Effects of aspirin on number, activity and inducible nitric oxide synthase of endothelial progenitor cells from peripheral blood1
Introduction
Vascular endothelial progenitor cells (EPC) are the precursors of endothelial cells. Increasing evidence suggests that circulating progenitor cells contribute to postnatal neovascularization. These cells home to sites of ischemia, adopt an endothelial phenotype, and contribute to new blood vessel formation, but the identity of the circulating cells that contribute to neovascularization is not entirely clear. Bone-marrow-derived hematopoietic progenitor cells can give rise to endothelial progenitor cells and contribute to endothelial recovery and new capillary formation after ischemia.
Aspirin (acetylsalicylic acid) is widely used in the primary and secondary prevention of vascular disease[1]. The anti-inflammatory effect of aspirin it is believed to complement its platelet inhibitory effect, and be due to the inhibition of cyclooxygenase, resulting in decreased thromboxane A2 production[2]. Although the major beneficial effect of aspirin is due to its inhibitory action on platelet aggregation, there is emerging evidence showing that other effects of aspirin on cells other than platelets may be equally important[3]. Therefore we investigated the effects of aspirin on the number and activity of endothelial progenitor cells from peripheral blood. Nitric oxide (NO) synthesized from L-arginine by inducible nitric oxide synthase (iNOS) is a very important signal pathway messenger in human endothelial cells[4,5]. We detected iNOS by western blotting and discussd the role of iNOS in these effects.
Materials and methods
Isolation and cultivation of EPC Human EPC were obtained from 6 healthy adults and cultured according to previously described techniques[6–8]. Written informed consent was obtained from all people involved in the study. Briefly, total mononuclear cells (MNC) were isolated from the blood of study subjects by Ficoll density gradient centrifugation. Cells were plated on culture dishes coated with human fibronectin (Chemicon) and maintained in Medium 199 (Sigma) supplemented with 20% fetal calf serum, penicillin (100 U/mL), and streptomycin (100 µg/mL). After 4 d of culture, nonadherent cells were removed by washing with phosphate-buffered saline (PBS), new media was added, and the culture was maintained through to d 7. Attached cells were stimulated with aspirin (Sigma; to achieve final concentrations of 1, 2, 5, and 10 mmol/L) for 3, 6, 12, and 24 h.
Cellular staining Fluorescent chemical detection of EPC was performed on attached MNC after 7 d in culture. Direct fluorescent staining was used to detect dual binding of fluorescein isothiocyanate (FITC)-labeled Ulex europaeus agglutinin (UEA-1; Sigma) and 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine (DiI)-labeled acetylated low-density lipoprotein (DiLDL; Molecular Probes). Cells were first incubated with DiLDL at 37 oC and later fixed with 2% paraformaldehyde for 10 min. After being washed, the cells were treated with UEA-1 (10 µg/mL) for 1 h. Samples were then viewed with an inverted fluorescent microscope (Leica) and a laser scanning confocal microscope (LSCM, Leica). Cells that were doubly fluorescent were identified as differentiating EPC[7–9]. Two or 3 independent investigators evaluated the number of EPC per well by counting 15 randomly selected high-power fields (×200) with an inverted fluorescent microscope.
Migration assay EPC migration was evaluated by using a modified Boyden chamber assay (Jiangsu Qilin Medical Equipment Factory, China). In brief, isolated EPC were detached using 0.25% trypsin, harvested by centrifugation, resuspended in 500 µL M199, and counted, then 2×104 EPC were placed in the upper chamber of a modified Boyden chamber. M199 and human recombinant VEGF (50 ng/mL) were placed in the lower compartment of the chamber. After 24 h incubation at 37 oC, the lower side of the filter was washed with PBS and fixed with 2% paraformaldehyde. For quantification, cells were stained with Giemsa solution. Cells migrating into the lower chamber were counted manually in 3 random microscopic fields (×200)[8,9].
