Toll-like receptor 7 and 9 expression in peripheral blood mononuclear cells from patients with chronic hepatitis B and related hepatocellular carcinoma
Introduction
Hepatitis B virus (HBV) infection is a major cause of chronic liver inflammation worldwide. Many chronic HBV carriers suffer from progressive liver diseases, such as liver cirrhosis (LC) and hepatocellular carcinoma (HCC) during their lifetime. The outcome of hepatitis B patients is closely linked to their immune status. Immunity plays a decisive role in host-virus interactions, and greatly influences viral replication and the clinical outcome of infection[1]. Chronic HBV infection is associated with T cell hyporesponsiveness or tolerance[2]. Thus, innate and/or adaptive immune responses are likely to be either absent or diminished when viral persistence follows HBV infections.
Toll-like receptors (TLR) 7 and 9 are members of the Toll-like family of receptors[3,4], and sense infection by detecting molecular structures of invading microbial pathogens and initiate innate immune responses[5]. These receptors mediate adaptive immune responses by activating immune cells, such as dendritic cells (DC) [4,6,7]. TLR7 and TLR9 are present on both immune and non-immune cells[8]. TLR9 recognizes unmethylated deoxycytidyl-phosphate-deoxyguanosine dinucleotides, which are common in bacterial and some viral nucleic acids[9], whereas TLR7 recognizes single-stranded RNA in the cytoplasm of infected cells during viral replication[10]. High expression levels of TLR9 were recently detected in clinical samples of lung and breast cancer and corresponding cell lines[11,12], suggesting a relationship between TLR9 and carcinogenesis.
Recent studies on TLR9 subfamily members describe the relationship between viral pathogens such as hepatitis C virus (HCV), herpes simplex virus, HIV, and these TLR[13–16]. It was demonstrated that the secretion of type I interferon (IFN) in response to these viruses is mediated by the TLR7/TLR9 pathway. However, the expression profiles of TLR7 and TLR9 in the peripheral blood mononuclear cells (PBMC) of patients with chronic HBV infection and related HCC have not been evaluated. Information about the regulation of TLR by viruses is also limited[10,17,18]. The current study was undertaken to investigate the expressions of TLR7 and TLR9 in the PBMC of chronic HBV and related HCC patients, and to analyze any relationship between the TLR expression and the disease states.
Materials and methods
Study patients The study patients included 11 healthy volunteers (normal controls, NC) and 41 patients at various stages of chronic HBV infection, including 19 with chronic hepatitis B (CHB), 11 with HBV-related LC, and 11 with HBV-related HCC. The patients were enrolled at the First Affiliated Hospital, School of Medicine, Zhejiang University (Hangzhou, China) from February 2006 to May 2006. Informed consent was obtained from each patient, and the study protocol was approved by the Ethics Committees of the School of Medicine, Zhejiang University. The clinical parameters of the patients are shown in Table 1.
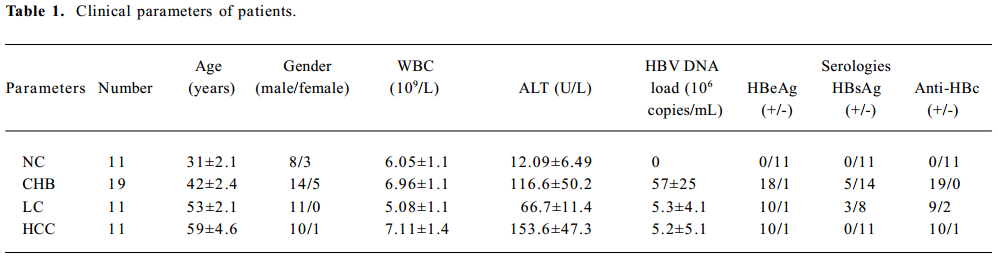
Full table
The diagnostic criteria conformed to “The guideline of prevention and treatment for chronic hepatitis B”[19]. The patients had not received any surgical treatment and had not received any antiviral treatment in the last month. All LC patients were in Child class B and C by Child-Pugh score evaluation. HCC patients were confirmed by liver tissue biopsy. All healthy volunteers were negative for HCV, HBV, and HIV.
