Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia1
Introduction
Several studies have reported that plant extracts have protective effects against ischemic damage in several organs such as the brain, heart and kidneys[1-3]. Licorice or Glycyrrhiza inflata, which is a commonly used herb, has a history of consumption for a few thousand years in both Eastern and Western cultures. It has been reported that major bioactive components of licorice are saponins, such as glycyrrhizin and glycyrrhetinic acids. In previous studies, it has been reported that glycyrrhizin has various desirable pharmacological properties such as anti-inflammatory effects[4], anti-viral effects[5] and free radical scavenging activity[6]. In recent studies it has been shown that 18-β glycyrrhetinic acid, a major component of licorice, reduces infarct size in isolated rabbit hearts[7], and that glycyrrhrizin shows a protective effect on ischemia/reperfusion or nephrotoxic injuries[8,9]. However, in some studies it was found that licorice interferred with steroid metabolism and might cause edema and hypertension[10].
The roasting process of licorice modifies the chemical composition and reduces the toxicity level[11]. Roasting of licorice under controlled conditions, including the roasting temperature and duration, results in the conversion of glycyrrhrizin to 18-β glycyrrhetinic acid[12]. Despite the considerable number of physiological studies that have been done on licorice, a comparative study between licorice and roasted licorice during in vivo ischemia has not been carried out. In the present study, therefore, we investigated the neuroprotective effects of licorice and roasted licorice on neuronal injury in the gerbil hippocampus induced by transient ischemia.
Materials and methods
Reagents Dried licorice roots were obtained from Dea Guang Medical (Chunchon, South Korea). Acetic acid was obtained from Merk (Ger, Germany). Glycyrrhetic acid (GA), glycyrrhetic acid monoglucuronide (GM) and glycyrrhizin (GL) were obtained from Sigma-Aldrich (St Louis, MO, USA). Acetonitrile was of HPLC grade, and all other chemicals were of analytical grade.
Roasting of licorice and GL Roasting of licorice was performed in a controlled manner using an oil bath. For dry-roasting, 20 g of sliced licorice was weighed into a rotary glass bottle (30 r/min) and placed in the heated oil bath at 150 °C for 100 min. To determine whether roasting of licorice caused the thermal decomposition of GL, the decomposition of GL in powder form was also studied by heating. For roasting, 10 mg of powder was weighed into a glass vial and placed in the heated oil bath at 150 °C for 30 min.
Preparation of samples of licorice and GL before and after roasting The GL samples (raw and roasted) were dissolved in methanol and the solutions were membrane-filtered (0.45μm). After dilution with methanol, the final concentrations of the prepared raw and roasted GL solutions for HPLC analysis were 1 and 5 mg/mL, respectively. Roasted licorice roots were first ground into fine powder using a laboratory blender (Waring Model 51BL30). The licorice powder (10 g) was refluxed in 50 mL of 95% ethanol for 2 h. Boiling stones were used during reflux to minimize bumping and sample loss. This extraction procedure was repeated three times. For comparison, 10 g of unprocessed licorice (raw licorice) was refluxed similarly. The ethanol extract was dried under vacuum at a low temperature (below 40 °C). The final concentrations of the prepared licorice solutions for HPLC analysis were 5 mg/mL.
HPLC analysis HPLC system consisted of two models of P-580 pumps, a model of ASI-100 Automated sample injector, a model of STH-585 column oven, and a model of UVD 170S UV-detector (Dionex, USA). The sample was separated on a reverse-phase Phenomenex Luna C18 column (4.6 mm?250 mm ID; 5 祄). The mobile phases consisted of 2% acetic acid and acetonitril as mobile phase A and B, respectively, which were degassed ultrasonically prior to use. The gradient was as follows: an isocratic elution with 20% acetonitril for 5 min, followed by a linear gradient elution with 20%?90% acetonitril for 50 min. The overall analysis time including re-equilibration was 65 min. The column was thermostated at 28 °C and a flow-rate of 0.8 mL/min was used. UV detection was operated at 254 nm.
