Effects of nine antihypertensive drugs on blood pressure variability in sinoaortic-denervated rats1
Introduction
Blood pressure (BP) is not constant and a spontaneous variation exists. This variation is defined as blood pressure variability (BPV). The arterial baroreflex acts as an effective buffer of short-term BP fluctuations and prevents excessive BP swings[1]. The baroreflex arc may be interrupted by sinoaortic denervation (SAD) or complete lesion of the nucleus tractus solitarus[2,3]. In these animals with baroreflex dysfunction, BPV is dramatically high[4]. Recently, SAD animals have been used as a high-BPV model for studying the physiology, pathology and pharmacology related to BPV.
It is reported that BPV is positively related to the severity of organ damage in hypertensive patients[5,6]. In animal studies, we confirmed that BPV was related to the severity of organ damage in spontaneously hypertensive rats (SHR)[4,7]. Furthermore, a positive relationship was also reported to exist in SAD rats[4,7]. This means that BPV alone without hypertension could induce organ damage as the average BP level is normal in SAD rats. Recently, we found that the importance of BPV for determining the organ damage was even greater than the BP level[8,9]. Obviously, an antihypertensive drug with a BP-stabilizing effect would benefit the hypertensives. Therefore, it is reasonable to take decreasing BPV into account in the treatment of hypertension and it is proposed that BPV reduction may be a new strategy for the treatment of hypertension[8]. Indeed, it has been found that BPV reduction has an important contribution to organ protection in the long-term treatment of nitrendipine or ketanserin in SHR[10,11].
Clinically, it will be important to know which antihypertensive drugs possess an effect on BPV reduction among more than 50 agents available. Therefore, the present work was designed to observe the effects of some representative antihypertensive drugs on BPV in SAD rats. Nine drugs were selected and included in this study: nifedipine, nitrendipine, amlodipine (calcium antagonists), clonidine (centrally acting agent), prazosin (α1 adrenoceptor blocker), atenolol (β1 adrenoceptor blocker), telmisartan (AT1 receptor blocker), captopril (angiotensin I converting enzyme inhibitor) and hydrochlorothiazide (diuretic).
Materials and methods
Animals and drugs Male Sprague-Dawley rats were provided by Sino-British SIPPR/BK Lab Animals (Shanghai, China). They were housed in controlled temperatures (22–25 ºC) and lighting (08:00 to 20:00 light; 20:00 to 08:00 dark), with free access to tap water and rat chow. All procedures were in accordance with institutional animal care guidelines.
Nifedipine, hydrochlorothiazide, prazosin, clonidine, telmisartan and captopril were purchased from Sigma Chemical Co (St Louis, MO, USA). Amlodipine was provided by Nanjing Pharmaceutical Co (Nanjing, China). Nitrendipine and atenolol were provided by Shanghai Second Pharmaceutical Co (Shanghai, China). Nifedipine, nitrendipine, telmisartan and hydrochlorothiazide were dissolved in the 0.5% carboxymethylcellulose sodium (CMC). Amlodipine, captopril, atenolol and clonidine were dissolved in distilled water. Prazosin was suspended in polyethylene glycol (PEG) and diluted with distilled water for a final concentration of 0.2 mg/mL (PEG, 0.5%)[12]. The doses used were as follows: nifedipine 3 mg/kg, nitrendipine 5 mg/kg, amlodipine 1 mg/kg, clonidine 10 µg/kg, prazosin 0.5 mg/kg, atenolol 20 mg/kg, telmisartan 20 mg/kg, hydrochlorothiazide 40 mg/kg and captopril 50 mg/kg. Drugs were administered by intra-gastric catheter implanted 3 days before the experiment.