Preparation of PBMC The PBMC were separated from 5 mL heparinized whole blood by centrifugation on a Lym-pholyte-H system (Cedarlane, Hornby, Canada) by gradient centrifugation. The PBMC were resuspended in ACK lysis buffer (0.15 mol/L NH4Cl, 1 mmol/L KHCO3, 0.1 mmol/L Na2 EDTA, pH 7.4) to lyse the red blood cells.
RNA isolation, reverse transcription, and quantitative real-time PCR Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) from 1×106~2×106 PBMC. Reverse transcription was performed using the RevertAid first-strand cDNA synthesis kit (MBI Fermentas,Vilnius, Lithuania). Real-time PCR was performed with the FastStart DNA SYBR premix ex Taq kit (Gene Home Biotechnology, Hangzhou, China) using an ABI 7500 system (Applied Biosystems, Foster, CA, USA). The primers were synthesized by Shanghai Yingjun Biotech (Shanghai, China) as follows: TLR7 forward, 5'-TGGAAATTGCCC TCGTTGTT-3', reverse, 5'-GTCAGCGCATCAAAAGCATT-3'; TLR9 forward, 5'-CCCGCTACTGGTGCTATCC-3', reverse, 5'-CCTTCCTCTTTCCACTCCC-3'; and β-actin forward, 5'-CCG-CCATGTAGGTCGCTAT-3', reverse, 5'-TGACACGCCATCA-CCAGAGT-3'. Thermocycling was performed at 95 °C for 20 s, followed by 40 cycles at 95 °C for 15 s, 58 °C for 10 s, and 72 °C for 40 s to measure the fluorescence signal. The dissociation stages, melting curves, and quantitative analyses of the data were performed using 7500 system SDS software v1.2.3 (Applied Biosystems, USA). The relative quantitation of target gene expression was evaluated using the comparative CT method as described by Ross et al[20].
Western blot analysis The cytoplasmic extracts were prepared by lysis in an lysis buffer containing 150 mmol/L NaCl, 10 mmol/L Tris-HCl (pH 7.9), 0.5%Triton X-100, 0.6% NP-40, and protease inhibitors (1 µg/mL leupeptin, 1 µg/mL pepstatin A, and 2 µg/mL aprotinin). The protein contents were determined using the DC protein assay kit (Bio-Rad, Richmond, CA, USA). The PBMC lysates were mixed with 2×SDS sample buffer. In total, 40 μg of protein was separated in a 10% polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). After blocking with 5% skim milk powder in TBST, the membrane was incubated with primary antibody, mouse anti-TLR9 mAb or rabbit anti-TLR7 polyclonal antibodies (Imgenex, San Diego, CA, USA) in TBST overnight at 4 °C. Subsequently, the membranes were washed in washing buffer (phosphate-buffered saline with 0.1% Tween-20) incubated with horseradish peroxidase-conjugated secondary antibody, rabbit antimouse, or goat anti-rabbit (Pierce, 1:10000 in blocking buffer) for 1 h at room temperature. The reactive bands were visualized with an EZ-ECL kit (Bioind, Kibbutz, Israel). A monoclonal anti-β-actin antibody was used as a control at a dilution of 1:400 (Santa Cruz, CA, USA).
Intracellular TLR staining and flow cytometry analysis A total of 6×106–10×106 PBMC were fixed and permeabilized with IntraPrep permeabilization reagent (Beckman Coulter, Fullerton, CA, USA). The cells were then stained with fluorescein-isothiocyanate-conjugated anti-TLR9 mAb or an unconjugated rabbit anti-TLR7 polyclonal antibody (Imgenex, San Diego, CA, USA). PE-conjugated goat antirabbit immunoglobulin G (Molecular Probes, Eugene, OR, USA) was used as a secondary antibody for TLR7 staining. Stained PBMC were analyzed on a FACSCalibur machine (BD Biosciences, San Jose, CA, USA). Data were analyzed using CellQuest software (BD Biosciences, San Jose, CA, USA)
Serum HBV–DNA assay The viral load of HBV–DNA in the serum samples was quantified using a high sensitivity fluorescent real-time PCR kit (PG biotech, Shenzhen, China) and amplified using a Light Cycler 2.0 instrument (Roche Applied Science, Basel, Switzerland). The detection sensitivity of the PCR assay was 4×102 copies/mL.
