IL-12p40 is not required for islet allograft rejection1
Introduction
CD4+ Th cells can differentiate into two subsets, Th1 and Th2 cells. Th1 cells produce cytokines such as interleukin (IL)-2, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α and often orchestrate the cellular immune responses noted in certain organ specific autoimmune diseases, allograft rejection, and delayed-type hypersensitivity responses. In contrast, Th2 cells secrete IL-4, IL-5, IL-6, and IL-10 cytokines and lead to the production of antibodies contributing to humoral immunity. The balance between Th1 and Th2 cells determines an overcome of immune response[1].
Previous studies have shown that allograft rejections including the heart, skin and islet are usually associated with a Th1-type response. In contrast, the tolerant allograft in recipients often manifested a Th2-type response[2–5]. When IFN-γ, gene knockout (KO) mice were used as graft recipients, the allograft survival was found to be highly conditional and both the host strain and experimental conditions could influence the results[6]. When IL-4-deficient mice were used as an allograft recipient accompanied by CTLA4/Fc treatment, the allograft rejection was inhibited as compared to the control, wild-type (WT) mice[7].
IL-12 and IL-23 are believed to be Th1 initiators playing an essential role in Th1 development[8–10]. The cytokines are composed of a p35 and p40 subunit for IL-12 and p19 and p40 subunit for IL-23. They are produced primarily by activated antigen-presenting cells, such as dendritic cells and macrophages, proinflammatory natural-killer (NK) and activated T cells, and induced cell-mediated immunity[11]. The p40 subunit KO mice results in a lack of IL-12 and IL-23 and consequently leads to a defect in inducing Th1 cell response in autoimmune diseases, such as experimental autoimmune encephalomyelitis (EAE)and experimental autoimmune uveoretinitis (EAU)[12,13]. The data demonstrate that in IL-12p40 transgenic mice the development of diabetes was exacerbated in non-obese diabetic (NOD) mice[14]. The heart allograft rejection was accelerated when recipient mice were injected with dendritic cell (DC) expressing IL-12p40[15]. It is unclear whether islet allograft survival is postponed in IL-12p40-deficient mice.
In this study, we investigated whether IL-12p40-deficient mice prevented islet allograft rejection in the streptozotocin (STZ)-induced diabetes mouse model. Our data showed that in an STZ-induced type-1 diabetes model, the survival time of islet allograft was similar in IL-12p40-deficient mice and WT mice.
Materials and methods
Mice Six-to eight-week-old female C57BL/6 (H2b), BALB/c (H2d), IL-12p40-deficient (P40KO,C57B6.129S1-Il12btm1Jm/J) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Animals were kept in a specific pathogen-free facility at the Chinese Academy of Sciences. Animal care and use were in compliance with institutional guidelines.
STZ treatment and diabetes induction Recipient mice were given diabetes by a single ip injection of STZ (150 mg/kg for C57 and 200 mg/kg for IL-12p40 deficient mice). STZ (Fluka, St Louis, MO) was dissolved in sodium citrate buffer (pH 4.5). Diabetes was defined as a plasma concentration of glucose >16.7 mmol/L within 2 d after the end of STZ treatment[16]. Plasma glucose concentration was measured using a Medisense Optium blood detector (Abbott Laboratories,MediSense products, Bedfold, USA).
Islet isolation and transplantation Islets were isolated from female BALB/c (H-2d) donors by using a filtered method as described in a previous study[17]. The pancreas was digested by collagenase P (Roche Diagnostics Corporation Indianapolis, IN, USA) for 12 min, and followed by filtering with a 300 µm strainer. The filtered part was poured through a 100 µm cell strainer (BD Falcon, Bedfold, USA) to enrich the islets. Finally, 500 islets (diameter varied between 100 µm and 300 µm) were handpicked and transplanted into the renal subcapsular space of STZ-induced diabetic recipients[18].
A graft transplantation was considered successful when the plasma glucose concentration was reduced to 16.7 mmol/L within 48 h. If not, the mouse was considered to be a technical failure and was excluded from analysis. Graft rejection was defined as the recurrence of a plasma glucose concentration >16.7 mmol/L on 2 successive days. Islet allograft function was confirmed by unilateral nephrectomy of the kidney bearing the transplant and documentation of the reappearance of diabetes.
