A20 overexpression under control of mouse osteocalcin promoter in MC3T3-E1 cells inhibited tumor necrosis factor-alpha-induced apoptosis1
Introduction
Bone mass is largely determined by the number of bone forming (osteoblasts) and bone resorbing (osteoclasts) cells present in the basic multicellular units responsible for the regeneration of the adult skeleton[1]. The osteoblast is the cell responsible for the synthesis of collagen and other bone proteins, and also has an important role in the subsequent mineralization of the matrix. Recent evidence has indicated that apoptosis plays a critical role during embryonic limb development, skeletal maturation, adult bone turnover by modeling and remodeling processes, and during fracture healing and bone regeneration. Apoptosis is the most common fate of osteoblasts during physiologic bone remodeling, thus the control of osteoblast apoptosis will affect the rate of bone formation and bone mass[2–4].
A20 is a cytoplasmic zinc finger protein that inhibits nuclear factor kappaB (NF-kappaB) activity and tumor necro-sis factor (TNF)-mediated programmed cell death (PCD)[5–9]. TNF dramatically increases A20 mRNA expression in all tissues. Previous studies have shown that protection against TNF cytotoxicity exists for endothelial cells, hepatocyte, carcinoma cells, and murine embryonic fibroblast cells[10–12].
In 2000, Lee et al[13] reported that mice deficient for A20 develop severe inflammation and cachexia, and die prematurely. A20-deficient cells failed to terminate TNF-induced NF-kappaB responses. These cells were also more susceptible than control cells to TNF-mediated PCD. Histological examination of 3- to 6-week-old A20–/– mice revealed severe inflammation and tissue damage in livers, kidneys, and intestines. The osseous parts of joints were thin and bone marrow was filled with inflammatory cells. Histological examination also showed that the trabecular bone was thinner in A20–/– mice than in A20+/+ mice. Failure to downregulate NF-kappaB transcriptional activity results in chronic inflammation and cell death. A20 is a potent inhibitor of NF-kappaB signalling, but its mechanism of action is unknown in the study. In 2004, a further study showed that A20 downre-gulates NF-kappaB signalling through the cooperative activity of its two ubiquitin-editing domains[8]. Boone et al[14] also reported mice developed spontaneous inflammation, if mice doubly deficient in either A20 and TNF or A20 and TNF receptor 1, indicated that A20 is also critical for the regulation of TNF-independent signals in vivo. However, the mechanism by which A20 is involved in bone loss is still unclear.
In present study, we constructed A20 expression vector under the control of mouse osteocalcin promoter. We then determined the specific expression of A20 in osteoblastic MC3T3-E1 cells using this vector, and investigated whether MC3T3-E1 cells stably over-expressed A20 preventing TNF-alpha-induced apoptosis.
Materials and methods
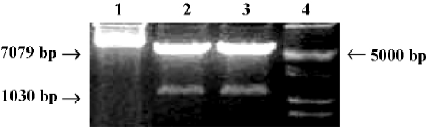
Construction of OC-A20 vector First, we designed the sense primer:5'-CATCGCGAGAATTGCTCATCGCAGCC-3' and reverse primer: 5'-CAGGTACCTGCACCCTCCAGC-ATCCA-3' containing the restricted sites of NruI and KpnI. The 1.3 kb DNA fragment of mouse osteocalcin gene 2 promoter, named plasmid pIIBS1.3 (a gift from Dr G KARSENTY, Baylor College of Medicine, Houston, Texas, USA), was obtained by PCR using the above primers. The plasmid pcDNA3-A20 containing the human A20 gene was cut with NruI, then restricted with KpnI (TaKaRa BIO, Dalian, China) to remove the CMV promoter fragment[15,16]. The1030 bp osteocalcin promoter fragment was released from plasmid pIIBS1.3 by digesting with NruI and KpnI to create the blunt end. Then osteocalcin promoter and pcDNA3-A20 were ligated to create a new plasmid containing the osteocalcin promoter, which was named A20 expression vector under the control of mouse osteocalcin promoter (OC-A20).