Culture of PC12 cells PC12 cells were grown in Dulbecco’s modified Eagle’s medium.
(DMEM) supplemented with 7% fetal calf serum, 7% horse serum, 100 μg/mL streptomycin, and 100 U/mL penicillin. The cell cultures were incubated at 37 °C in an atmosphere of 6% CO2. Medium was changed twice weekly, and the cultures were split at a 1:6 ratio once a week. Cells were washed twice with warm DMEM (without phenol red), then treated with serum-free medium. In all experiments, cells were treated with licorice before hypoxia.
Hypoxia On the day of experiment, culture media were replaced with glucose-free DMEM, then gassed with 85% N2, 10% H2, and 5% CO2 for various periods in the absence or presence of various doses of licorice extract (raw and roasted).
Lactate dehydrogenase (LDH) release assay After hypoxia for 1 or 2 h, the supernatant of cultured PC12 cells was collected for assay of LDH release. The reaction was initiated by mixing 0.1 mL of cell-free supernatant with potassium phosphate buffer containing nicotinamide adnine dinucleotide (NADH) and sodium pyruvate in a final volume of 0.2 mL in a 96-well plate. The rate of absorbance was read at 490/630 nm on an automated SpectraMAX 340 microtiter plate reader. Data were expressed as the mean percent of viable cells versus hypoxia control.
Experimental animals The progeny of male Mongolian gerbils (Meriones unguiculatus) were obtained from the Experimental Animal Center, Hallym University, Chunchon, South Korea. The animals were housed in a temperature (23 °C) and humidity (60%) controlled room with a 12-h light/12-h dark cycle and provided with food and water ad libitum. Experimental procedures and animal care conformed to the Institutional Guidelines that are in compliance with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication N
Induction of ischemia The gerbils were divided into 4 groups: normal group, vehicle (saline)-treated group, raw licorice-treated group and roasted licorice-treated group. At least 21 d before surgery, 50 and 100 mg/kg of the extract of raw or roasted licorice were injected orally using the jonde every day until gerbils were killed. Gerbils weighing 65?75 g were anesthetized with a mixture of 2.5% isoflurane (Baxtor, USA) in 33% oxygen and 67% nitrous oxide. A midline ventral incision was made in the neck. Both common carotid arteries were isolated, freed of nerve fibers, and occluded with non-traumatic aneurysm clips. Complete interruption of blood flow was confirmed by observing the central artery in the eyeball using an ophthalmoscope. After 5 min of occlusion, the aneurysm clips were removed from the common carotid arteries. Restoration of blood flow (reperfusion) was observed under the ophthalmoscope. We maintained the body (rectal) temperature under free-regulating or normothermic (37±0.5 °C) conditions with a rectal temperature probe (TR-100; YSI, USA) and thermometric blanket before, during, and after the surgery until the animals fully recovered from anesthesia. Normal animals served as controls.
Histological analysis by cresyl violet staining Seven animals in each group were anesthetized with pentobarbital sodium and perfused transcardially with 0.1 mol/L phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4) at the designated times after the surgery. Brains were removed and post-fixed in the same fixative for 6 h. The brain tissues were cryoprotected by infiltration with 30% sucrose solution overnight. Thereafter the tissues were frozen and serially cut into 30-祄 thick coronal sections on a cryostat and the sections were collected in 6-well plates containing PBS. The sections were stained with cresyl violet acetate according to the previously published procedures[13].
The number of cresyl violet-positive neurons was counted by two blinded observers at the same time using an image analyzing system equipped with a computer-based CCD camera (Software: Optimas 6.5, CyberMetrics, USA). The number of cresyl violet-positive neurons in a 1-mm diameter of the hippocampus was counted in 10 sections for each animal. The number of cresyl violet-positive neurons was compared to that of the sham-operated group.