Sinoaortic denervation At the age of 10–11 weeks, sinoaortic denervation was performed in Sprague-Dawley rats according to the method described in a previous study[13]. Rats were anesthetized with a mixture of ketamine (50 mg/kg) and diazepam (5 mg/kg), intraperitoneally. Atropine sulfate (0.5 mg/kg) was then administered intraperitoneally. Each animal was fixed in a supine position and a 2.5 cm midline incision was made in the neck. After bilateral isolation of the neck muscles, aortic baroreceptor denervation was carried out bilaterally by cutting the superior laryngeal nerves near the vagi, removing the sympathetic trunk, and sectioning aortic depressor nerves. The carotid sinus baroreceptors were denervated bilaterally by stripping the carotid bifurcation and its branches, followed by the application of 10% phenol in 95% ethanol solution to the external, internal, and common carotid arteries and the occipital artery. The incision was sutured closed and each rat was injected intramuscularly with 60000 units of penicillin G.
Blood pressure measurement Four weeks after sinoaortic denervation, rats were anesthetized as mentioned. A polyethylene catheter (PE-10 connected to PE-50) was chronically inserted into the lower abdominal aorta via the left femoral artery for the measurements of BP and heart period (HP), and another catheter (PE-50) was inserted into the left femoral vein for the examination of baroreflex function. The third catheter (PE-50) was inserted into the stomach for drug administration. These catheters were tunneled subcutaneously, exteriorized between the scapulae, and fixed on saddle. After 2 days of recovery, BP was continuously recorded in conscious unrestrained rats with a computerized technique (MPA 2000M, Alcott Biotech, Shanghai, China)[13,14]. The BP signals, transmitted to the electric signals by a transducer, were digitized and processed on a personal computer, which calculated online the BP and HP beat by beat. The average value was used as an index of BP, and the standard deviation (SD) of beat-to-beat BP values as an index of BPV including systolic (SBPV) and diastolic BPV (DBPV)[14]. The same method was used for the calculation of HP. In SAD rats, arterial baroreflex function for heart-rate control was also assessed by intravenous injection of phenylephrine, 2 to 5 µg/kg. If SAD rats exhibit a bradycardia of less than 20 beats/min, they were considered as successful baroreceptor denervation and included in the study[13,14].
Protocol After approximately 4-h habituation to BP recording environment, the BP signal was recorded for half an hour as the basal (pre-drug) value. The drug was then administered via the catheter of gastric fistula. One and a half hours after administration of nifedipine, nitrendipine, clonidine, atenolol, hydrochlorothiazide and captopril, 2.5 h after administration of prazosin and 3.5 h after administration of amlodipine and telmisartan, the BP signal was recorded for half an hour as post-drug value.
Statistical analysis Data were expressed as mean±SEM. Comparisons between values obtained in the same group before and after drug administration were made using the paired t - test. P<0.05 was considered statistically significant.
Results
Effects of nine antihypertensive drugs on BP in SAD rats The effects of nine antihypertensive drugs on BP in conscious SAD rats are presented in Figure 1. It was found that SBP was significantly decreased after the administration of all of these nine drugs. This decrease in SBP was approximately 20 mmHg (19–25 mmHg) for nifedipine, nitrendipine, amlodipine, clonidine, prazosin, atenolol, and telmisartan; and was approximately 16 mmHg for hydrochlorothiazide and captopril. DBP was also found to be significantly reduced by these nine drugs in SAD rats.