Specific expression of OC-A20 in MC3T3-E1 cells MC3T3-E1 cells (a gift of Dr WB XIA, Beijing Union Hospital, Beijing, China) and mouse embryo fibroblast NIH3T3 cells were grown in a-modified minimal essential medium (a-MEM) supplemented with 10% heat-inactivated FBS and kanamycin (60 µg/mL) (Gibco Invitrogen, Grand Island, NY, USA) at 37 °C in a humidified atmosphere of 5% CO2. The day before transient transfection, both kinds of cells were plated on 6-cm-diameter dishes at a density of 4×105 cells per dish. MC3T3-E1 cells and NIH3T3 cells were transfected with OC-A20 (5 µg/100-mm dish), respectively, by lipofection according to standard procedures (Invitrogen, Carlsbad, CA, USA). After 8 h, the transfection medium was replaced with fresh medium (10% FBS) overnight. The expression of A20 mRNA of MC3T3-E1 and NIH3T3 cells transfected with OC-A20 was determined by reverse transcription-polymerase chain reaction (RT-PCR).
Cell culture and stable transfection MC3T3-E1 cells were grown in a-MEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and kanamycin (60 µg/mL) at 37 °C in a humidified atmosphere of 5% CO2. The medium was changed twice weekly. The day before transfection, cells were plated on 6-cm-diameter dishes at a density of 4×105 cells per dish. MC3T3-E1 cells were transfected with the A20 plasmid containing mouse osteocalcin gene 2 promoter (5 µg/100-mm dish) by lipofection according to standard procedures described by the manufacturer, and with 5 ug of the plasmid pCMV-pcDNA3 alone to generate control. After 8 h, the transfection medium was replaced with fresh medium (10% FBS) and cultured overnight. The cells were then cultured in α-MEM supplemented with 10% FBS and 60 µg/mL kanamycin and exposed to 400 µg/mL of geneticin G418 (Gibco). After 2 weeks of selection (the medium was replaced with fresh 10% FBS medium each 72 h) and several resistant clones were obtained. The survival clones were subcultured before reaching confluency. Expression of A20 mRNA and A20 protein in the MC3T3-E1 cells was determined by RT-PCR and Western blot analysis, respectively.
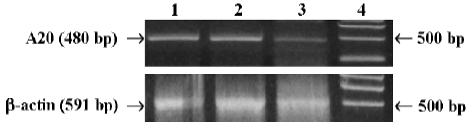
Analysis of A20 mRNA endogenous expression in MC3T3-E1 cells Total RNA was isolated from mouse MC3T3-E1 cells with or without TNF-alpha (1000 u/mL, Peproteck, UK) for 1 h and 2 h, with Trizol Reagent (Gibco Invitrogen, Japan) according to the manufacturer’s instruc-tions, and then identified by electrophoresis, and cDNA was synthesized using random primer. For detection of A20, the primers of mice A20 were used (sense primer: 5'-TTTGAGC-AATATGCGGAAAGC-3', reverse primer: 5'-AGTTGTCCCA-TTCGTCATTCC-3'). PCR was performed as follows: denaturing at 94 °C for 1 min, annealing at 62 °C for 1.5 min, synthesis at 72 °C for 2.5 min, for total of 30 cycles. The PCR reaction generated a 480-pb fragment of murine A20 and a 591-bp fragment of murine β-actin (sense primer: 5'-AACGA-GCGGTTCCGATGCCCTGAG-3', reverse primer: 5'-TGTCG-CCTTCACCGTTCCAGT- T-3')[17]. The RT-PCR products were determined by electrophoresis in 1.5% denaturing agarose gel.