Assay of SOD1 activity Seven animals in each group were used for SOD1 activity measurement. The activity was measured by monitoring the capacity to inhibit the reduction of ferricytochrome c by xanthine/xanthine oxidase as described by McCord and Fridovich[14]. The samples were separated by electrophoresis in 10% native polyacrylamide gels and visualized as described by Beauchamp and Fridovich[15]. Briefly, the gel was soaked in 2.45 mmol/L nitroblue tetrazolium solution for 15 min, and then in 28 mmol/L N,N,N',N'-tetramethylethylene diamine, 28μmol/L riboflavin, and 0.36 mmol/L potassium phosphate buffer (pH 7.8) for 30 min. The gel was then exposed to a fluorescence light source until the bands showed maximum resolution.
Western blotting of SOD1 protein Seven animals in each group (the same animals as in the analysis of SOD1 activity) were used for an immunoblotting study. The hippocampus were removed and sectioned into 400-μm thick coronal slices on a Vibratome (Leica, Germany), and the hippocampal CA1 region was dissected with a surgical blade. The tissues were homogenized using an electrical homogenization machine in 50 mmol/L PBS (pH 7.4) containing 0.1 mmol/L egtazic acid (pH 8.0), 0.2% NP-40, 10 mmol/L edetic acid (pH 8.0), 15 mmol/L sodium pyrophosphate, 100 mmol/L β-glycerophosphate, 50 mmol/L NaF, 150 mmol/L NaCl, 2 mmol/L sodium orthvanadate, 1 mmol/L PMSF, and 1 mmol/L DTT. After centrifugation at 10 000×g, the protein concentration was determined in the supernatants by using the Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce Chemical, USA). Aliquots containing 20μg total protein were boiled in loading buffer containing 150 mmol/L Tris (pH 6.8), 3 mmol/L DTT, 6% SDS, 0.3% bromophenol blue, and 30% glycerol. Each aliquot was then loaded onto a 10% polyacryamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Schleicher and Schuell, USA). To reduce background staining, the filters were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 min, followed by incubation with rat anti-mouse SOD1 antiserum (1:400) with peroxidase conjugated horse anti-mouse IgG (Sigma, USA), and then with ECL kit (Amersham, USA).
For quantitative analysis of the Western band of SOD1 in the hippocampus, video images were digitized into an array of 512 pixels×512 pixels. Each pixel resolution was of 256 gray levels. The intensity of western band of SOD1 was expressed as a relative optical density (ROD) value which was transformed from mean gray values using the formula: ROD=lg (256/mean gray). A background parameter was obtained from each section out of the immunolabeled structures and subtracted from obtained ROD values of each group. ROD values are informed as ROD units. The bands of Western blot study were scanned and a ROD value was obtained using Scion Image software (Scion Corp, USA).
Statistical analysis Inter-animal differences in each group, as well as inter-experiment differences, were not statistically significant. Values shown represent the mean of experiments performed for each hippocampal area. All data obtained from the quantitative data are expressed as mean±SD and analyzed using one-way ANOVA to determine statistical significance. Bonferroni’s test was used for post-hoc comparisons. P<0.05 or 0.01 was considered statistically significant.