ELISA The sample of mouse serum was collected and frozen at -70 °C until it was detected. Briefly, 20 µg/mL of EQ31-BSA (the C-peptide conjugated with BSA carrier) was coated with 100 µL/well in a 96-well Nunc Immunoplate (Nunc, Roskilde, Denmark) overnight at 4 °C. The plates were blocked with blocking buffer (PBS with 3% gelatin) for 2 h at 37 °C. Sample 100 µL (containing 90 µL serum and 10 µL C-peptide antibody) was added and incubated at 37 °C for 2 h. Then a second antibody horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin (Ig)-G (BD PharMingen, San Diego, CA, USA) at 100 µL/well was added and OD values were measured with a microplate autoreader (Biotek, Vermont, USA) at 450 nm. The standard curve for C peptide was created with different concentrations of C peptide and was used to measure C peptide concentrations in tested sera samples by competitive ELISA.
Immunohistopathology Islet graft was embedded in optimal cutting temperature compound (OCT) and frozen at -20 °C. The sections of 10-µm thickness were kept at -70 °C until staining. CD4+ and CD8+ cells were determined by immunohistochemistry with the specific antibodies (BD PharMingen).
Real-time PCR Total RNA was isolated from graft. An mRNA expression of IFN-γ and IL-4 was determined by real-time PCR using SYBR Green Master Mix (Applied Biosystems, Foster City, USA) as described in a previous study[19]
Statistical analysis The log-rank test of nonparametric analysis (Instat software; Graph Pad, San Diego, CA, USA)was used to analyze graft survival data. The differences in graft survival were analyzed by a Kaplan-Meier test. The experiments were usually repeated three times.
Results
Establishment of islet allograft model Initially, C57BL/6 and IL-12p40-deficient mice served as recipient mice and STZ was used to induce diabetes in the mice. We found that the different genetic background had a great impact on diabetes induction, which was consistent with other’s reports[20]. Based on our primary experiments, the optimal dose of STZ to induce diabetes in C57BL/6 mice is 150 mg/kg, but it was 200 mg/kg in IL-12p40-deficient mice.
After STZ ip injection, the glucose in the blood increased rapidly and remained at a relative high level. However, it remained normal in the vehicle control group. Based on this protocol, the diabetes was developed within a few days in STZ-treated mice. If the sick mice were not treated with islet transplantation, the mice began to die on d 10 after STZ treatment (Figure 1A).
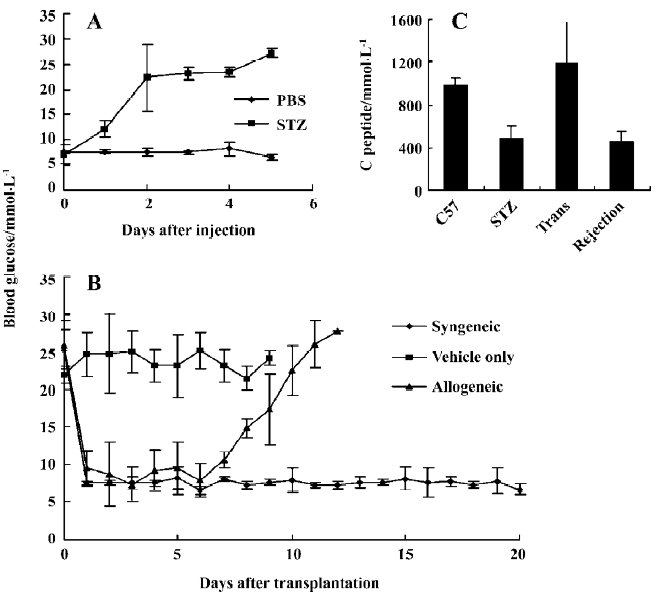
Five hundred islets freshly isolated from BALB/c or C57BL/6 mice were transplanted into the renal subcapsular space of C57BL/6 mice. Then the glucose in the blood was detected. The data showed that, after transplantation of the islets, the enhanced glucose in the recipient mice dropped to the normal level within 2 d (Figure 1B). It was obvious that the glucose concentration was increased and exceeded 16.7 mmol/L on d 10 following the islet transplantation and remained hyperglycemic when the islet allograft from Balb/c mice (allogeneic group) was rejected completely. But the islets from C57BL/6 mice (syngeneic group) functioned well and the blood glucose remained normal until 20 d after islet transplantation. In contrast, when Hanks’ solution served as a control (vehicle group) in the transplantation, the hyperglycemia was not changed.