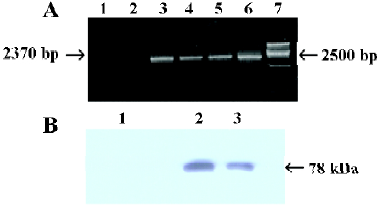
Total RNA extraction, RT-PCR and Western blot analysis of MC3T3-E1 cells-transfected stably with A20 Total RNA of MC3T3-E1 cells-transfected with OC-A20 or pcDNA3-null plasmid DNA was extracted with Trizol Reagent according to the manufacturer’s instruction, and then identified by electrophoresis. Random hexamer primers (Promega, Madison, WI, USA) were employed for cDNA preparation using the moloney murine leukemia virus (MMLV) reverse transcriptase (Promega). PCR was performed as follows: denaturing at 94 °C for 1 min, annealing at 62 °C for 1.5 min, synthesis at 72 °C for 2.5 min (for 10 min in last cycle), for total of 30 cycles. The PCR reaction generated a 2370-bp fragment of human A20 (sense primer: 5'-CGGTACCGC-ACAATGGCTGAACAAGTCCTTCCTC-3', reverse primer: 5'-CGTCTAGAGTTAGCCATACATCT-GCTTGAACTG-3'), and a 591-bp fragment of murine β-actin by previously described primers.
For Western blot, the transfected cells were collected, suspended in suspending buffer (NaCl 0.1 mol/L, Tris-HCl 0.01 mol/L, pH 7.6, egtazic acid 1 mmol/L, aprotinin 1 mg/L, and phenylmethyl sulfonylfluoride (PMSF)(100 mg/L), then 2×SDS loading buffer was immediately added with equal volume, and the mixture was boiled for 10 min. After centrifugation, the lysates were sonicated for 10 s and incubated on ice for 10 min. A sample (30 UL) was resolved on 10% SDS-polyacrylamide gel electrophoresis. Proteins were electrotransferred onto a nitrocellulose membrane. The membranes were blocked with 5% fat-free milk and probed with mouse anti-human A20 antibody (1/1000, Oncogene, CA, USA). The blots were washed and exposed to horseradish peroxidase (HRP)-conjugated antimouse IgG secondary antibody (Santa Cruz Biotechnology, USA) and then developed using the enhanced chemiluminescence (ECL) reagent (PerkinElmer, Boston, MA, USA). The molecular mass of the protein was estimated relative to the pre-stained size marker.
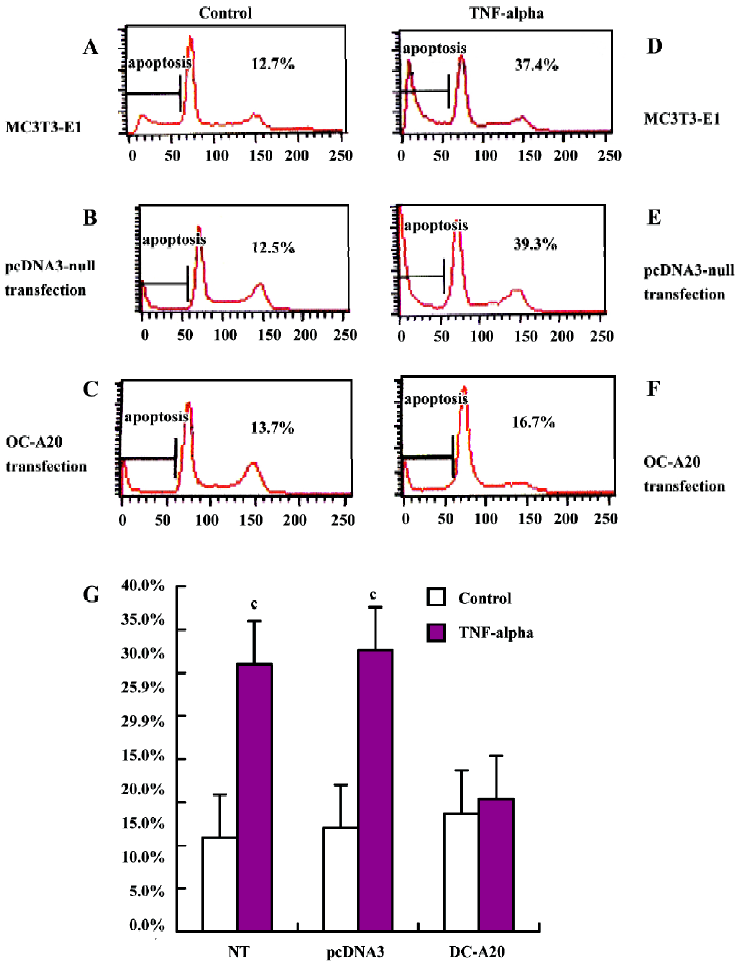
Flow cytometric analysis of apoptosis MC3T3-E1 cells transfected stably with OC-A20 and pcDNA3-null were plated on 6-cm-diameter dishes at a density of 4×105 cells per dish cells. After the cells were cultured in the presence of 10% FBS medium for 48 h, they were divided into two groups: cells cultured in the presence or absence of TNF-alpha (1000 u/mL)-treated and control groups. After 8 h, cells were harvested, dispersed, fixed in 70% ethanol, and suspended into FACS buffer (1% FBS, 0.05% NaN3 in PBS pH 7.0), and then washed twice in FACS buffer before fixing with 2% paraformaldehyde in PBS for 15 min at 4 °C For reading, cells were suspended in 300 µL FACS buffer and processed with a FACS (Becton Dickinson, Rahway, New Jersey, USA).
