Mitosis orientation in prostate epithelial cells changed by endocrine effect
Introduction
Androgens are known to play an important role in benign prostatic hyperplasia (BPH) and prostatic carcinoma (PCa)[1,2], but their mechanism have not been fully understood[3]. Androgens could probably affect the structure of tissues, but it never been reported that they could change the mitosis orientation in prostate epithelial cells. The present study provides a further observation on the role of androgens in androgen-related diseases, such as BPH and PCa.
The development and maturation of the male urogenital system depends on the normal function of androgens[4]. However, it has not been reported how the orientation of the mitosis in prostate epithelial cells alters after hormonal level regulation. Androgens may affect not only simple processes, such as proliferation, cell differentiation, and cell–cell or cell–extracellular matrix interrelation, but also the structure and orientation of the tissues or their components. Our study found that testosterone propionate (TP) could adjust mitosis orientation in prostate epithelial cells, as discussed later.
Materials and methods
Experimental animals All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources Commission on Life Sciences, America. Male Sprague-Dawley rats (190–210 g) were obtained from the Shanghai SIPPR-BK (Institute of Planned Parenthood Research-BK Laboratory Animal Limited Company, Shanghai, China). The animals were housed under the same conditions with free access to water and food. The experiments were started at 1 month, and then, all animals were castrated under anesthesia with ketamine.
Treatments The castrated rats were treated with a single subcutaneous injection of TP in olive oil at a dose 0.5 mg per rat or benzogynestry (E2) in olive oil at a dose 5 µg per 100 g body weight. There were 8 rats in the control group and TP-treated or E2-treated group. Prostate, a specimen of the liver, a specimen of skin, a segment of the jejunum, and segment of the colon were removed from untreated and TP- or E2-injected groups at 24, 36, and 48 h. All of the animals were injected intraperitoneally with bromodeoxyuridine (BrdU; Sigma, St Louis, MO, USA) dissolved in saline 2 h before the tissues were removed. The dose of BrdU for the rats was 2 mg per 100 g body weight[5]. All tissue specimens were removed under anesthesia, then fixed and embedded in paraffin. The paraffin-blocked section was consecutively cut at 5 μm thickness for iron hematoxylin-eosin (HE) and immunohistochemical staining.
Cell proliferation assay Cell proliferation was determined using BrdU. BrdU was detected using immunohistochemical staining as described. After deparaffination and rehydration of the sections, slides were placed in sodium citrate solution (0.01 mol/L, pH 6.0) and heated to 96–100 °C for 25 min. After cooling, the sections were put into 5% bovine serum albumin for 20 min. Then the sections were incubated for 2 h with a primary anti-BrdU mouse monoclonal antibody (Boster Biotechnology, Wuhan, Hubei, China), diluted at 1:100 in Tris-buffered saline[6], and then covered with coverslips. In the sections, positive staining was a red precipitate localized in the nucleus. Stained BrdU cells were counted under a light microscope. The sections of obtained tissues (prostate, liver, skin, jejunum, and colon) were examined in randomly selected areas. No less than 1000 cells were viewed in each tissue per animal.
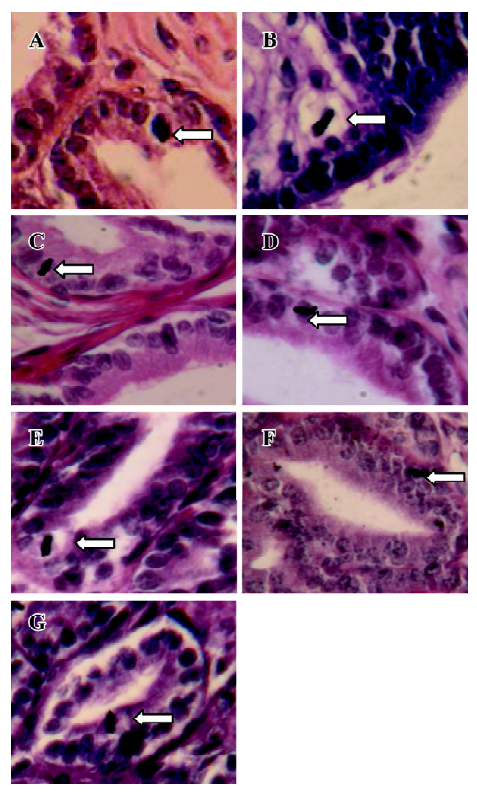
Orientation of mitoses In the tissue sections stained with iron HE, mitotic figures were viewed under a light microscope and the orientation of mitosis was determined. Mitoses with the poles disposed from 0 to 45 degrees to the basement membrane of prostate epithelia were regarded as parallel oriented. Mitoses with poles aligned from 45 to 90 degrees to the plane of the basement membrane were considered as perpendicular oriented. In the prostate, only the prostate epithelial cells were examined.
Statistics Data were expressed as mean±SD. One-way ANOVA and χ2-test were used to evaluate significant differences between the groups.
