaravone reduces ischemia-reperfusion injury mediators in rat liver. J Surg Res 2007;137:69-74.
- Hiranuma S, Ito K, Noda Y, Ozasa H, Koike Y, Horikawa S. Amelioration of hepatic ischemia/reperfusion injury in the remnant liver after partial hepatectomy in rats. J Gastroenterol Hepatol 2007;22:2167-72.
- Yilmaz T, Kukner AS, Aydemir O, Ozercan HI, Naziroglu M. Aprotinin reduces ischemia-reperfusion injury in the retina of guinea pigs. Eur J Ophthalmol 2003;13:642-7.
- Nayak MS, Kita M, Marmor MF. Protection of rabbit retina from ischemic injury by superoxide dismutase and catalase. Invest Ophthalmol Vis Sci 1993;34:2018-22.
- Chida M, Suzuki K, Nakanishi-Ueda T, Ueda T, Yasuhara H, Koide R, et al. In vitro testing of antioxidants and biochemical end points in bovine retinal tissue. Ophthalmic Res 1999;31:407-15.
- Dilsiz N, Sahaboglu AM, Yildiz Z, Reichenbach A. Protective effects of various antioxidants during ischemia-reperfusion in the rat retina. Graefes Arch Clin Exp Ophthalmol 2006;4:627-33.
- Amemiya S, Kamiya T, Nito C, Inaba T, Kato K, Ueda M, et al. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur J Pharmacol 2005;516:125-30.
- Dong J, Takami Y, Tanaka H, Yamaguchi R, Jingping G, Chun Q, et al. Protective effects of a free radical scavenger, MCI-186, on high-glucose-induced dysfunction of human dermal microvascular endothelial cells. Wound Repair Regen 2004;12:607-12.
- Block F, Schwarz M. The b-wave of the electroretinogram as an index of retinal ischemia. Gen Pharmacol 1998;30:281-7.
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 2004;23:91-147.