Cell adhesion assay EPC were washed with PBS and gently detached with 0.25% trypsin. After centrifugation and resuspension in M199 with 5% fetal bovine serum, identical cell numbers were replated onto fibronectin-coated culture dishes and incubated for 30 min at 37 oC. Adherent cells were counted by independent blinded investigators[8,10].
EPC proliferation assay The effect of aspirin on EPC proliferation was determined by 3-(4,5-dimethyl-2 thiazoyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. After being cultured for 7 d, EPC were digested with 0.25% trypsin and then cultured in serum-free medium in 96-well culture plates (200 µL per well). EPC were supplemented with 10 µL MTT (5 g/L) and incubated for another 6 h. Then the supernatant was discarded by aspiration and the EPC preparation was shaken with 200 µL Me2SO for 10 min, before the optical density (OD) value was measured at 490 nm[8].
In vitro vasculogenesis assay The in vitro vasculogenesis assay was performed with an in vitro Angiogenesis Assay Kit (Chemicon) according to the manufacturer’s instructions. Briefly, ECMatrix solution was thawed on ice overnight, then mixed with 10× ECMatrix diluent and placed in a 96-well tissue culture plate at 37 oC for 1 h to allow the matrix solution to solidify. EPC were harvested as described earlier and replated (1×104 cells per well) on top of the solidified matrix solution. Cells were incubated at 37 oC for 24 h. Tubule formation was inspected under an inverted light microscope at 200× magnification. Tubule formation was defined as development of a structure with a length at least 4 times its width[8,11,12]. Five independent fields were assessed for each well, and the average number of tubules per 200× field was determined.
Western blot analysis for iNOS The cell monolayers were washed 3 times with PBS and lysed with RIPA buffer containing phenylmethylsulfonyl fluoride and aprotinin as protease inhibitors. The cell lysates (30 µg total protein) were denatured and subjected to 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to nitrocellulose membranes by electroblotting. The membranes were soaked in a blocking solution containing PBS with 5% non-fat dried milk and 0.05% Tween 20 for 1 h at room temperature. The membranes were incubated with iNOS monoclonal antibodies (Santa Cruz Biotechnology) for 2 h and then with peroxidase-conjugated secondary antibodies for 2 h at room temperature. The bands corresponding to iNOS were detected using a chemiluminescence reagent (Amersham).
Statistical analysis Data are expressed as mean±SD. We used one-way ANOVA and the independent-samples t-test to analyze the differences in variables. The differences were considered significant if the P<0.05. All statistical analyses were performed with SPSS 11.5.
Results
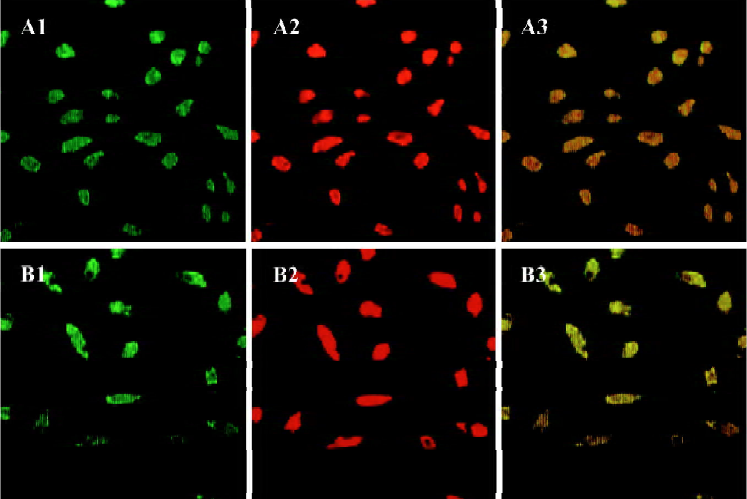
Characteristics of human EPC Total MNC that were isolated and cultured for 7 d were spindle-shaped, with an endothelial cell-like morphology. EPC were characterized as adherent cells that were double-positive for DiLDL-uptake and lectin binding under a laser scanning confocal microscope (Figure 1). We and other investigators have previously demonstrated that endothelial progenitor cells isolated in this fashion also exhibit many other endothelial characteristics, including expression of CD31, von Willebrand factor, and vascular endothelial growth factor receptor 2[7–9,11].