Statistical analysis Statistical analyses (non-parametric tests, Mann-Whitney U-test) were performed using SPSS software version 13.0 (SPSS, Chicago, IL, USA). Correlation analyses were calculated according to the Spearman-Rho method. A P-value of less than 0.05 was considered to be significant.
Results
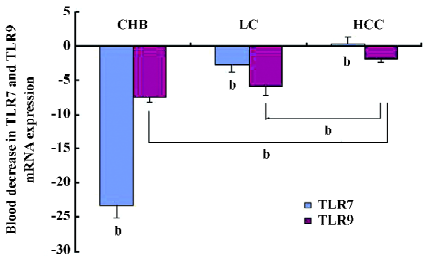
Decreased expression of TLR7 and TLR9 mRNA in PBMC of patients As indicated in Figure 1, the patient group with the lowest TLR7 and TLR9 expressions in PBMC was the CHB group. The expressions of TLR7 and TLR9 mRNA decreased more than 23.2- and 7.6-fold, respectively, compared with the NC group. Between the NC and HCC groups, the decrease of the TLR7 mRNA expression was not significantly different compared with the NC group (P>0.05). The decrease in the TLR7 mRNA expression in the LC and HCC groups differed 2.8- to -0.34-fold, respectively, compared to the NC group (P>0.05). A similar but weaker trend was also observed in the decrease of TLR9 mRNA expression among the groups. The expression of TLR9 mRNA decreased in the LC and HCC patients by more than 6- and 1.7-fold, respectively, compared with the NC group.
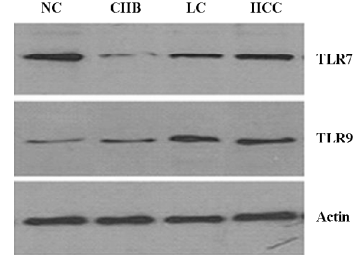
Western blot analysis of TLR7 and TLR9 in PBMC There was a significant decrease in the TLR7 expression of the CHB group compared with controls, and an increased TLR7 expression in various patient stages (Figure 2). In contrast, the TLR9 expression significantly increased in the 3 patient groups compared with the NC group. An upward tendency was observed in the CHB, LC, and HCC groups.
Flow cytometry analysis of TLR7 and TLR9 expressions in PBMC Mean fluorescence intensity (MFI) values corresponding to TLR7 were downregulated in all patient groups compared with the control group (P<0.05, Figure 3). The MFI±SEM values were: NC=196.6±14.2, CHB=70.1±3.9, LC=79.7±3.7, and HCC=83.8±3.2. The results showed no significant differences among the 3 patient groups (P>0.05).
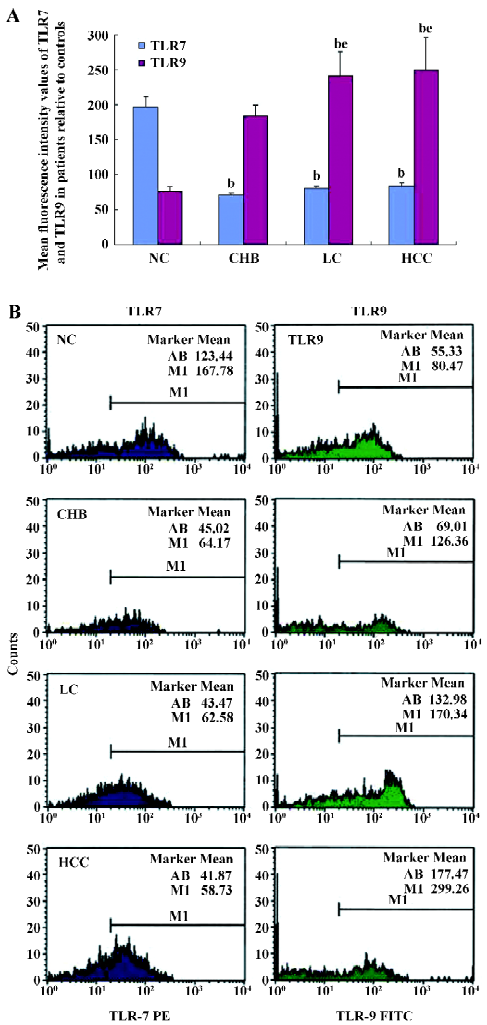
The MFI values corresponding to TLR9 increased in all the patient groups compared with the NC group (P<0.05). The MFI±SEM values were: NC=76.2±6.2, CHB=183.7±15.1, LC=240.8±34.4, and HCC=250.2±45.5. The TLR9 expression in the HCC patient group was upregulated (P<0.05) compared to the CHB patient group.