To confirm the islet rejection, the left kidneys from islet transplanted mice were removed on d 5 (non-rejected islet) and d 12 following the transplantation. The tissue histopathology was stained with HE. Only intact islets could be seen in the tissues of non-rejected islets, but could not be seen in rejected islet (data not shown).
It was reported that the concentration of C-peptide in the blood was reduced when the diabetes occurred[21]. The concentration of C-peptide was detected in the mice serum from different types of the disease. The C-peptide concentration was consistent with diabetes. In control C57BL/6 mice, the C-peptide secretion was normal. After diabetes was induced, the C-peptide concentration was decreased. When islet transplantation was successful, the secretion of the C-peptide was rescued and then it returned to low levels after the allograft was rejected completely. The data indicates that C-peptide concentration is a good marker to reflect the stature of insulin secretion in vivo (Figure 1C).
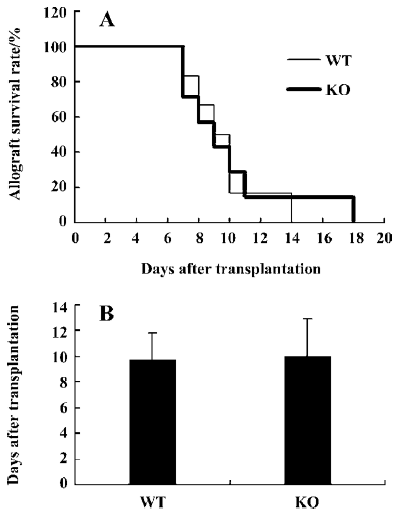
Role of IL-12p40 gene in islets allograft transplantation Islets were isolated from major histocompatibility complex (MHC)-mismatched BALB/c mice and then they were transplanted into IL-12p40- deficient or wild-type C57BL/6 mice. As shown in Figure 2A and 2B, the rejection rate and survival mean days for islet transplantation were similar between WT and IL-12p40-deficient mice. In the control group, after islet transplantation, the mean survival time was 9.7±2.1 d (n=6). Similarly, it was 10.0±2.9 d (n=7) in the IL-12p40-deficient mice. The results suggested that after islet transplantation, the C57BL/6 WT and IL-12p40-deficient mice rejected the allograft at a similar rate, which was not as anticipated.
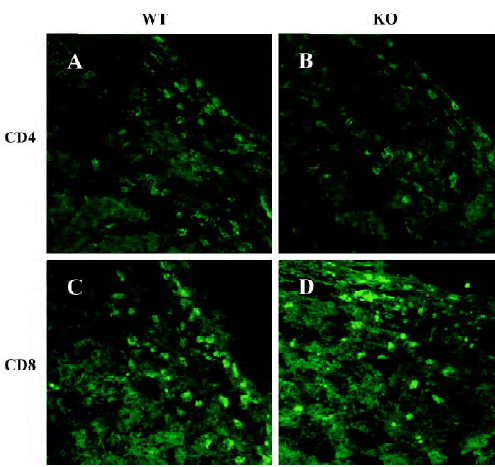
Immunohistopathology Previous studies have revealed that T cells play an important role during the islet allograft rejection and that the deficiency of IL-12p40 can affect the function of CD4+ and CD8+ T cells. In this study, we observed the infiltration of CD4+ and CD8+ T cells in the allograft. On d 9 after transplantation, the infiltration of CD4+ and CD8+ cells in the grafts was similar in the recipients of WT and IL-12p40-deficient mice (Figure 3).