TUNEL detection of apoptosis Apoptotic cells were also determined by terminal dUTP nick endo-labeling (TUNEL) staining using an in situ cell death detection kit (Roche Diagnostics, Switzerland) according to the manufacturer’s protocol. MC3T3-E1 cells (500/cm2) transfected stably with OC-A20 and pcDNA3-null were cultured on Labtek chambers in the presence of 10% FBS medium were treated with TNF-alpha (1000 u/mL; as treatment group) or vehicle (as control group) for 8 h, respectively, and then fixed with paraformaldehyde at room temperature for 25 min. Endogenous peroxidase was quenched with 3% H2O2, and the cells were permeabilized with 0.2% Triton X-100, at 4 °C for 5 min and incubated for 1 h at 37 °C with the TUNEL reaction mixture containing the terminal deoxynucleotidyl transferase. Incorporated fluorescein was detected by sheep anti-fluorescein antibody conjugated with horseradish peroxidase. The cells were detected using TUNEL assay using an in situ cell death detection kit. TUNEL-positive cells were detected by brown nuclei and nuclear fragmentation. We counted the total of TUNEL positive cells in different groups from four independent experiments. In each experiment group, the TUNEL positive cells were counted from 10 different visual fields in fluorescence microscope, and data from four independent experiments were pooled and are given as the percentage of apoptosis (mean±SD).
DNA ladder analysis MC3T3-E1 cells transfected stably with OC-A20 and pcDNA3-null were stimulated with TNF-alpha (1000 u/mL) for 8 h. Genomic DNA was isolated using the phenol-chloroform extraction method and the DNA were separated by electrophoresis on a 1.5% agarose gel.
Statistical analysis Student’s t-test was used to assess the statistical significance of differences by the software SPSS11.0. A P-value of less than 0.05 was considered to be statistically significant. Data were expressed as mean±SD.
Results
A20 weakly endogenous expression in MC3T3-E1 cells Weak endogenous expression of A20 in MC3T3-E1 cells was analyzed by RT-PCR using mice A20 primer. But A20 mRNA was rapidly induced (within 2h) in MC3T3-E1 cells after TNF-alpha stimulation (Figure1).
