Influences of 3-methylcholanthrene, phenobarbital and dexamethasone on xenobiotic metabolizing-related cytochrome P450 enzymes and steroidogenesis in human fetal adrenal cortical cells1
Introduction
Cytochrome P450 (CYP) isoforms are key enzymes responsible for the metabolism of xenobiotics and anabolism of steroid hormones[1]. The human adult adrenals are considered as the main organs in steroid hormone synthesis, CYP isoforms expressed in human adult adrenals have been demonstrated to provide these functions[2]. The human fetal adrenal system is one of the most important endocrine systems and is indispensable in sustaining biological homeostasis. In particular, steroid hormones play a key role in reproductive systems and influence the growth, differen-tiation, and function of many target cells. The majority of enzymes needed to produce steroid hormones, including progestins, androgens, estrogens, glucocorticoids and mineralocorticoids, are members of the CYP superfamily[3]. Previous research has demonstrated that steroidogenesis-related CYP11A1, CYP17, CYP21, CYP11B1, and CYP11B2 were expressed in human fetal adrenals[4]. Our previous study using RT-PCR technique demonstrated the expressions of CYP1A1, 2C8-19, and 3A7 mRNA in human fetal adrenals[5]. However, few data are available concerning the correlations among xenobiotics, xenobiotic metabolizing-related CYP1, 2, 3 and steroidogenesis in human fetal adrenals during the fetal development with respect to possible toxicological significance. In the present paper, we study the steroidogenesis and inducibility of CYP1, 2, and 3 isoforms by 3-methylcholanthrene, phenobarbital, and dexamethasone in human fetal adrenal cells in vitro, in order to explore the influence and possible mechanism of xenobiotics on fetal adrenal steroidogenesis.
Materials and methods
Samples Human fetal specimens were obtained by therapeutic and legal abortion from second trimester tissues (20–24 weeks of gestation, females), approved by the Academic Committee and the Ethics Committee of Medical College of Wuhan University.
Chemicals 3-Methylcholanthrene, phenobarbital, dexamethasone, bovine serum albumin (BSA), 7-ethoxy-resorufin, isocitric acid, isocitric acid dehydrogenase, collagenase I and dimethyl sulfoxide were purchased from Sigma-Aldrich Corporation (St Louis, Missouri). All other chemicals and reagents were of Acceptance Requirement grade.
Cell preparation, culture and treatment Primary human fetal adrenal cortical cells, consisting mainly (90%–95%) of fetal zone cells, were prepared and cultured as described in a previous study[6,7]. The gland was decapsulated to remove most of the definitive zone, and the remaining fetal zone was minced and digested with 0.4 mg/mL collagenase I in phosphate-buffered saline enriched 10% fetal calf serum (ES Cell qualified, Gibco Company, Los Angeles, USA) at 37 ºC for 15 min. Nondigested tissues were allowed to settle, the supernatants were diluted with modified McCoy’s 5A medium (Gibco Company, Los Angeles, USA), which contained 10% fetal calf serum and penicillin/streptomycin. The mixtures were centrifuged at 67×g for 5 min at 4 ºC to pellet the cells. The pelleted cells were suspended in medium and repelleted as described above.
The cells were plated at a density of approximately 5×106 cells/mL on 12-well plates and incubated overnight in a humidified atmosphere of 5% CO2 at 25 ºC or cell attachment. Three replicates were used. After 48 h, the medium was renewed (2 mL/well), the administration was carried out with the agents (3-methylcholanthrene 0–2 µmol/L in 1% Me2SO, phenobarbital 0–1 mmol/L in H2O or dexamethasone 0–100 µmol/L in H2O) for a 24 h period. The medium were removed and kept frozen at -30 ºC until assayed for the steroids. The fetal zone cells were rinsed twice with pre-cold phosphate-buffered solution. Pre-cold TEN buffer (pH 7.8) 0.4 mL, which contained Tris-HCl 40 mmol/L, EDTA 1 mol/L and NaCl 150 mmol/L, was added and then the mixture was kept on ice for 5 min. The cells were harvested with a rubber scalpel and centrifuged at 151×g for 5 min, and then suspended in Tris buffer (pH 7.8, 20 mmol/L) and sonicated thrice in ice water for 5 s by a W375 sonicator (Heat Systems-Ultrasonics. Inc., Plainview, NY). The cell extracts were used for enzyme assay and protein determination.