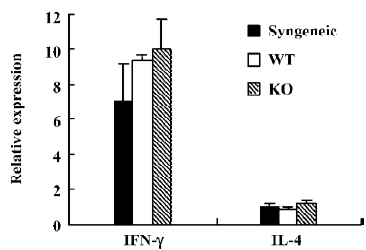
IL-4 and IFN-γ expression in the graft As Th1 and Th2 cells are believed to play a crucial role in determining the islet rejection, IL-4 and IFN-γ expressions in infiltrating T cells in the grafts were measured by real-time PCR. As we expected, IL-4 and IFN-γ expressions were similar in the grafts from WT and IL-12p40-deficient mice (Figure 4).
Discussion
Since the discovery of the cross-regulating Th1 and Th2 phenotypes, there has been great anticipation that a Th1 to Th2 immune deviation may be critical in the acquisition of transplantation tolerance. This is because IL-12 is a major inducer and a pivotal regulator in the generation of Th1 cells[22]. It is also believed that IL-12 plays a key role in the destruction of insulin-producing cells during the development of autoimmune diabetes with a higher production of IFN-γ and lower production of IL-4, which is representative of a Th1-type response[23–25]. We are interested in studying the roles of IL-12 and IL-23 in allograft rejection, which is regarded as a Th1 cell-mediated response[26,27]. Allograft rejection in unmodified recipients is often associated with a Th1-type response. Moreover, allograft recipients that are treated with tolerant immunosuppressive regimens often manifest a Th2-type response during the treatment period[3–5].
In the present study, we addressed whether Th1 to Th2 immune deviation in IL-12p40-deficient mice could eliminate the severity of allograft rejection based on the fact that Th1 cells are essential in induced autoimmune diseases[28] and the observation in CCR5–/– mice of an obvious prolongation of islet allograft survival and a switch to Th2 response[18]. We predicted that IL-12p40 might play a determining role in islet allograft rejection in a STZ-induced diabetic mouse model. Surprisingly, islet allograft from BALB/c mice survived well in IL-12p40- deficient mice (C57BL/6 background) as compared to wild-type control mice, their mean survival time was similar and the values were 10.0±2.9 d and 9.7±2.1 d, respectively. In addition, there was no significant difference in the infiltration of CD4+ and CD8+ T cell and the cytokines expression (IFN-γ and IL-4) in transplanted grafts.
According to our observations, although tolerance therapy in some animal models often skewed the immune activation toward a Th2-dominated response[26,27], a Th1 to Th2 immune deviation does not uniformly permit the acquisition of transplant tolerance. This new finding leads us to make an explanation about the mechanisms of allograft rejection[29,30]. First, we have noticed that the induction of permanent engraftment or a state of allograft tolerance through the administration of long-acting Th2 cytokines (ie, IL-4-Ig and IL-10-Ig fusion proteins) or immune deviation via the application of IL-12 antagonists have failed in the heart allograft[30,31]. It is obvious that the strategies, which are extremely effective in dampening autoimmunity, have not been proven to be effective in transplantation[32–36]. In accordance with our results, although anti-IL-12 was used to deviate Th1 to Th2, anti-IL-12 treatment was totally ineffective in prolonging the engraftment of MHC-mismatched islet allografts. While the impact of anti-IL-12 upon the pattern of cytokine expression was consistent, the differences were noted in the impact of such therapy upon the duration of engraftment in MHC-mismatched versus MHC-matched conditions. It is concluded that regarding to the model of Ag presentation and the responding T cell clone number, the allograft response to MHC-matched rather than minor Ag-mismatched allografts may more closely resemble the response to auto-antigens[37]. It should be pointed out that a manifestation of a Th2-type response is not equal to an induced Th1 to Th2 immune deviation manipulation. If they have equal effects in vivo, logically, the Th2 biased polarization is only to induce weak tolerance rather than a sufficient condition to block immune response.