Construction of OC-A20 vector and specific expression in MC3T3-E1 cells The new OC-A20 vector containing human A20 gene and mouse osteocalcin gene 2 promoter were digested with NruI and KpnI restriction enzyme, and 1030 bp and 7079 bp DNA fragments were obtained by PCR-RFLP (Figure 2). The identity of the OC-A20 vector was confirmed by DNA sequence analysis (data not shown). After MC3T3-E1 and NIH3T3 cells were transiently transferred with OC-A20, A20 mRNA expression was not found in NIH3T3 cells. A significant expression of A20 mRNA was detected in MC3T3-E1 cells (Figure 3).

Establishment of stably transfected cells Using RT-PCR and Western blot analysis, our results showed that the expression of A20 mRNA and A20 protein was significantly higher in MC3T3-E1 cells transfected stably with OC-A20, but the expressions of A20 mRNA and A20 protein were not found in MC3T3-E1 cells transfected with pcDNA3-null (Figure 4).
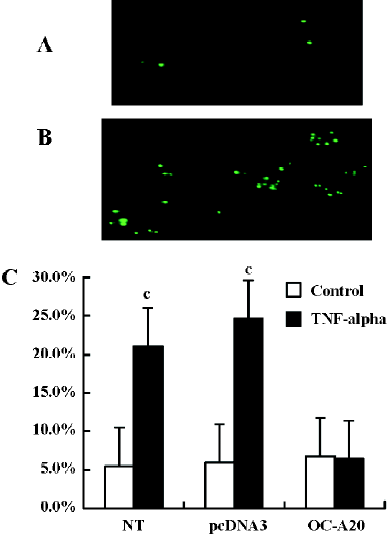
A20 anti-apoptotic effects Apoptotic cells were quantified as the proportion of cells that had a DNA content of less than 2N(sub-G1DNA content).As shown in Figure 5, after treatment with TNF-alpha (1000 u/mL) for 8 h, the apoptosis of OC-A20-transfected cells was only 15.4%±1.3%, but significant apoptosis was observed in the non-transfected cells (31.0%±7.5%) and pcDNA3-null transfected cells (32.6%±7.0%) at the same TNF-alpha concentration. Data from six independent experiments were pooled and were given as the percentage of apoptosis. There was a significant difference of apoptosis between the OC-A20-transfected group and pcDNA3-null-transfected group (P<0.001) (Figure 5).
We assessed DNA fragmentation in individual transfected cells by specific labeling of double-strand DNA breaks using the TUNEL methods. TUNEL staining of pcDNA3-null-transfected cells and OC-A20-transfected cells treated with TNF-alpha (1000 u/mL) for 8 h was conducted. Exposure of pcDNA3-null-transfected cells to TNF-alpha showed more DNA fragmentation compared to OC-A20-transfected cells in vitro. Data from four independent experiments were pooled and were given as the percent of apoptosis. The difference in apoptosis percent between the OC-A20-transfected group and pcDNA3-null-transfected group was significant (P<0.001) (Figure 6).
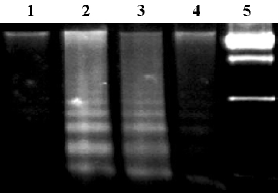
To assay for DNA degradation, DNA was analyzed by agarose gel electrophoresis. TNF-alpha treatment resulted in the formation of a DNA ladder in non-transfected group or pcDNA3-null-transfected group at 8 h, but no ladder was demonstrated in the OC-A20-transfected cells (Figure 7).
Discussion
A20 was originally described as a TNF-alpha-inducible zine finger protein in endothelial cells[18]. Stable overexpression of A20 plays a role of resistance to TNF-induced apoptosis in some cell lines. Several researchers have reported that the antiapoptotic gene A20 protects β cells against apoptosis in islets[15,19,20]. It has also been identified that A20 protects some cell lines against TNF cytotoxicity, such as human breast carcinoma cells, murine fibrosarcoma cells, and murine embryonic fibroblast cells[11,12,21]. Both mouse osteoblast cells and murine embryonic fibroblast cells develop from mesenchymal progenitor. We presume that a similar anti-apoptotic effect of A20 may also exist in osteoblastic cells.