Correlation between the TLR protein expression and serum HBV–DNA levels The expression of the TLR7 protein was negatively correlated with the serum copies of HBV–DNA (r=–0.669, P<0.01), and the upregulation of the TLR9 protein was positively correlated with the serum copies of HBV–DNA (r=0.563, P<0.01).
Discussion
After recognizing particular microbial molecules, subsets of immune cells in PBMC equipped with the corresponding set of TLR release immunological repertoire, such as IFN-α, TNF-α, and interleukin-6, which directly regulate immunocompetent cells[8]. TLR7 and TLR9 are members of the TLR9 subfamily[21,22]. Recent research indicates that the ligands for TLR3, TLR4, TLR5, TLR7, and TLR9 can inhibit HBV replication in the livers of HBV transgenic mice[23], suggesting that TLR7 and TLR9 may play a role in regulating HBV. The high expression of TLR9 was recently detected in clinical samples of lung and breast cancer and corresponding cell lines[11,12], suggesting a possible role in carcinogenesis. If TLR7 and TLR9 are associated with the recognition of specific viral components, it is important to characterize TLR7 and TLR9 expression in the PBMC of patients with chronic HBV infection and HBV-related HCC to further understand pathogen-host interactions and predicted outcomes.
The results of our study clearly show decreased TLR7 at the mRNA and protein levels, and TLR9 expression at the mRNA level in patient PBMC compared with the healthy controls. These results are different from the upregulated TLR after other virus infections, as shown in previous studies[24,25]. HBV may elicit a factor that inhibits TLR7 expression and TLR9 mRNA in the PBMC of patients, resulting in immune escape and even immunological tolerance. Because the same decreased TLR7 and TLR9 expressions of plasmacytoid DC were also found in further studies, the alteration of TLR7 and TLR9 expressions even impair the IFN-α production of pDC (unpublished data).
The regulation of eukaryotic gene expression is the result of complex, multi-level processes; mRNA levels do not always correspond to the actual expressed protein levels[26–28]. The increase of the TLR9 protein might be related to post-transcriptional processes and might be involved in the immune surveillance of HCC. This also may reflect negative feedback inhibition from the TLR9 protein to the mRNA or other regulatory influences which are not currently understood[29].
TLR9 is mostly localized in the endoplasmic reticulum[30] and traffics to endosomal and lysosomal compartments after cellular activation[31,32]. Some studies also showed that a fraction of the TLR9 expresses on the cell surface, in the gastric epithelium[33], intestinal epithelial cells[34], and some peripheral blood mononuclear cells[35]. It was no doubt that FACS data indicated the functional cellular expression of TLR while the Western blot analysis showed the total TLR expression, including the superficial pool. These results reflect another level of regulation that is protein trafficking. The TLR9 expression in the HCC group was the highest, which may be directly related to carcinogenesis of HCC. The statistical analysis indicated no difference in the TLR9 levels among the HCC and LC groups. If the sample size was enlarged, the results may be different. The expression of TLR7 was not different among the groups of patients, suggesting that unlike TLR9, TLR7 has no correlation with HCC.
Finally, we were interested in understanding whether changes in TLR7 and TLR9 expressions were correlated with the levels of serum HBV–DNA. Positive results suggested that the TLR expression was related to the state of HBV replication. We conclude that HBV might produce an inhibitor when it is replicating. However, the expression of TLR9 was influenced by chronic HBV infection and the carcinogenesis of HCC, which may further complicate the situation.
In summary, this study is the first investigation of the expression of TLR7 and TLR9 of PBMC in CHB, LC, and HCC patient groups. A larger scale clinical investigation should be undertaken to determine whether TLR7 and TLR9 expressions may be used to evaluate patient immunity and predict HCC outcomes.
