Design and screening of antisense oligodeoxynucleotides against PAI-1 mRNA in endothelial cells in vitro1
Introduction
As more and more gene sequences, mostly of unknown biological function, become available through genome sequencing efforts, antisense oligonucleotides (ASODNs) are widely used for the elucidation of gene and protein function and as therapeutic agents in clinical trials[1,2]. But not all ASODN are equally effective in their ability to inhibit gene expression and protein synthesis[3,4]. To date, the screening of multiple sequences has been the most familiar way to identify effective antisense sequences.
Type-1 plasminogen activator inhibitor (PAI-1) is the physiological inhibitor of both tissue-type (t-PA) and urokinase-type plasminogen activator and plays an important role in the process of fibrinolysis and thrombus formation. PAI-1 is expressed in vascular endothelial cells and smooth muscle cells. Overexpression of PAI-1 is strongly associated with life-threatening thrombotic diseases[5–8] in atherosclerosis, myocardial infarction, deep-vein thrombosis and gram negative sepsis.
The aim of this study is to design some ASODNs that hybridize to various target sites of PAI-1 mRNA and evaluate their inhibitory effect on PAI-1 expression in cultured human umbilical vein endothelial cells (HUVEC), which will then provide a basis for further screening of thrombolytic ASODN.
Materials and methods
ASODNs design and modification PAI-1 mRNA reported by Ginsburg et al (Genebank accession: M16006)[9] was used as a target sequence for design of ASODNs. The secondary structure of PAI-1 mRNA was simulated by software of RNA-structure 3.6[10]. Twenty seven antisense sequences targeted to different sites of PAI-1 mRNA were designed (Table 1), including two against the initiation site, twelve against the translation area and thirteen against the 3' side. The ASODNs were synthesized by a DNA synthesizer (ABI 3900, Weiter-stadt, Germany) and modified by phosphoramidite solid-phase approach[11]. The sulphurization step was performed by means of bis(O,O-disopropoxy phosphinothioyl) disulfide. After standard cleavage from the support, the protection oligonucleotide was subjected to double reverse phase HPLC purification, followed by Na+-ion exchange. Their purity was routinely controlled by polyacrylamide gel electrophoresis (PAGE) and was not lower than 95%.
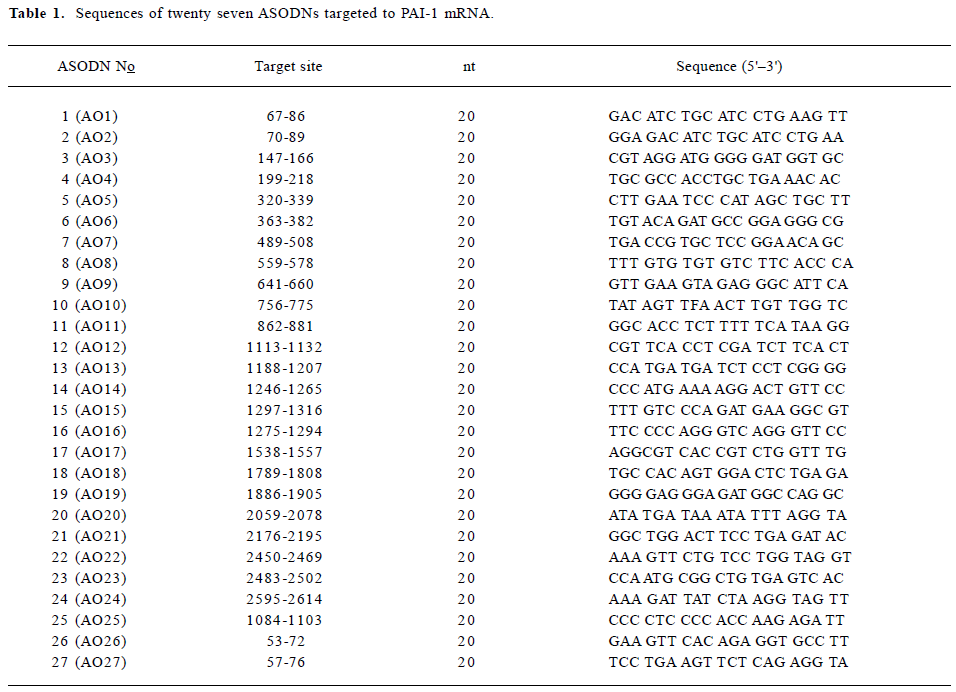
Full table
Cell culture and ASODNs delivery HUVEC was kindly provided by Dr Xiao NAN (Surgery Institute of Third Military Medical University, Chongqing, China). HUVEC were grown to confluence on 48 wells of fibronectin-coated culture plates with DMEM medium supplemented with 10% fetal calf serum (Hyclone, Logan, UT, USA), 100 U/mL penicillin and 100 µg/mL streptomycin for 48 h before transfection of ASODNs. The media was replaced with serum-free media containing 10 µg/mL lipofectin (Invitrogen, Carlsbad, CA, USA) and various concentrations of ASODNs (0.0, 0.1, 0.25, 0.5, and 1.0 µmol/L), and were incubated for 6 h at 37 °C. Cells were stimulated by transforming growth factor β1 (TGF-β1, Roche Diagnostics, Mannheim, Germany) with a concentration of 0.5 ng/mL (for increasing PAI-1 concentration in media, as normal concentration is extremely low) for 48 h before collecting media. The control group was treated only with TGF-β1 0.5 ng/mL without the use of ASODNs. The HUVEC were used for total RNA extraction and the media were stored at -70 °C until assay for PAI-1 antigen and activity.