The evidence indicates that Th2 cytokine production to either graft survival or rejection is based on assessing intragraft cytokine gene expression by RT-PCR or identifying graft infiltrating cells that stain for cytokine protein by immunohistochemistry within allografts. As mentioned by Piccotti et al[29], these assays do not take into account the antigen specificity of the cells producing cytokines. Limiting dilution analysis studies have revealed that the frequency of donor-specific T cells infiltrating grafts is very low. Hence, RT-PCR may detect cytokine mRNA produced by irrelevant cells that are trafficking through the graft. We also found that the cytokines such as IFN-γ and IL-4 expressed by the grafts had no difference. This observation suggested that the evaluation of intragraft cytokine profiles for Th1/Th2 dominance should be viewed carefully. We accepted that all of the cytokine profile reflected a Th2 bias in the graft location, there was no solid evidence to indicate that Th2 cells are really protective as we had previously thought.
It should be pointed out that IL-12p40 is the common-chain of IL-12 and IL-23. Thus, the IL-12p40-deficient mice have both cytokines knocked out. Recently, the important role of IL-23 in autoimmune diseases and pathological inflammatory processes has been unraveled. IL-23 drives the development of a novel T-cell subset characterized by the production of IL-17 (ThIL-17), which plays a central role in mediating chronic inflammatory responses[38]. It is not clear whether IL-23 will have any impact on allograft rejection. Our results suggested that a lack of IL-23 in p40-deficient mice did not inhibit islet transplantation.
In conclusion, the islet allograft survival is not prolonged in IL-12p40-deficient mice as compared to WT controls. We conclude that Th cell activation rather than Th1/Th2 polarization plays the major role in controlling allograft rejection. The precise mechanism for the failure of Th1 to Th2 immune deviation in allograft rejection in MHC-mismatched allograft recipients will require further investigation.
References
- Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145-73.
- Tanigawa K, Ohguni S, Kawaguchi M, Kato Y, Tanigawa K, Ohguni S, et al. Insulin release from the pancreas and fuel metabolism during late gestation in chemically diabetic rats. Endocrinol Jpn 1990;37:709-17.
- Onodera K, Hancock WW, Graser E, Lehmann M, Sayegh MH, Strom TB, et al. Type 2 helper T cell-type cytokines and the development of “infectious” tolerance in rat cardiac allograft recipients. J Immunol 1997;158:1572-81.
- Qin L, Chavin KD, Ding Y, Tahara H, Favaro JP, Woodward JE, et al. Retrovirus mediated transfer of viral IL-10 gene prolongs murine cardiac allograft survival. J Immunol 1996;156:2316-23.
- Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB. CD28–B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med 1995;181:1869-74.
- Nicolls MR, Coulombe M, Diamond AS, Beilke J, Gill RG. Interferon-gamma is not a universal requirement for islet allograft survival. Transplantation 2002;74:472-7.
- Nickerson PW, Zheng XX, Steiger J, Steele AW, Steurer W, Roy-Chaudhury P, et al. Prolonged islet allograft acceptance in the absence of interleukin-4 expression. Transpl Immunol 1996;4:81-5.
- Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol 1995;54:5071-9.
- Ha SJ, Kim DJ, Baek KH, Yun YD, Sung YC. IL-23 induces stronger sustained CTL and Th1 immune responses than IL-12 in hepatitis C virus envelope protein 2 DNA immunization. J Immunol 2004;172:525-31.
- Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 1996;4:471-81.
- Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol 2003;24:207-12.
- Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol 2002;169:7104-10.
- Tarrant TK, Silver PB, Chan CC, Wiggert B, Caspi RR. Endogenous IL-12 is required for induction and expression of experimental autoimmune uveitis. J Immunol 1998;161:122-7.
- Nitta Y, Kawamoto S. IL-12 plays a pathologic role at the inflammatory loci in the development of diabetes in NOD mice. J Autoimmun 2001;16:97-104.
- Sun W, He X, Guo Z, Wang Q, Li X, Rayner J, et al. IL-12p40-over expressing immature dendritic cells induce T cell hypo-responsiveness in vitro but accelerate allograft rejection in vivo: role of NK cell activation and interferon-gamma production. Immunol Lett 2004;94:191-9.