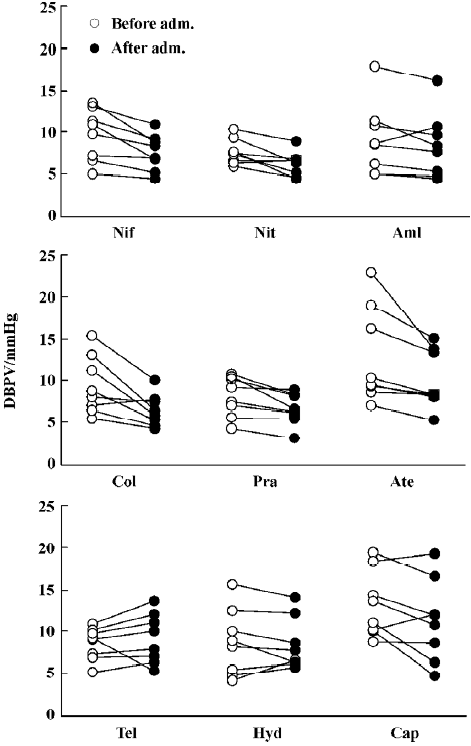
Effects of nine antihypertensive drugs on BPV in SAD rats The effects of nine antihypertensive drugs on SBPV in SAD rats are shown in Figure 2. It was found that nifedipine, nitrendipine, amlodipine, clonidine, prazosin and atenolol all significantly decreased SBPV. Nifedipine decreased SBPV from 11.0±1.2 mmHg to 9.1±0.9 mmHg. Nitrendipine decreased SBPV from 9.7±0.6 mmHg to 7.7±0.6 mmHg. Amlodi-pine decreased SBPV from 11.8±2.2 mmHg to 10.0±1.8 mmHg. Clonidine decreased SBPV from 11.8±1.4 mmHg to 8.2±0.8 mmHg. Prazosin decreased SBPV from 12.4±1.4 mmHg to 8.9±0.6 mmHg. Atenolol decreased SBPV from 13.1±1.8 mmHg to 9.5±1.0 mmHg. Although telmisartan, hydrochlorothiazide and captopril significantly decreased SBP to a similar extent with nifedipine and other drugs as shown in Figure 1, the values of SBPV were unchanged (Figure 2).
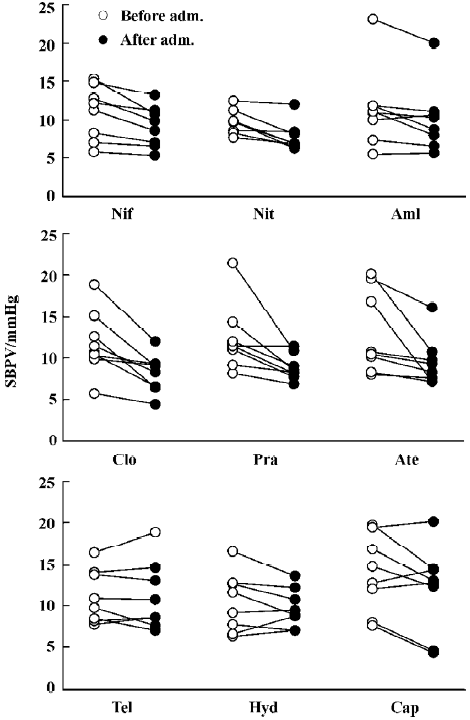
Effects of nine antihypertensive drugs on DBPV in SAD rats Figure 3 shows the results of 9 antihypertensive drugs on DBPV in SAD rats. It was found that nifedipine, nitrendi-pine, amlodipine, clonidine, prazosin and atenolol all significantly decreased DBPV, similar to SBPV. DBPV was decreased from 9.7±1.1 mmHg to 7.6±0.8 mmHg for nifedipine. DBPV was decreased from 7.6±0.5 mmHg to 6.2±0.5 mmHg for nitrendipine. DBPV was decreased from 9.3±1.8 mmHg to 8.1±1.6 mmHg for amlodipine. DBPV was decreased from 9.4±1.2 mmHg to 6.5±0.7 mmHg for clonidine. DBPV was decreased from 8.1±0.8 mmHg to 6.6±0.6 mmHg for prazosin. DBPV was decreased from 12.8±2.0 mmHg to 10.1±1.2 mmHg for atenolol. Although telmisartan, hydrochlorothiazide and captopril significantly decreased DBP to a similar extent with nifedipine and other drugs as shown in Figure 1, the values of DBPV were unchanged (Figure 3).