PAI-1 antigen assay PAI-1 antigen in conditioned medium was determined by specific enzyme-linked immuno-sorbent assay (ELISA)[12] kits (Diagnostica Stago, American Bioproducts, Parsipanny, New Jersey, USA). In brief, 50 µL of media was incubated with PAI-1 monoclonal antibodies that precoated the flat bottom of 96-well cell culture plates (Corning Laboratories, Corning, NY, USA) for 1 h at 37 °C. Peroxidase conjugated anti-PAI-1 antibody 0.1 mL was added to all wells and incubated for 1 h at 37 °C. Tetramethyl-benzidine (TMB) substrate 0.1 mL was added to all wells and incubated for 10 min at room temperature. Stop solution 0.1 mL was added to each well. The PAI-1 Ag in the media was measured by reading the absorbance at 450 nm and calculated against standard regression line.
PAI-1 activity assay PAI-1 activity was measured by amidolytical assay[13]. One unit PAI-1 activity was defined as the amount of PAI that inhibited one international unit of human single-chain t-PA.
PAI-1 mRNA expression by RT-PCR The primers were designed by computer assistance according to the gene bank. PAI-1: forward, 5'-CGGAGCACGGTCAAGCAAGTG-3'; reverse, 5'-GTTGAGGGCAGAGAGAGGCGC-3', the size of amplified fragment is 401 bp. Internal control GAPDH: forward, 5'-CCATGGAGAAGGCTGGGG-3'; reverse, 5'-CAA-AGTTGTCA-TGGATGACC-3'; the size of amplified fragment is 195 bp. Total RNA from each sample was isolated using TRIzol solution (Invitrogen). Total RNA was quantified with the ratio of absorption values of RNA samples at 260 nm and 280 nm. For each sample, 4 µg of total RNA was reverse transcribed into the first strand of cDNA in a 20-µL reaction system at 37 °C for 50 min. Then polymerase chain reaction was performed from the synthesized cDNA in a 50 µL solution containing 3 µL of cDNA, 1 µL of 25 mmol primers (up-stream and down-stream) of PAI-1, 0.5 µL of 25 mmol primers (up-stream and down-stream) of GAPDH, 10 mmol dNTP 1 µL, 25 mmol MgCI2 4 µL, 10×buffer 5 µL, 0.3 µL of Taq DNA polymerase (Qiagen, Valenca, CA, USA). Amplification was performed in a thermal cycler (Bio-Rad, Alfred Nobel Drive Hercules, CA, USA) under the following conditions: 26 cycles of denaturation at 94 °C for 50 s, annealing at 59 °C for 45 s, extension at 70 °C for 40 s, followed by a final extension for 5 min. PCR product 10 µL was electrophoresed on a 2% agarose gel, and stained with EB. The PAI-1 mRNA level in each sample was semi-quantified by comparing the intensities of PAI-1 mRNA band with those of the internal control GAPDH band.
Statistics assay Data were shown as mean±SD. Statistical analysis was performed by analysis of variance (ANOVA). The level of statistical significance was chosen as P<0.05.