Analysis of steroids Commercially available, direct competitive radioimmunoassay kits (North Institute of Biological Technology, Beijing, China) were used for the measurements of medium cortisol, aldosterone, testosterone and progesterone. The lower limit of quantification of cortisol, aldosterone, testosterone and progesterone was 10 ng/mL, 3.75 pg/mL, 20 pg/mL, 5 pg/mL, respectively. The correlation coefficient was 0.9815, 0.9834, 0.9715, and 0.9834, respectively, for the working curve of quantification of cortisol, aldosterone, testosterone and progesterone
Enzyme assays Activity of 7-ethoxyresorufin O-dealkylase (EROD) was assayed using SHIMADZU RF-540 Fluorescence Spectrophotometer (Kyoto Japan). In brief, the total reaction contained cell extract protein (0.2 mg protein), Tris·HCl 50 mmol/L (pH 7.8), MgCl2 2.5 mmol/L, KCl 50 mmol/L, BSA 12 mg/ml, 7-ethoxyresorufin 2 µmol/L and an NADPH generating system (NADP+ 0.4 mmol/L, isocitric acid 10 mmol/L and isocitric acid dehydrogenase 0.6 units). The reaction was initiated with the NADPH-generating system and stopped by the addition of ice-methanol 2.5 mL at 30 min. Fluorescent intensity of the supernatants was read at λem 586 nm and λex 530 nm against a standard of resorufin.
Activities of benzphetamine, aminopyrine and erythromycin N-demethylase were determined as previously described. In brief, the assay mixture contained cell extract protein (0.2 mg protein), Tris·HCl 50 mmol/L (pH 7.8), MgCl2 10 mmol/L, KCl 150 mmol/L, and substrate. The final concentration of erythromycin, benzphetamine and aminopyrine was 0.4 mmol/L, 2 mmol/L, and 8 mmol/L, respectively. The reaction was initiated with the NADPH-generating system and stopped by the addition of 25% ZnSO4 and saturated Ba(OH)2 at 60 min. The supernatant, after centrifugation, was incubated with the Nash reagent (Wiltshire SP4 0JG. England) at 60 ºC for 20 min and its color was measured at 415 nm spectrophotometrically.
Statistical analysis Data were analyzed using one-way ANOVA test and differences were considered statistically significant if the P value was less than 0.05.
Results
Inducibility of CYP isoforms The activities of benzphetamine and aminopyrine N-demethylase were increased in the cultural fetal adrenal cortical cells treated with phenobarbital (0.25–1 mmol/L) for 24 h, and the maximal induction was 1.96- and 3.40-fold, respectively (P<0.05, P<0.01; Figure 1). Dexamethasone (25–100 µmol/L) also increased the activity of erythromycin N-demethylase, and this increase showed a concentration-effect relationship with a maximal induction of 4.74-fold (P<0.01; Figure 2). The activity of 7-ethoxyresorufin O-dealkylase was undetected in the cells treated without and with 3-methylcholanthrene (0.5–2 µmol/L).
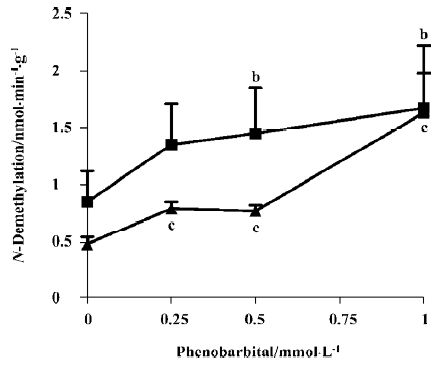
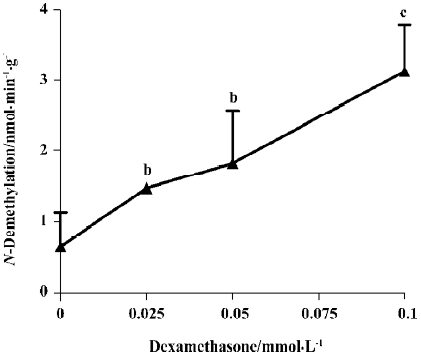
Steroidogenesis The results (Table 1) showed that the contents of medium cortisol, aldosterone and progesterone have decreased to 51%, 21% (P<0.01) and 13% (P<0.05) of control, respectively after treatment with 3-methylcholanthrene (0.5–2 µmol/L). Cortisol, aldosterone and progesterone concentrations have also decreased to 32%, 30%, and 39% of control respectively, with phenobarbital (0.25–1 mmol/L). Dexamethasone (25–100 µmol/L) had enhanced the production of cortisol and progesterone remarkably, almost 14.90- and 3.62-fold higher than their controls (P<0.01). However, the trend of testosterone concentration was uncertain after 3-methylcholanthrene, phenobarbital or dexamethasone treatment.