Results
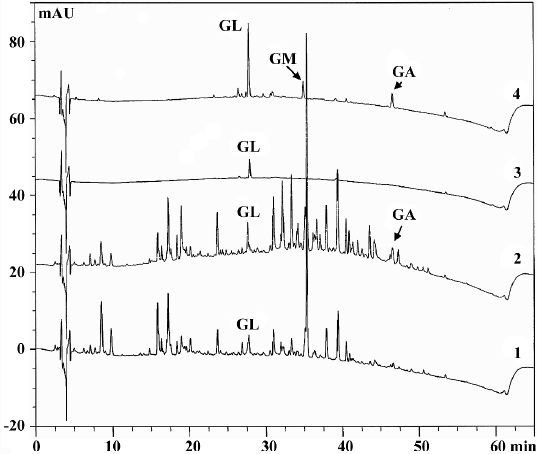
Effects of roasting on licorice and GL The major peaks in licorice extracts were completely separated by HPLC analysis. The comparative non-polar components which were eluted after 30 min were significantly increased. The untreated GL showed only the GL peak. After being roasted at 150 °C for 30 min, two new peaks appeared (Figure 1). Upon spiking with standard solution (GA and GM), the new peak was identified to have resulted from GA and GM (data not shown). The appearance of GM and GA was also confirmed by the UV contour plot obtained by the PDA detector (data not shown). The fact that the formation of GM and GA was attributed to thermal decomposition of GL was the same as previous reports[12,15]. It was reported that sugar chains in the saponin and glycosidic flavonoid constituents in licorice were hydrolyzed step-by-step during roasting through hydrothermolysis[16].
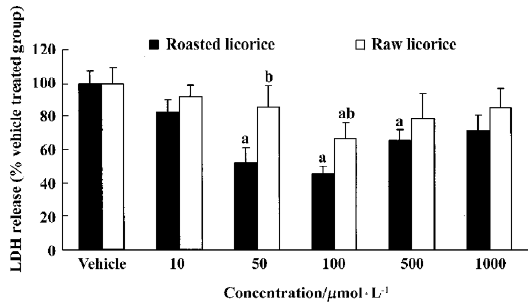
LDH release inhibition by raw and roasted licorice Raw and roasted licorice effectively protected PC12 cells from hypoxic damage. In the raw licorice-treated group, LDH release was decreased by 9%‒33% at concentrations of 10‒1000μmol/L, while in the roasted licorice-treated group, LDH release was decreased by 17%‒49% at concentrations of 10‒1000μmol/L (Figure 2).
Protective effects of raw and roasted licorice on ischemic pyramidal cells In the vehicle-treated group, the percentage of cresyl violet-positive pyramidal cells in the CA1 region was 11.5% compared with the control group at d 4 after ischemia/reperfusion (Figures 3B, 4B, 5). In 50 mg/kg raw