Effect of aspirin on number of EPC Incubation of isolated human MNC with aspirin decreased the number of EPC in a concentration- and time-dependent manner (Figure 2).

Effects of aspirin on the proliferative capacity of isolated EPC Incubation of isolated human MNC with aspirin decreased EPC proliferative capacity in a concentration- and time-dependent manner (Figure 3).

Effects of aspirin on the migratory capacity of isolated EPC Incubation of isolated human MNC with aspirin decreased EPC migratory capacity in a concentration- and time-dependent manner (Figure 4).
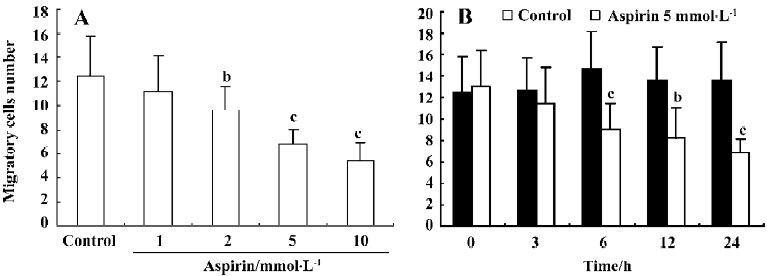
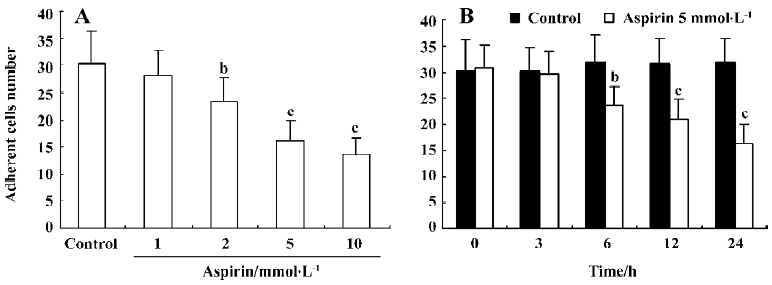
Effect of aspirin on the adhesive capacity of isolated EPC Incubation of isolated human MNC with aspirin decreased EPC adhesive capacity in a concentration- and time-dependent manner (Figure 5).
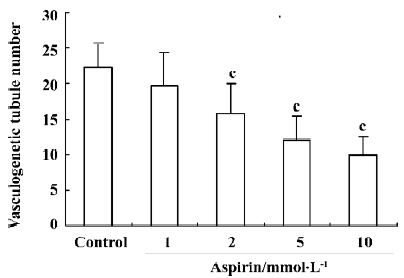
Effects of aspirin on EPC in vitro vasculogenesis Incubation of isolated human MNC with aspirin decreased the in vitro vasculogenesis capacity of EPC cells in a concentration-dependent manner (Figure 6).
Effects of aspirin on EPC iNOS Western blot analysis showed that the expression of iNOS was significantly decreased by aspirin in a concentration-dependent manner (Figure 7), strongly suggesting that the inhibitory effect of aspirin is mediated through decreasing NO.

Discussion
There is strong evidence that EPC plays a significant role in neovascularization and re-endothelialization, particularly under ischemic conditions. Recently, it has been noted in animal and human subjects that EPC contribute up to 25% of endothelial cells in newly formed vessels[13,14]. Thus, increasing the number of circulating EPC by transplantation of hematopoietic stem cells or by injection of in vitro differentiated EPC has been shown to improve neovascularization of ischemic hindlimbs[15], accelerate blood flow in diabetic mice[16], and improve cardiac function[17]. Moreover, Vasa et al have recently reported that patients with coronary heart disease (CHD) have reduced levels and functional impairment of EPC, which correlate with risk factors for CHD[9]. In addition, we have previously observed that hyperho-mocysteine, a major risk factor for cardiovascular diseases, induces a reduction in EPC levels with decreased functional activity in vitro[8]. Therefore, stimulation of mobilization and/or differentiation of EPC may provide a useful novel therapeutic strategy to improve postnatal neovascularization and re-endothelialization in patients with CHD.