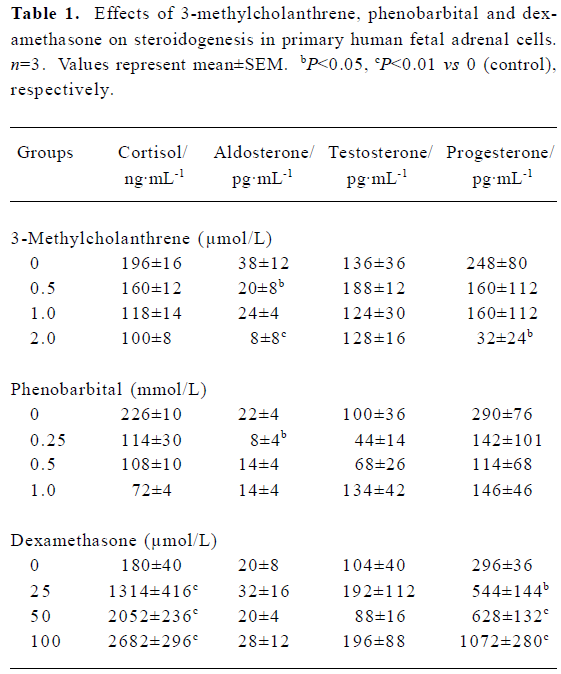
Full table
Discussion
CYP1, 2, and 3 families are the most important CYP isoforms in xenobiotic metabolism. 3-Methylcholanthrene, phenobarbital and dexamethasone are considered as the classical inducers of CYP1A, CYP2B/2C, and CYP3A, respectively. 7-Ethoxyresorufin, benzphetamine, aminopyrine and erythromycin are classical substrates of CYP1, 2, and 3. Our previous results obtained by RT-PCR technique demonstrated the expression of CYP1A1 mRNA[5]. However, in this study, whether 3-methylcholanthrene was administrated or not, EROD activity was undetected in the cultured fetal zone cells. This might be attributed to a lower enzyme activity of CYP1A in human fetal adrenal cortical cells. Meanwhile, both N-demethylations of benzphetamine and aminopyrine had increased when phenobarbital was added, erythromycin N-demethylation had also increased by the treatment with dexamethasone. All these observations suggested that the fetal adrenal CYP isoforms were capable of catalyzing the metabolism of xenobiotics, and possess inducibility.
During pregnancy, cortisol may influence placental function and uterine blood flow. If cortisol concentration was changed, it would affect the growth and development of the fetus or induce the onset of preterm labor[8]. Progesterone has suppressive actions on lymphocyte proliferation and on the immune system to prompt the developing fetus and placenta. A higher progesterone level was reported to display a delay in parturition of several days[9]. Aldosterone can sustain a homeostatic mechanism for the conservation of sodium for both mother and fetus[10]. Therefore, it can be presumed that any changes of steroid hormone level caused by xenobiotics might affect the growth and development of the fetus. The adrenal steroid hormones are produced in the multi-step pathways involving different CYP isoforms, such as CYP11A1, CYP17, CYP21, CYP11B1, and CYP11B2[4], and steroidogenic acute regulatory (StAR) protein and CYP11A1 is a rate-limiting step in steroidogenesis. It was found that steroidogenesis of fetal adrenals could be affected by multiple factors, such as estrogen[11], nicotine[12] and ethanol[13], which are the substrates and inducers of CYP1, 2, and 3. In the present work, we observed the steroidogenesis in human fetal adrenal cortical cells, and paid more attention to the treatments of 3-methylcholanthrene, phenobarbital and dexamethasone, which have not been reported in the published literature. As shown in the results, the production of cortisol, aldosterone and progesterone were decreased to some extent after 3-methylcholanthrene and phenobarbital treatments. Meanwhile, dexamethasone remarkably increased the levels of cortisol and progesterone. Recently, our research further demonstrated that exposure to nicotine (CYP1A1 inducer) in utero during the middle and last gestation decreased the expression of StAR protein and CYP11A1 mRNA of fetal adrenals in rats (data not shown). These results suggested that xenobiotics could interfere with adrenal steroidogenesis during the fetal development, and a possible pathway may be CYP isoform activation induced by xenobiotics. However, few investigations were focused on the interfering mechanism of xenobiotics on the steroidogenesis. The latest research of Aluru et al (2005) found that β-naphthoflavone, another specific inducer of CYP1A, significantly elevated the expression of CYP1A1 gene and protein in the kidney, and depressed StAR protein and CYP11A1 in rainbow trout, which suggests an Ah receptor-mediated impairment of inter-renal steroidogenesis[14].