- Phillips NE, Markees TG, Mordes JP, Greiner DL, Rossini AA. Rossini blockade of CD40-mediated signaling is sufficient for inducing islet but not skin transplantation tolerance. J Immunol 2003;170:3015-23.
- Salvalaggio PR, Deng S, Ariyan CE, Millet I, Zawalich WS, Basadonna GP, et al. Islet filtration: a simple and rapid new purification procedure that avoids ficoll and improves islet mass and function. Transplantation 2002;74:877-9.
- Abdi R, Smith RN, Makhlouf L, Najafian N, Luster AD, Auchincloss H Jr, et al. The role of CC chemokine receptor 5 (CCR5) in islet allograft rejection. Diabetes 2002;51:2489-95.
- Konnai S, Usui T, Ohashi K, Onuma M. The rapid quantitative analysis of bovine cytokine genes by real-time RT-PCR. Vet Microbiol 2003;94:283-94.
- Mabley JG, Hasko G, Liaudet L, Soriano F, Southan GJ, Salzman AL, et al. NFkappaB1 (p50)-deficient mice are not susceptible to multiple low-dose streptozotocine-induced diabetes. J Endocrinol 2002;173:457-64.
- Grajwer LA, Pildes RS, Horwitz DL, Rubenstein AH. Control of juvenile diabetes mellitus and its relationship to endogenous insulin secretion as measured by C-peptide immunoreactivity. J Pediatr 1977;90:42-8.
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol 1995;13:251-76.
- Rabinovitch A, Suarez-Pinzon WL, Sorensen O. Interleukin-12 mRNA expression in islets correlates with beta-cell destruction in NOD mice. J Autoimmun 1996;9:645-51.
- Rothe H, Burkart V, Faust A, Kolb H. Interleukin-12 gene expression is associated with development of diabetes mellitus in non-obese diabetic mice. Diabetologia 1996;39:119-22.
- Trembleau S, Penna G, Bosi E, Mortara A, Gately MK, Adorini L. Interleukin-12 administration induces T helper type I cells and accelerates autoimmune diabetes in NOD mice. J Exp Med 1995;181:817-21.
- Takeuchi T, Lowry RP, Konieczny B. Heart allografts in murine systems: the differential activation of Th2-like effector cells in peripheral tolerance. Transplantation 1992;53:1281-94.
- Chen N, Gao Q, Field EH. Prevention of Th1 responses is critical for tolerance. Transplantation 1996;61:1076-83.
- Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 development pathways: application to autoimmune disease therapy. Cell 1995;80:707-18.
- Piccotti JR, Chan SY, VanBuskirk AM, Eichwald EJ, Bishop DK. Are Th2 helper T lymphocytes beneficial, deleterious, or irrelevant in promoting allograft survival? Transplantation 1997;63:619-24.
- Zheng XX, Steele AW, Hancock WW, Stevens AC, Nickerson PW, Roy-Chaudhury P, et al. Strom. A noncytolytic IL-10/Fc fusion protein prevents diabetes, blocks autoimmunity, and promotes suppressor phenomena in NOD mice. J Immunol 1997;158:4507-13.
- Piccotti JR. IL-12 antagonism induces Th2 responses, yet exacerbates cardiac allograft rejection. J Immunol 1996;157:1951-7.
- Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in NOD mice. J Exp Med 1996;184:1093-9.
- Williamson E, Garside P, Bradley JA, More IA, Mowat AM. Neutralizing IL-12 during induction of murine acute graft-versus-host disease polarizes the cytokine profile toward a Th2-type alloimmune response and confers long-term protection from disease. J Immunol 1997;159:1208-15.
- Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin-12. J Exp Med 1995;181:381-6.
- Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin-12 abrogate established experimental colitis in mice. J Exp Med 1995;182:1281-90.
- Gavett SH, O’Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med 1995;182:1527-36.
- Li XC, Zand MS, Li Y, Zheng XX, Strom TB. On histocompatibility barriers, Th1 to Th2 immune deviation, and the nature of the allograft responses. J Immunol 1998;161:2241-7.
- Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 2004;202:96-105.