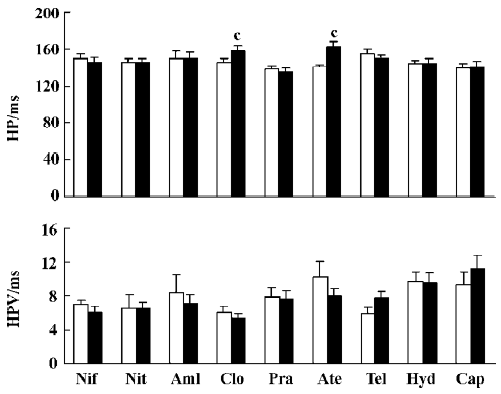
Effects of nine antihypertensive drugs on HP and HPV after sinoaortic denervation Among these nine antihypertensive drugs, only clonidine and atenolol prolonged HP in SAD rats. There were no significant changes in HPV for any drugs studied in SAD rats (Figure 4).
Discussion
During the past four decades, several classes of antihypertensive agents-calcium channel blockers, centrally acting antihypertensive agents, α1-adrenoceptor antagonist, β-blockers, AT1 receptor blockers, diuretics and angiotensin-converting enzyme inhibitors have been shown to effectively reduce elevated blood pressure and associated cardiovascular events[15–18]. The present work for the first time systematically studied and clearly demonstrated the effects of these representative drugs on reducing BP and BPV in SAD rats. The main finding of the present work may be summarized as follows: (1) calcium antagonists (nifedipine, nitrendipineand amlodipine) significantly decreased BPV in SAD rats; (2) antihypertensive drugs inhibiting sympathetic nervous system (clonidine, prazosin and atenolol) significantly decreased BPV in SAD rats; (3) other antihypertensive drugs, such as telmisartan, hydrochlorothiazide and captopril, did not significantly influence BPV in SAD rats.
Dihydropyridine calcium antagonists are the most widely used antihypertensive drugs. According to their action duration, they are divided into three subclasses. Nifedipine, nitrendipine and amlodipine are the representative drugs for the short-, medium- and long- lasting drugs, respectively. In the present work, it was found that all three drugs significantly decreased BPV in SAD rats. This means that the effects of calcium antagonists on BPV does not relate to the action duration but relate to the intrinsic property of calcium antagonism. Generally speaking, the stability of BP is maintained by arterial baroreflex in physiological conditions. Hemodynamic studies in conscious rats with neonatal chemical sympathectomy, SAD, and acute neurohumoral blockade have revealed that one major source of hemodynamic perturbations is the myogenic response of vascular smooth muscle cells[19–21]. Therefore, it is not surprising that all three calcium antagonists used decreased BPV in SAD rats by preventing this myogenic response[19].
Antihypertensive drugs inhibiting the sympathetic nervous system used in the present work were prazosin, atenolol and clonidine. All of these three drugs significantly decreased BPV in SAD rats. These results suggest that activation of the sympathetic nervous system is involved in the increased BPV after SAD. As prazosin and atenolol represent α- and β-receptor antagonists, respectively, it seems that both α- and β-receptors are involved in the SAD-induced high BPV. It is well known that arterial baroreflex modulates sympathetic and parasympathetic nervous systems and keeps the balance between these two autonomic nervous systems. The loss of this balance after SAD operation is often characterized by an over-activation of sympathetic nervous systems and a deactivation of parasympathetic nervous systems probably contributing to the elevation of BPV.
The present work also revealed that captopril and telmisartan did not influence BPV in SAD rats. This means that the activation of the renin-angiotensin system is not important in the maintenance of high BPV induced by SAD in rats. In addition, hydrochlorothiazide did not modify BPV in SAD rats. This suggests that elevated BPV may mainly be due to the variation of peripheric resistance and not to the variation of blood volume.