Results
Effects of ASODNs on PAI-1 Ag After 48-h transfection, in comparison to control group (290.0±57.2 ng/mL), all of the ASODNs did not significantly inhibit PAI-1 Ag at a concentration of 0.1 µmol/L. ASODN 1, 7, 14, and 15 could significantly decrease PAI-1 Ag at concentrations of 0.25, 0.5 and 1.0 µmol/L (inhibition rate: 25.9%–78.7%). ASODN 8 could also remarkably inhibit PAI-1 Ag at concentration of 0.5 and 1.0 µmol/L (inhibition rate: 60.8%, 65.1%, respectively). The inhibitory effect was in a dose-independent manner (Table 2). These data suggested that five among twenty seven designed ASODNs significantly inhibited PAI-1 Ag expression and AO14 exhibited the best inhibitory effect.
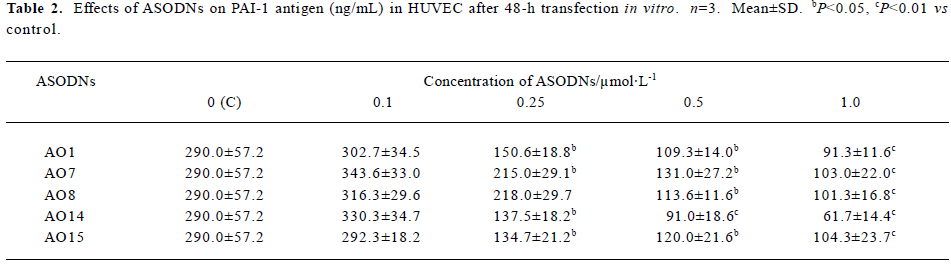
Full table
Effects of ASODNs on PAI-1 activity After 48-h trans-fection, compared to control group (15.0×10-2±1.5×10-2 AU/mL), at a concentration of 0.1 µmol/L, all of the ASODNs did not significantly inhibit PAI-1 activity. At 0.25 µmol/L, ASODN 1, 14, and 15 significantly reduced PAI-1 activity (inhibition rate: 41.3%, 40.0%, 33.3%, respectively). At 0.5 and 1.0 µmol/L, ASODN 1, 7, 8, 14, and 15 remarkably decreased PAI-1 activity (inhibition rate: 40.0%–52.7%), These data (Table 3) suggested that five ASODNs that inhibited PAI-1 Ag could also inhibit PAI-1 activity and AO14 showed the best inhibitory effect.
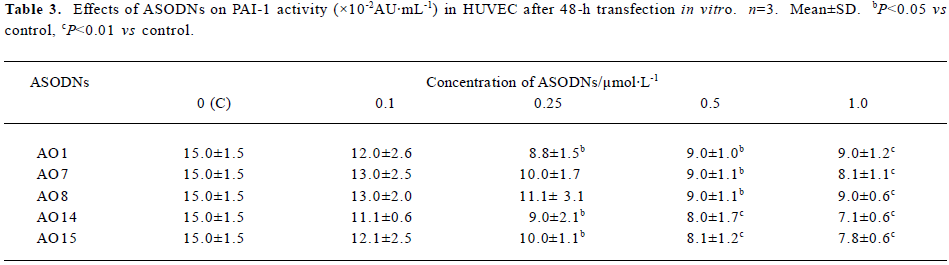
Full table
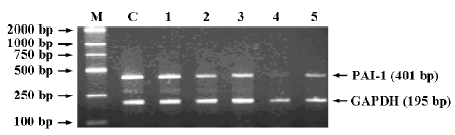
Effects of ASODNs on expression of PAI-1 mRNA After 48-h transfection, at a concentration of 1.0 µmol/L, ASODN 1, 7, 8, 14, and 15 significantly decreased PAI-1 mRNA expression induced by TGF-β1. The inhibition rates were 25.3%, 43.3%, 40.0%, 86.3%, 61.4%, respectively, when compared with the control (Figure 1).
Specific inhibitory effects of ASODNs on PAI-1 activity Positive results of decrease PAI-1 expression by five ASODNs in cultured endothelial cells prompted us to investigate further the specificity of action of the ASODNs. Because AO14, among twenty seven designed ASODNs, was confirmed to have the best efficiency in inhibiting PAI-1 mRNA, PAI-1 Ag and PAI-1 activity, we designed and synthesized corresponding control oligonucleotides of AO14, including sense (AO28), scrambled (AO29), and an oligonucleotide with two mismatches (AO30). Their nucleotide sequences are listed in Table 4. Transfection (1.0 µmol/L) of these control oligonucleotides to HUVEC by lipofectin was under the same experimental condition as AO14. The results showed that sense, scrambled, and mismatched oligonucleotides of AO14 did not have an inhibitive effect on PAI-1 activity after 48-h of transfection (Table 4). This suggests that the inhibitory effect of AO14 on PAI-1 activity was indeed of specific antisense origin.