licorice-treated group, the number of cresyl violet-positive neurons showed no difference to that of the vehicle-treated group (P>0.05; Figures 3E, 4E, 5). In 100 mg/kg raw licorice-treated group, the number of cresyl violet-positive neurons was slightly increased (P<0.05; Figures 3F, 4F, 5).
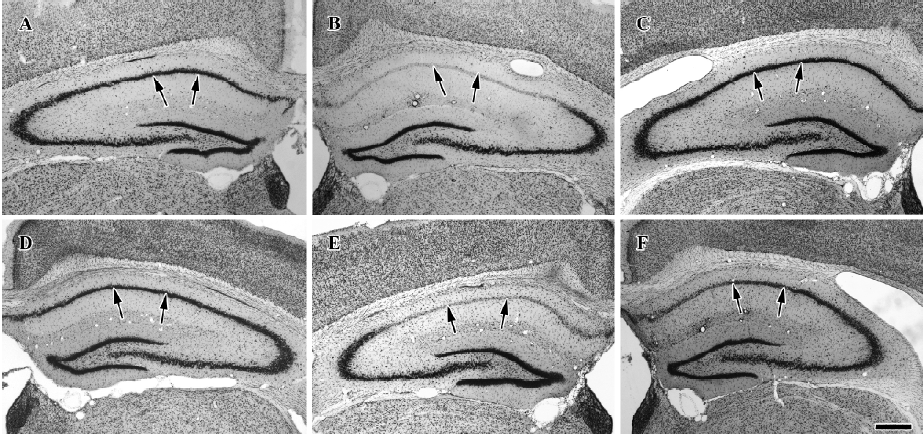
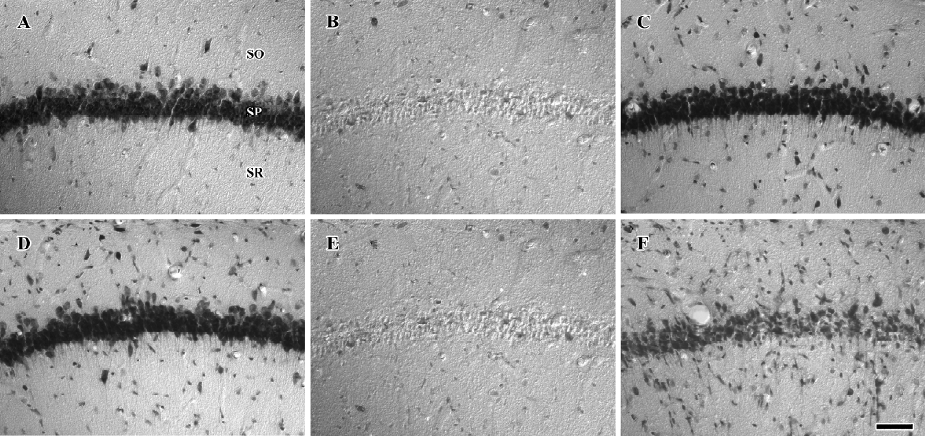
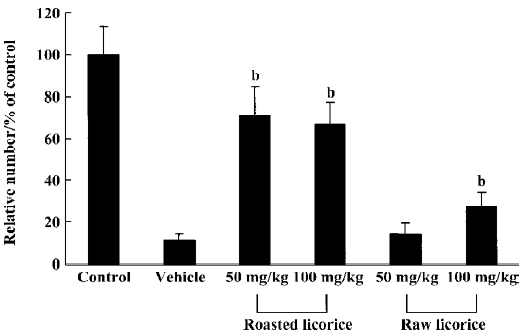
In contrast, in the roasted licorice-treated group, abundant CA1 pyramidal cells were detected in the hippocampal CA1 region after ischemia/reperfusion. In the 50 mg/kg roasted licorice-treated group, approximately 71.4% of CA1 pyramidal cells were stained with cresyl violet (Figures 3C, 4C, 5). In the 100 mg/kg roasted licorice-treated group, approximately 66.4% cresyl violet-positive neurons were detected in the striatum pyramidal of the CA1 region (Figures 3D, 4D, 5).
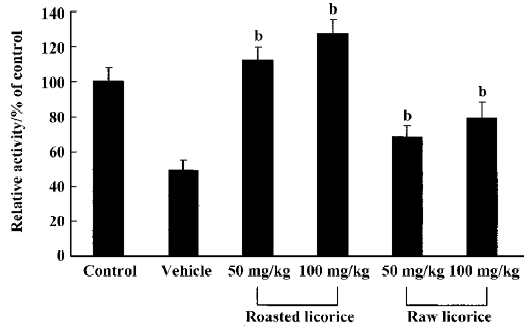
Changes in SOD1 activity In the vehicle-treated group, SOD1 activity was significantly decreased compared with the control group. The SOD1 activity was slightly increased in the raw licorice-treated groups and significantly increased in the roasted licorice-treated groups compared with that in vehicle-treated group (Figure 6).
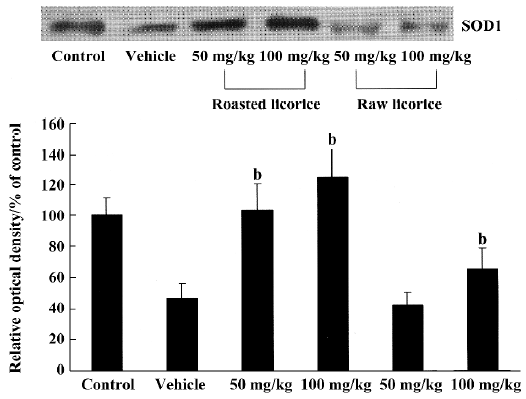
Changes in SOD1 protein contents In the vehicle-treated and raw licorice-treated groups, SOD1 protein contents were significantly decreased compared with the control group (P<0.05). But in the roasted licorice-treated groups, SOD1 protein contents were not significantly altered compared with the control group (P>0.05; Figure 7).