Results
Iron HE staining of rat prostates The prostate of all of the castrated rats had typically atrophic morphology. At 24, 36, and 48 h after a single injection of TP, a picture of early proliferative phenomenon was observed. Mitoses were rarely found in the prostate epithelial cells of castrated rats. Therefore, the percentage of mitotic cells was very low. However, a single injection of TP or E2 increased the proliferative activity of the prostate. All mitoses found in the prostate epithelial cells of the castrated rats with TP treatment were oriented parallel to the basement membrane (Figure 1), but mitoses found in the prostate epithelial cells of the castrated rats untreated or treated with E2 were oriented perpendicular to the basement membrane (Figure 1). TP treatment resulted in marked changes in mitosis orientation in the prostate epithelial cells of the prostate. TP treatment resulted in marked changes in mitosis orientation in the prostate epithelial cells. At 24, 36, and 48 h after TP injection, 7.1%, 7.9%, and 7.5% cells’ mitosis orientation were aligned parallel to the basement membrane of the epithelial cells in all prostate epithelial cells, respectively. However, in other tissues (liver, jejunum, colon, and skin), TP or E2 treatment had no effect on the orientation of cell division.
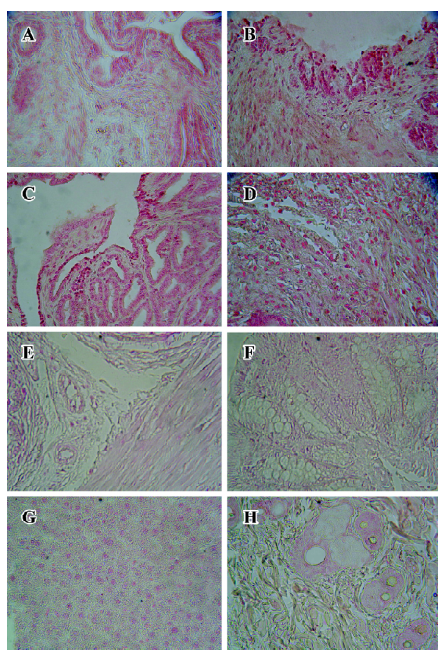
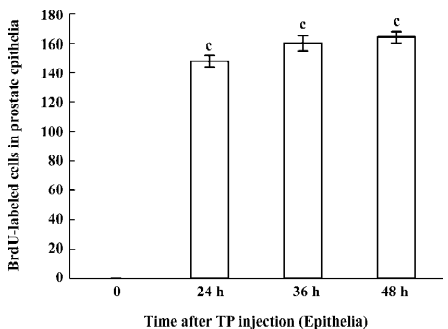
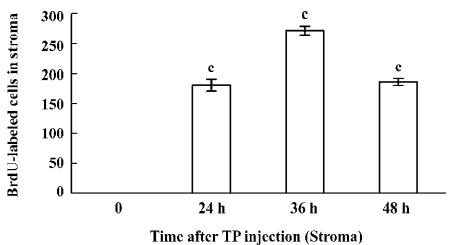
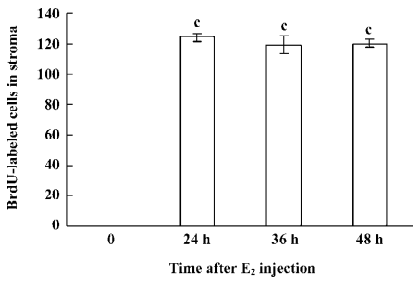
Immunohistochemical staining The BrdU-labeled cells were specific for abnormal hyperplasia[7]. A number of positive cells were observed in the areas of prostate epithelial cells and stroma, which correlated with the degree of proliferation. No positive cells were observed in the untreated group (control group; Figure 2). At 24, 36, and 48 h after a single injection of TP, the BrdU-labeled positive cells could be seen throughout the stroma and epithelial cells of the prostate (Figure 2); however, at 24, 36, and 48 h after a single injection of E2, the positive cells could only be seen in the stroma of the prostate (Figure 2). At 24, 36, and 48 h after TP injection, BrdU-labeled positive cells were found not only in the stroma of the prostate, but also in the prostate epithelial cells (Figures 3, 4), and there were significant differences between the control and TP groups (P<0.01). In the rats treated with E2 after 24, 36, and 48 h, BrdU-labeled positive cells were only found in the stroma of the prostate (Figure 5) and there was significant differences between the control and E2 groups (P<0.01). However, BrdU-labeled cells were hardly seen in other tissues (liver, jejunum, colon, and skin) treated with TP or E2 (Figure 2).
Discussion
The present study shows a novel effect of TP. TP remarkably altered the mitosis orientation in the prostate epithelial cells. A single injection of TP increased the proliferation and appearance of parallel-oriented mitoses. The effect of TP on mitosis orientation has also been examined in other tissues, such as liver, jejunum, colon, and skin tissues through immunohistochemical examination and iron HE staining. No noticeable effects were found in these tissues. Therefore, TP may only affect the mitosis orientation in certain organs. In this case, the effect of TP on mitosis orientation might be considered specific for target organs, at least for the prostate.