Aspirin is widely used in the primary and secondary prevention of vascular disease. The anti-inflammatory effect of salicylates is believed to complement their platelet inhibitory effect and is thought to be due to the inhibition of cyclooxygenase, resulting in decreased thromboxane A2 production. Aspirin inhibits cyclooxygenase by acetylating the serine residue in the active site of the enzyme[18,19]. Bernhardt et al[20] have reported that high concentrations of aspirin can inhibit smooth muscle cell proliferation. Marra et al[21] have shown that aspirin inhibits the proliferation of smooth muscle cells through inhibition of cyclin-dependent kinases, which hyperphosphorylate the retinoblastoma protein. Oberle et al[22] have discovered that aspirin increases the resistance of endothelial cells to assault by free radicals through ferritin synthesis. An antiarrhythmic effect of aspirin has been found in platelet-depleted dogs subjected to coronary occlusion[23]. Kharbanda et al[24] have reported a protective effect of aspirin against endothelial dysfunction. Husain et al[25] have reported that improvement of endothelial dysfunction with aspirin may improve vasodilation, reduce thrombosis, and inhibit the progression of athero-sclerosis. Ranganathan et al[26] have reported that aspirin inhibits the synthesis of protein and DNA by upregulation of p53 expression in endothelial cells. p53 is an inhibitor of cyclin-dependent kinases (Cdk)[27]. Activation of Cdk is important in the hyperphosphorylation of retinoblastoma protein, which activates the transcription of several genes required for the progression of the cell cycle[28]. The present study shows that aspirin (1) decreases the number of EPC; (2) decreases the proliferative, migratory, adhesive, and in vitro vasculogenesis capacity of EPC; and (3) decreases EPC production of iNOS in a concentration- and time-dependent manner. Katsuyama et al[29] showed that aspirin dose-dependently inhibited cytokine-stimulated NO production and iNOS protein expression. NO can enhance EPC proliferation and function through activating the PI3K/Akt signal pathway[30,31].
Circulating EPC are constantly exposed to inflammatory factors, such as cytokines and oxidized lipoproteins. Atherosclerosis in general is indicative of a low grade inflammatory process, and atherosclerotic plaques that are prone to rupture and result in acute coronary syndromes reveal an intense inflammatory state[32,33]. Therefore, the effects of aspirin on EPC in the inflammatory condition must be studied further. Aspirin is a cornerstone of therapy in acute coronary syndromes[1,3], and has been shown to reduce atherosclerosis-related events in a multitude of clinical studies[34]. Although most of the benefits of aspirin have been ascribed to its anti-platelet aggregatory properties, non-platelet-mediated effects are also thought to contribute to its salutary effect.
References
- Awtry EH, Loscalzo J. Aspirin. Circulation 2000;101:1206-15.
- Aspirin Weissmann G. Sci Am 1991;264:84-90.
- Mehta JL. Salutary effects of aspirin in coronary artery disease are not limited to its platelet inhibitory effects. Clin Cardiol 1998;21:879-84.
- Guo X, Chen LW, Liu WL, Guo ZG. High glucose inhibits expression of inducible and constitutive nitric oxide synthase in bovine aortic endothelial cells. Acta Pharmacol Sin 2000;21:325-8.
- Natarajan R, Jones DG, Fisher BJ, Wallace TJ, Ghosh S, Fowler Iii AA. Hypoxia inducible factor-1: regulation by nitric oxide in posthypoxic microvascular endothelium. Biochem Cell Biol 2005;83:597-607.