Acknowledgements
We thank Prof Yong-min TANG for his expert technical assistance with the flow cytometry. We are grateful to Prof Fang XU and Dr Ping LI for reviewing this manuscript and for their valuable advice.
References
- Visvanathan K, Lewin SR. Immunopathogenesis: role of innate and adaptive immune responses. Semin Liver Dis 2006;26:104-15.
- Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995;13:29-60.
- Medzhitov R, Janeway C Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev 2000;173:89-97.
- Werling D, Jungi TW. TOLL-like receptors linking innate and adaptive immune response. Vet Immunol Immunopathol 2003;91:1-12.
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol 2005;17:1-14.
- Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002;20:197-216.
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004;5:987-95.
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 2002;168:4531-7.
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003;21:335-76.
- Andersen JM, Al-Khairy D, Ingalls RR. Innate immunity at the mucosal surface: role of toll-like receptor 3 and toll-like receptor 9 in cervical epithelial cell responses to microbial pathogens. Biol Reprod 2006;74:824-31.
- Droemann D, Albrecht D, Gerdes J, Ulmer AJ, Branscheid D, Vollmer E, et al. Human lung cancer cells express functionally active Toll-like receptor 9. Respir Res 2005;6:1.
- Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa T, Gehrs B, Rosenthal E, et al. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res 2006;4:437-47.
- Schlaepfer E, Audige A, Joller H, Speck RF. TLR7/8 triggering exerts opposing effects in acute versus latent HIV infection. J Immunol 2006;176:2888-95.
- Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol 2005;26:221-9.
- Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, et al. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity 2005;23:465-78.
- Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 2004;103:1433-7.
- Huang YH, Chou MH, Du YY, Huang CC, Wu CL, Chen CL, et al. Expression of toll-like receptors and type 1 interferon specific protein MxA in biliary atresia. Lab Invest 2006;87:66-74.
- Lesmeister MJ, Bothwell MR, Misfeldt ML. Toll-like receptor expression in the human nasopharyngeal tonsil (adenoid) and palantine tonsils: a preliminary report. Int J Pediatr Otorhino-laryngol 2006;70:987-92.
- Chinese Society of Hepatology and Chinese Society of Infectious Diseases CMA. The guideline of prevention and treatment for chronic hepatitis B. Chin J Hepatol 2005;13:881-90.
- Ross JW, Ashworth MD, Hurst AG, Malayer JR, Geisert RD. Analysis and characterization of differential gene expression during rapid trophoblastic elongation in the pig using suppression subtractive hybridization. Reprod Biol Endocrinol 2003;1:23.
- Chuang TH, Ulevitch RJ. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw 2000;11:372-8.
- Du X, Poltorak A, Wei Y, Beutler B. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw 2000;11:362-71.
- Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol 2005;79:7269-72.
- An H, Xu H, Yu Y, Zhang M, Qi R, Yan X, et al. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-kappaB, ERK and p38 MAPK signal pathways. Immunol Lett 2002;81:165-9.
- McKimmie CS, Johnson N, Fooks AR, Fazakerley JK. Viruses selectively upregulate Toll-like receptors in the central nervous system. Biochem Biophys Res Commun 2005;336:925-33.
- McBride S, Walsh D, Meleady P, Daly N, Clynes M. Bromo-deoxyuridine induces keratin protein synthesis at a posttranscriptional level in human lung tumour cell lines. Differentiation 1999;64:185-93.
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 1999;19:1720-30.
- Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis 1997;18:533-7.
- Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, et al. Discordant protein and mRNA expression in lung adeno-carcinomas. Mol Cell Proteomics 2002;1:304-13.
- Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol 2004;173:1179-83.
- Bauer S. Toll-erating self DNA. Nat Immunol 2006;7:13-5.
- Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol 2006;7:49-56.
- Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Muller-Hermelink HK, et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol 2004;136:521-6.
- Lee J, Rachmilewitz D, Raz E. Homeostatic effects of TLR9 signaling in experimental colitis. Ann N Y Acad Sci 2006;1072:351-5.
- Eaton-Bassiri A, Dillon SB, Cunningham M, Rycyzyn MA, Mills J, Sarisky RT, et al. Toll-like receptor 9 can be expressed at the cell surface of distinct populations of tonsils and human peripheral blood mononuclear cells. Infect Immun 2004;72:7202-11.