These results may be helpful in the selection of antihypertensive drugs. For a hypertensive patient with high BPV, a calcium antagonist, a drug inhibiting sympathetic nervous system, or the combination of two drugs from these classes would be proposed. However, it should be noted that the mechanisms for increased BPV in SAD rats and in hypertensive patients or rats may be different. In SAD rats, the increased BPV is induced purely by the loss of baroreflex control, while the mechanism for increased BPV seen in hypertension is more complicated.
We conclude that calcium antagonists and drugs inhibiting the sympathetic nervous system significantly decreased BPV in SAD rats. This suggests the involvement of the myogenic response of vascular smooth muscle cells and activation of sympathetic nervous system in SAD-induced high BPV.
References
- Jordan J, Tank J, Shannon JR, Diedrich A, Lipp A, Schroeder C, et al. Baroreflex buffering and susceptibility to vasoactive drugs. Circulation 2002;105:1459-64.
- Norman RA Jr, Coleman TG, Dent AC. Continuous monitoring of arterial pressure indicates sinoaortic denervated rats are not hypertensive. Hypertension 1981;3:119-25.
- Buchholtz RA, Nathan NA. Chronic lability of the arterial pressure produced by electrolytic lesions of the nucleus tractus solitarii in the rat. Circ Res 1984;54:227-38.
- Su DF, Miao CY. Blood pressure variability and organ damage. Clin Exp Pharmacol Physiol 2001;28:709-15.
- Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens 1987;5:93-8.
- Mancia G, Frattola A, Parati G, Santucciu C, Ulian L. Blood pressure variability and organ damage. J Cardiovasc Pharmacol 1994;24:S6-11.
- Miao CY, Su DF. The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens 2002;20:1865-72.
- Su DF, Miao CY. Reduction of blood pressure variability: a new strategy for the treatment of hypertension. Trends Pharmacol Sci 2005;26:388-90.
- Miao CY, Xie HH, Zhan LS, Su DF. Blood pressure variability is more important than blood pressure level in determination of end-organ damage in rats. J Hypertens 2006;24:1137-46.
- Liu JG, Xu LP, Chu ZX, Miao CY, Su DF. Contribution of blood pressure variability to the effect of nitrendipine on end-organ damage in spontaneously hypertensive rats. J Hypertens 2003;21:1961-7.
- Xie HH, Shen FM, Cao YB, Li HL, Su DF. Effect of low-dose ketanserin on blood pressure variability, baroreflex sensitivity and end-organ damage in spontaneously hypertensive rats. Clin Sci 2005;108:547-52.
- Gabor R, Marta M, Mark K, Emil M, Laszlo D. Experimental orthostasis elicits sustained hypertension, which can be prevented by sympathetic blockade in the rats. J Cardiovasc Pharmacol 2005;45:354-61.
- Fu YJ, Shu H, Miao CY, Wang MW, Su DF. Restoration of baroreflex function by ketanserin is not blood pressure dependent in conscious freely moving rats. J Hypertens 2004;22:1165-72.
- Xie HH, Miao CY, Jiang YY, Su DF. Synergism of atenolol and nitrendipine on hemodynamic amelioration and organ protection in hypertensive rats. J Hypertens 2005;23:193-201.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560-71.
- Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995-1003.
- William C. Are there benefits to specific antihypertensive drug therapy? AM J Hypertens 2003;16:315-55.
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A, et al. The felodipine event reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005;23:2157-72.
- Juline C. Pharmacological attenuation of blood pressure variability. Acta Pharmacol Sin 2005;26:1288-9.
- Burattini R, Borgdorff P, Westerhof N. The baroreflex is counteracted by autoregulation, thereby preventing circulatory instability. Exp Physiol 2004;89:397-405.
- Kornet L, Hoeks APG, Janssen BJA, Houben AJ, De Leeuw PW, Reneman RS. Neural activity of the cardiac baroreflex decreases with age in normotensive and hypertensive subjects. J Hypertens 2005;23:815-23.