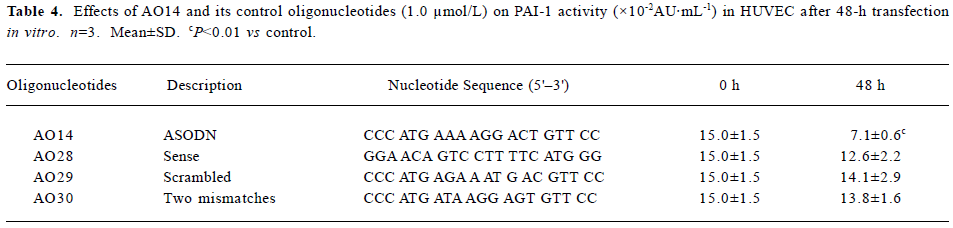
Full table
Discussion
Antisense strategy is a novel approach for inhibiting target gene expression by ASODNs complementary to preselected regions of mRNA by Watson-Crick base pairing. So ASODNs have been proposed as potential therapeutic agents for inhibiting candidate genes that may account for specific diseases[1,2]. But not every site of mRNA sequence is accessible to hybridization with ASODNs. A major obstacle in employing ASODN is the election of target sites within nucleotide sequences for effective inhibition of expression[14]. Several reports suggest that the most effective target sites are either the 5'-nontranslated sequences or the ATG start site for translation[15]. In the present study, we designed twenty seven ASODNs (20 nt) that are complementary to PAI-1 mRNA local sites, including the initiation site, translation field and 3' side, and transfected them respectively into cultured HUVEC by lipofectin. The results showed that five among twenty seven designed ASODNs significantly decreased PAI-1 mRNA, PAI-1 antigen and PAI-1 activity. The effective target sites were located as follows: 1 (total 2) at initiation site, 3 (total 12) at translation field and 1 (total 13) at 3' side. The results showed that the efficiency of ASODNs against the initiation site (1/2) and translation field (3/12) were higher than 3' side (1/13) and 5' side (0/2), which showed that not only the initiation site but also the translation field were promising sites for ASODNs design (because the 5' side of PAI-1 mRNA is too short, we designed only two ASODNs there).
PAI-1 is one of the most important factors in the pathogenesis of thrombosis[5–8]. Overexpression of PAI-1 is associated with thrombotic disease in atherosclerosis, myocardial infarction, and gram negative sepsis. A similar effect was also observed in transgenic mice, indicating that individuals with elevated concentrations of the inhibitor in their blood tend to be at risk of developing thrombotic problems. However, some researchers have shown that neutralization of plasma PAI-1 by monoclonal antibody[16], small molecular weight inhibitors[7,17] or synthetic peptides[18] would remarkably increase fibriolysis and protect against thrombus formation. In the present study, twenty seven ASODNs against PAI-1 mRNA were designed and transfected to HUVEC in vitro. The results showed that ASODN 1, 7, 8, 14, and 15 were effective in inhibiting the increase in PAI-1 antigen and PAI-1 activity, which was induced by TGF-β1 after 48 h of transfection, and AO14 showed the best inhibitory effect. In addition to determination of the protein level under suppression of the translation process by antisense constructs, RT-PCR of mRNA for PAI-1 was performed for mRNAs isolated from HUVEC untreated (control) and treated with ASODN 1, 7, 8, 14, and 15. Only cells treated with ASODN 1, 7, 8, 14, and 15 showed a decrease in PAI-1 mRNA. These results indicate that inhibition of PAI-1 biosynthesis occurs at the mRNA level. Among five effective ASODNs, AO14 was found to be the most efficient inhibitor of PAI-1 synthesis in cultured HUVEC. For further determination of the antisense mechanism of sequence-specific ASODN, the control sequences of AO14, including sense, scramble, and mismatch sequences (Table 4), were tested under the same experimental conditions. The results indicated that the control sequences of AO14 did not significantly inhibit PAI-1 activity. This shows that the inhibitory efficacy of AO14 was not only in a dose-dependent manner but also in a sequence-specific manner in HUVEC in vitro.
The remarkable advantage of antisense strategy in specificity of action suggests that the prevention and treatment of thrombotic disease by ASODNs may be a potential method that is worthy of further exploration.