In conclusion, the present study found the influences of 3-methylcholanthrene, phenobarbital, and dexamethasone on steroidogenesis in human fetal adrenal cortical cells, which might be mediated by adrenal xenobiotic metabolizing-related CYP isoform activation. Our results provide an important clue for elucidating the pathogenesis of some diseases and for guiding clinical medication. For example, phenobarbital and dexamethasone applied in human neonatal hyperbilirubinemia[15] and fetal lung fibroblasts[16], respectively, might interfere with steroidogenesis and affect the growth and development of the fetus. Further studies are required to explore the molecular mechanism of xenobiotics on fetal adrenal StAR and CYP11A1 expressions.
References
- Guengerich FP. Human cytochrome P-450 enzymes. Life Sci 1992;50:1471-8.
- Sanderson T, Vandenberg M. Interactions of xenobiotics with the steroid hormone biosynthesis pathway. Pure Appl Chem 2003;75:1957-71.
- Suzuki T, Sasano H, Takeyama J. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol 2000;53:739-47.
- Rehman KS, Carr BR, Rainey WE. Profiling the steroidogenic pathway in human fetal and adult adrenals. J Soc Gynecol Investing 2003;10:372-80.
- Wang H, Peng RX, Yu JP, Zhang YH. Expression and characteristics of cytochrome P-450 1, 2 and 3 in human fetal adrenals. Asia Pac J Pharmacol 2000;14:33-8.
- Mesiano S, Jaffe RB. Interaction of insulin-like growth factor-II and estradiol directs steroidogenesis in the human fetal adrenal toward dehydroepiandrosterone sulfate production. J Clin Endocri Metab 1993;77:754-8.
- Parker CR, Stankovic AK, Falany CN, Faye PO, Grizzle WE. Immunocytochemical analyses of dehydroepiandrosterone sulfotransferase in cultured human fetal adrenal cells. J Clin Endocrinol Metab 1995;80:1027-31.
- McLean M, Smith R. Corticotropin-releasing hormone in human pregnancy and parturition. Trends Endocrinol Metab 1999;10:174-8.
- Piekorz RP, Gingras S, Hoffmeyer A. Regulation of progesterone levels during pregnancy and parturition by stat5 and 20α-hydroxysteroid dehydrogenase. Mol Endocrinol 2005;19:431-7.
- Lisurek M, Bernhardt R. Modulation of aldosterone and cortisol synthesis on the molecular level. Mol Cell Endocrinol 2004;215:149-59.
- Albrecht ED, Henson MC, Walker ML, Pepe GJ. Modulation of adrenocorticotropin-treated baboon fetal adrenal dehydroepian-drosterone formation in vitro by estrogen at mid- and late-gestation. Endocrinology 1990;126:3083-8.
- Sarasin A, Schlumpf M, Muller M. Adrenal-mediated rather than direct effects of nicotine as a basis of altered sex steroid synthesis in fetal and neonatal rat. Reprod Toxicol 2003;17:153-62.
- Khisti RT, Kumar S, Morrow AL. Ethanol rapidly induces steroidogenic acute regulatory protein expression and translocation in rat adrenal gland. Eur J Pharmacol 2003;473:225-7.
- Aluru N, Renaud R, Leatherland JF, Vijayan MM. Ah receptor-mediated impairment of interrenal steroidogenesis involves StAR protein and P450scc gene attenuation in rainbow trout. Toxicol Sci 2005;84:260-9.
- Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbiliru-binemia. N Engl J Med 2001;344:581-90.
- Wen FQ, Kohyama T, Skold CM. Glucocorticoids modulate TGF-beta production by human fetal lung fibroblasts. Inflammation 2003;27:9-19.