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593-600.
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 2000;97:3422-7.
- Chen JZ, Zhu JH, Wang XX, Zhu JH, Xie XD, Sun J, et al. Effects of homocysteine on number and activity of endothelial progenitor cells from peripheral blood. J Mol Cell Cardiol 2004;36:233-9.
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001;89:E1-E7.
- Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, et al. Statin therapy accelerates reendotheli-alization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 2002;105:3017-24.
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002;106:2781-6.
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 2002;109:337-46.
- Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization. Exp Hematol 2002;30:967-72.
- Suzuki T, Nishida M, Futami S, Fukino K, Amaki T, Aizawa K, et al. Neoendothelialization after peripheral blood stem cell transplantation in humans. A case report of a Tokaimura nuclear accident victim. Cardiovasc Res 2003;58:487-92.
- Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 2000;105:1527-36.
- Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest 2000;106:571-8.
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430-6.
- Roth GJ, Majerus PW. The mechanism of the effect of aspirin in human platelets. J Clin Invest 1975;56:624-32.
- Lecomte M, Laneuville O, Ji C, DeWitt DL, Smith WL. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J Biol Chem 1994;269:13207-15.
- Bernhardt J, Rogalla K, Luscher TF, Buhler FR, Resink TJ. Acetylsalicylic acid, at high concentrations, inhibits vascular smooth muscle cell proliferation. J Cardiovasc Pharmacol 1993;21:973-6.
- Marra DE, Simoncini T, Liao JK. Inhibition of vascular smooth muscle proliferation bysodium salicylate mediated by upregulation of p21Waf1 and p27Kip1. Circulation 2000;102:2124-30.
- Oberle S, Polte T, Abate A, Podhaisky HP, Schroder H. Aspirin increases ferritin synthesis in endothelial cells. Circ Res 1998;82:1016-20.
- Moschos CB, Lahiri K, Peter A, Jesrani MU, Regan TJ. Effect of aspirin upon experimental coronary and non-coronary thrombosis and arrhythmia. Am Heart J 1972;84:525-30.
- Kharbanda RK, Walton B, Allen M, Klein N, Hingorani AD, MacAllister RJ, et al. Prevention of inflammation induced endothelial dysfunction. Circulation 2002;105:2600-4.
- Husain S, Andrews NP, Mulcahy D, Panza JA, Quyyumi AA. Aspirin improves endothelial dysfunction in atherosclerosis. Circulation 1998;97:716-20.
- Ranganathan S, Joseph J, Mehta JL. Aspirin inhibits human coronary artery endothelial cell proliferation by upregulation of p53. Biochem Biophys Res Commun 2003;301:143-6.
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997;88:323-31.
- La Thangue NB. E2F and the molecular mechanisms of early cell-cycle control. Biochem Soc Trans 1996;24:54-9.
- Katsuyama K, Shichiri M, Kato H, Imai T, Marumo F, Hirata Y. Differential inhibitory actions by glucocorticoid and aspirin on cytokine-induced nitric oxide production in vascular smooth muscle cells. Endocrinology 1999;140:2183-90.
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI3-kinase/Akt pathway. J Clin Invest 2001;108:391-7.
- Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 2001;103:2885-90.
- Mehta JL, Li DY. Inflammation in ischemic heart disease; response to tissue injury or a pathogenetic villain. Cardiovasc Res 1999;43:291-9.
- Kinlay S, Selwyn AP, Libby P, Ganz P. Inflammation, the endothelium, and the acute coronary syndromes. J Cardiovasc Pharmacol 1998;32 Suppl 3:S62-S66.
- Antiplatelet Trialists’ Collaboration. Prevention of death, myocardial infarction and stoke by antiplatelet therapy: collaborative meta-analysis of 266 trials involving 200,000 patients at high risk of occlusive vascular events. Br Med J 2002;324:71-86.