Discussion
In the present study, we examined the effects of raw or roasted licorice on LDH release in normoxic and hypoxic PC12 cells because the release of LDH implied neuronal damage. We found that roasted licorice at 50‒500μmol/L significantly reduced LDH release in hypoxic PC12 cells. A weak, but statistically significant protection was also observed in hypoxic PC12 cells treated with 100μmol/L of raw licorice. In addition, 100‒1000μmol/L of raw or roasted licorice caused cytotoxic effects in normoxic PC12 cells. Therefore, the optimal concentration of neuroprotection for raw and roasted licorice was 50‒100μmol/L. In addition, we found that 50 mg/kg or 100 mg/kg of raw or roasted licorice, which is the commonly used dose in herbal medicine, exerted neuroprotective effects in an in vivo ischemic model.
We found that roasted licorice had a neuroprotective effect against transient forebrain ischemia. However, raw licorice had no significant effect against ischemic damage. In the process of roasting licorice, almost all polar components were not altered, while the non-polar components were significantly increased. In previously published studies, it has been reported that licorice root has an effect on the anti-apoptotic protein Bcl-2, which is a 26-kDa protein that blocks cell death by inhibiting cytochrome c release from mitochondria, a critical event in the apoptotic pathway[17,18]. In our study, SOD1 activity and protein content in the roasted licorice-treated group were significantly increased compared to those in the vehicle-treated group. This result suggests that roasted licorice has neuroprotective effects through antioxidant activity.
The biopharmaceutical properties of roasted licorice have been previously examined to clarify the influence of roasting on the bioavailability of glycyrrhizin after oral administration of the extract. Among polar components of licorice, glycyrrhizin is a major component. It is taken orally and is transformed (hydrolysed) by intestinal bacteria into the active metabolite glycyrrhetic acid, non-polar component[19]. This glycyrrhetinic acid is widely used as a gap junction inhibitor that is effective in the micromolar concentration range. Glycyrrhetinic acid seems to act through changes in phosphorylation and/or connexin assembly and inhibition of sarcolemmal Ca2+ currents and of mRNA synthesis. In our study, we also observed that the increased non-polar compounds found in roasted licorice contained glycyrrhizin-degraded products such as glycyrrhetinic acid and glycyr-rhetinic acid monoglucuronide.
We observed in the present study that 18-β glycyrrhetinic acid strongly protected hippocampal CA1 pyramidal neurons from ischemic damage. This result is supported by a previous report that carbenoxolone, the succinyl ester of 18-β glycyrrhetinic acid has protective effects against ischemic damage in middle cerebral artery occlusion models[20].
In conclusion, roasted licorice has a significant neuroprotective effect against ischemic damage through anti-oxidant effects. In addition, the neuroprotective effect of roasted licorice is associated with non-polar compounds including glycyrrhetinic acid monoglucuronide and its degradation product, glycyrrhetinic acid.
Acknowledgements
The authors would like to thank Mr Suek HAN, Mr Seung-uk LEE and Ms Hyun-sook KIM for their technical help during this study.
References
- Peng H, Li YF, Sun SG. Effects of Ginkgo biloba extract on acute cerebral ischemia in rats analyzed by magnetic resonance spectroscopy. Acta Pharmacol Sin 2003;24:467-71.
- Zhang YY, Li PF, Li D. Effect of Ginkgo biloba leaf extract on electroencephalography of rat with cerebral ischemia and reperfusion. Acta Pharmacol Sin 2003;24:157-62.
- Wong TM, Wu S, Yu XC, Li HY. Cardiovascular actions of Radix Stephaniae Tetrandrae: a comparison with its main component, tetrandrine. Acta Pharmacol Sin 2000;21:1083-8.