Both TP and E2 induced proliferation in the prostate, but the results were different. The BrdU-labeled cells were found in both the epithelia and stroma of the prostate in TP-treated rats. BrdU-labeled cells were only found in the stroma of the prostate in E2-treated rats. Androgens could affect not only the stroma, but also the epithelia; however, the roles of other steroids, especially estrogens, are still unknown. Bektic et al[8] first reported the effects of estrogen on global gene expression in human prostatic stroma cells. Scarano et al[9] described the effects of chronic estradiol treatment on guinea pig prostatic stroma. The present study found that a single injection of E2 could cause proliferation of prostatic stroma.
To our knowledge, it is has not reported that TP can cause changes in mitosis orientation in prostate epithelial cells. Although the underlying mechanism is not fully understood, the effects are most likely related to the androgen receptors (AR), because mitosis orientation was not observed in the control or E2-treated groups. Androgens enhanced the transcription of genes involved in cellular proliferation by binding and activation, such as the mitogenic growth factors epidermal growth factor and insulin-like growth factor-I[10]. This process may be related to the Wnt signaling pathway, which controls mitosis orientation[10,11]. Moreover, a SHya may be a useful biomaterial to regulate Wnt signaling in tissue engineering[12]. In addition, there was significant crosstalk between these signaling pathways in the regulation of proliferation and differentiation in the prostate cells. For example, β-catenin is not only utilized in the prostate epithelium as an important co-activator of AR signaling[13], but also the key component in the Wnt signaling pathway[14]. Therefore, Wnt signaling, β-catenin, and the crosstalk with androgen signaling and other pathways may help us understand the process. Therefore, further studies are needed to explain the mechanism and these observations, and finally, for treating androgen-dependent diseases such as BPH and PCa.
Acknowledgements
We would like to thank Gui-lin HE, Xiu-rong JIANG, Gui-ming LIU, Shu-wu XIE, Zhi-ling LI, Li MA, Wen-juan HU, and Ying LU for technical assistance.
References
- Qian LH, Wang XL, Tu ZH. Inhibition of regrowth of prostatic glandular cells by epristeride. Acta Pharmacol Sin 2001;22:847-50.
- Chu JH, Sun ZY, Meng XL, Wu JH, He GL, Liu GM, et al. Differential metastasis-associated gene analysis of prostate carcinoma cells derived from primary tumor and spontaneous lymphatic metastasis in nude mice with orthotopic implantation of PC-3M cells. Cancer Lett 2006;233:79-88.
- Meigs JB, Mohr B, Barry MJ, Collins MM, McKinlay JB. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol 2001;54:935-44.
- Griffiths K. Estrogens and prostatic disease. International Prostate Health Council Study Group. Prostate 2000;45:87-100.
- Gunin AG. Estrogen changes mitosis orientation in the uterine epithelial cells. Eur J Obstet Gynecol Reprod Biol 2001;97:85-9.
- Gunin AG, Sharov AA, Nikolaev DV. Two month glucocorticoid treatment increases estradiol-induced stromal and myometrial cell proliferation in the uterus of ovariectomized rats. Eur J Obstet Gynecol Reprod Biol 2000;88:171-9.
- Knol JA, Walker SC, Roberison JM, Yang Z, DeRemer S, Stetson PL, et al. Incorporation of 5-bromo-2''-deoxyuridine into colorectal liver metastases and liver in patients receiving a 7-day hepatic arterial infusion. Cancer Res 1995;55:3687-91.
- Bektic J, Wrulich OA, Dobler G, Kofler K, Ueberall F, Culig Z, et al. Identification of genes involved in estrogenic action in the human prostate using microarray analysis. Genomics 2004;83:34-44.
- Scarano WR, Cordeiro RS, Góes RM, Carvalho HF, Taboga SR. Tissue remodeling in guinea pig lateral prostate at different ages after estradiol treatment. Cell Biol Int 2005;29:778-84.
- Ho E, Boileau TW, Bray TM. Dietary influences on endocrine-inflammatory interactions in prostate cancer development. Arch Biochem Biophys 2004;428:109-17.
- Schlesinger A, Shelton CA, Maloof JN, Meneghini M, Bowerman B. Wnt pathway components orient a mitotic spindle in the early Caeonrhabditis elegans embryo without requiting gene transcription in the responding cell. Genes Dev 1999;13:2028-38.
- Nagira T, Nagahata-Ishiguro M, Tsuchiya T. Effects of sulfated hyaluronan on keratinocyte differentiation and Wnt and Notch gene expression. Biomaterials 2007;28:844-50.
- Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, . Linking beta-catenin to androgen-signaling pathway. J Biol Chem 2002; 277: 11 336–44.
- Verras M, Sun Z. Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett 2006;237:22-32.