References
- Aboul-Fadl T. Antisense oligonucleotides: the state of the art. Curr Med Chem 2005;12:2193-214.
- Gleave ME, Monia BP. Antisense therapy for cancer. Nat Rev Cancer 2005;5:468-79.
- Peyman A, Helsberg M, Kretzschmar G, Mag M, Grabley S, Uhlmann E. Inhibition of viral growth by antisense oligonucleotides directed against the IE110 and the UL30 mRNA of herpes simplex virus type-1. Biol Chem 1995;376:195-8.
- Monia BP, Johnston JF, Geiger T, Muller M, Fabbro D. Antitumor activity of a phosphorothioate antisense oligodeoxynucleo-tide targeted against C-raf kinase. Nat Med 1996;2:668-75.
- Nordenhem A, Leander K, Hallqvist J, de Faire U, Sten-Linder M, Wiman B. The complex between tPA and PAI-1: risk factor for myocardial infarction as studied in the SHEEP project. Thromb Res 2005;116:223-32.
- Rupin A, Martin F, Vallez MO, Bonhomme E, Verbeuren TJ. Inactivation of plasminogen activator inhibitor-1 accelerates thrombolysis of a platelet-rich thrombus in rat mesenteric arterioles. Thromb Haemost 2001;86:1528-31.
- Liang A, Wu F, Tran K, Jones SW, Deng G, Ye B, et al. Characterization of a small molecule PAI-1 inhibitor, ZK4044. Thromb Res 2005;115:341-50.
- Hasenstab D, Lea H, Clowes AW. Local plasminogen activator inhibitor type 1 overexpression in rat carotid artery enhances thrombosis and endothelial regeneration while inhibiting intimal thickening. Arterioscler Thromb Vasc Biol 2000;20:853-9.
- Ginsburg D, Zeheb R, Yang AY, Rafferty UM, Andreasen PA, Nielsen L, et al. cDNA cloning of human plasminogen activator-inhibitor from endothelial cells. J Clin Invest 1986;78:1673-80.
- Mathews DH, Burkard ME, Ferier SM, Wgatt JR, Turner DH. Predicting oligonucleotide affinity to nucleic acid targets. RNA 1999;5:1458-69.
- Stec WJ, Uznanski B, Wilk A, Hirschbein BL, Fearon KL, Bergot BJ. Bis(O,O-diisopropoxy phosphinothioyl) disulfide–a highly efficient sulfurizing reagent for cost-effective synthesis of oligo(nucleoside phosphothioate)s. Tetrahedron Lett 1993;34:5317-20.
- Declerck PJ, Alessi MC, Werstreken M, Kruithof EKO, Juhan-Vague I, Collen D. Measurement of plasminogen activator inhibitor-1(PAI-1) in biological fluids, with a murine monoclonal antibody based enzyme-linked immunosorbent assay. Blood 1988;71:220-5.
- Eriksson E, Ranby M, Gyzander E, Risberg B. Determination of plasminogen activator inhibitor in plasma using tPA and a chromogenic single-point poly-D-lysine stimulated assay. Thromb Res 1988;50:91-105.
- Patzel V, Steidl U, Kronenwett R, Haas R, Sczakiel G. A theoretical approach to select effective antisense oligodeoxyribonucleo-tides at high statistical probability. Nucleic Acids Res 1999;27:4328-34.
- Chiang MY, Chan H, Zounes MA, Freier SM, Lima WF, Bennett CF. Antisense oligonucleotides inhibit intercellular adhesion molecular 1 expression by two distinct mechanisms. Biol Chem 1991;266:18162-71.
- Biemond BJ, Levi M, Coronel R, Janse MJ, ten Care JW, Pannekoek H. Thrombolysis and reocclusion in experimental jugular vein and coronary artery thrombosis: effects of a plasminogen activator inhibitor type 1 neutralizing monoclonal antibody. Circulation 1995;91:1175-81.
- Crandall DL, Elokdah H, Di L, Hennan JK, Gorlatova NV, Lawrence DA. Characterization and comparative evaluation of a structurally unique PAI-1 inhibitor exhibiting oral in-vivo efficacy. J Thromb Haemost 2004;2:1422-8.
- Eitzman DT, Fay PW, Lawrence DA, Francis-Chmura AM, Shore JD, Olson ST, et al. Peptide-mediated inactivation of recombinant and platelet plasminogen activator inhibitor-1 in vitro. J Clin Invest 1995;95:2416-20.