- Kang JS, Yoon YD, Cho IJ, Han MH, Lee CW, Park SK, et al. Glabridin, an isoflavan from licorice root, inhibits inducible nitric-oxide synthase expression and improves survival of mice in experimental model of septic shock. J Pharmacol Exp Ther 2005;312:1187-94.
- Ohuchi K, Kamada Y, Levine L, Tsurufuji S. Glycyrrhizin inhibits prostaglandin E2 production by activated peritoneal macrophages from rats. Prostaglandins Med 1981;7:457-63.
- Abdugafurova MA, Li VS, Sherstnev MP, Atanaev TB, Isamuk-harmedov AS, Bachmanova GI. Antioxidative properties of glycyrrhyzic acid salts and their effect on the liver monooxygenase system. Vopr Med Khim 1990;36:29-31.
- Miura T, Ohnuma Y, Kuno A, Tanno M, Ichikawa Y, Nakamura Y, et al. Protective role of gap junctions in preconditioning against myocardial infarction. Am J Physiol Heart Cir Physiol 2003;286:H214-21.
- Nagai T, Egashira T, Yamanaka Y, Kohno M. The protective effect of glycyrrhizin against injury of the liver caused by ischemia-reperfusion. Arch Environ Contam Toxicol 1991;20:432-6.
- Yokozawa T, Liu ZW, Chen CP. Protective effects of Glycyrrhizae Radix extract and its compounds in a renal hypoxia (ischemia)-reoxygenation (reperfusion) model. Phytomedicine 2000;6:439-45.
- Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CH, Edwards CR. Mineralocorticoid activity of liquorice: 11-beta-hydroxysteroid dehydrogenase deficiency comes of age. Lancet 1987;2:821-4.
- Majima T, Yamada T, Tega E, Sakurai H, Saiki I, Tani T. Pharmaceutical evaluation of liquorice before and after roasting in mice. J Pharm Pharmacol 2004;56:589-95.
- Sung MW, Li PC. Chemical analysis of raw, dry-roasted, and honey-roasted licorice by capillary electrophoresis. Electrophoresis 2004;25:3434-40.
- Hwang IK, Eum WS, Yoo KY, Cho JH, Kim DW, Choi SH, et al. Copper chaperone for Cu,Zn-SOD supplement potentiates the Cu,Zn-SOD function of neuroprotective effects against ischemic neuronal damage in the gerbil hippocampus. Free Radic Biol Med 2005;39:392-402.
- McCord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244:6049-55.
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 1971;44:276-87.
- Kuwajima H, Taneda Y, Chen WZ, Kawanishi T, Hori K, Taniyama T, et al. Variation of chemical constituents in processed licorice roots: quantitative determination of saponin and flavonoid constituents in bark removed and roasted licorice roots. Yakugaku Zasshi 1999;119:945-55.
- Reed JC. Double identity for proteins of the Bcl-2 family. Nature 1997;387:773-6.
- Rafi MM, Vastano BC, Zhu N, Ho CT, Ghai G, Rosen RT, et al. Novel polyphenol molecule isolated from licorice root (Glycrrhiza glabra) induces apoptosis, G2/M cell cycle arrest, and Bcl-2 phosphorylation in tumor cell lines. J Agric Food Chem 2002;50:677-84.
- Akao T, Hayashi T, Kobashi K. Intestinal bacterial hydrolysis is indispensable to absorption of 18β-glycyrrhetic acid after oral administration of glycyrrhizin in rats. J Pharm Pharmacol 1994;46:135-7.
- Hosseinzadeh H, Nassiri Asl M, Parvardeh S. The effects of carbenoxolone, a semisynthetic derivative of glycyrrhizinic acid, on peripheral and central ischemia-reperfusion injuries in the skeletal muscle and hippocampus of rats. Phytomedicine. 2005;12:632-7.