In an attempt to clarify the role of A20 in osteoblasts, we constructed osteoblast-specific A20 expression vector, which induces A20 expression under the control of murine osteocalcin promoter. Ducy and Karsenty[22] have reported that a short promoter fragment of mouse osteocalcin gene 2(mOG2, -147/+13 fragment) displays osteoblast-specific activity in DNA transfection experiments. They identified that this promoter fragment contains two cis-acting elements, which are called OSE1 and OSE2, fulfilling the definition of an osteoblast-specific activator of transcription. These two elements may be important at several stages of osteoblast differentiation. They then cloned the mOG2 promoter containing 1.3 kb DNA fragments, named the recombine vector as pIIBS1.3[22]. In the present study, we constructed the A20 expression vector under the control of mouse osteocalcin promoter by fusing a 1030-bp fragment of mouse osteocalcin promoter with human A20 complementary DNA. The genomic structure of OC-A20 vector was confirmed through PCR-RFLP and DNA sequence analysis. Using this expression vector, the MC3T3-E1 cells and mouse embryo fibroblast NIH3T3 cells were transiently transfected and the results demonstrated the specific expression of A20 in osteoblastic MC3T3-E1 cells.
Grey et al[19] reported on the induced expression of the anti-apoptotic gene A20 in cells of islets in response to IL-beta. But little is currently known about the expression of cytoprotective genes in osteoblast cells. Moreover, to our knowledge, there are no reports regarding the expression of the A20 gene in mouse osteoblast. We raised the question whether osteoblast cells are able to have a protective response to inflammation. In the present study, we found that expression of A20 is rapidly induced in mouse MC3T3-E1 cells in response to TNF-alpha. The rapid expression of A20 in osteoblast suggested that it may be a component of their physiological protective response to injury[23].
Before clarifying the anti-apoptotic effect of A20 in osteoblastic MC3T3-E1 cells, we established the cell line which stably over-expressed A20 protein during osteoblast differentiation. The establishment of MC3T3-E1 cell line stable over-expression A20 can diminish errors in independent experiments of different times. By FACS, TUNEL and DNA ladder analysis, we found that A20 could protect the MC3T3-E1 cells from TNF-alpha induced apoptosis in vitro. The mechanism of action has not yet been completely clarified. Previous studies showed that A20 might interfere with TNF-receptor associated death domain (TRADD) binding to the TNF-receptor 1 and might therefore negatively regulate TNF-induced cytotoxicity[5–8,12–14,24].
Apoptosis plays a critical role during bone turnover and bone loss. There are many molecules that are involved in apoptosis, and the fate of the cell depends on how these molecules interact with each other. The functional significance of osteoblast death in the postnatal skeleton and adult skeleton for normal bone homeostasis or in response to pharmacologic agents is not presently well understood. Exploration to determine the selective pathways of apoptosis, which have been identified in other cells and organs, have only been started in the bone. The understanding that A20 protects osteoblasts against apoptosis will be leveraged into potential targets for new therapeutic strategies towards osteoporosis.
At present, the drugs for anti-osteoporotic therapy mainly focus on inhibiting osteoclast function and decreasing bone turnover. Thus the search for drugs that promote differentiation, proliferation and postpone apoptosis of osteoblasts is still in its infancy. In our study, we have constructed an osteoblast-specific expression vector, which was under the control of mouse osteocalcin promoter, and have confirmed the anti-apoptotic effect of A20 in TNF-alpha-induced apoptosis of osteoblast in vitro. Further study is necessary to identify the biological function of A20 in vivo. The present study has also established a novel experimental basis for the long-standing goal to exploit and obtain anti-osteoporotic drugs.
Acknowledgments
We are grateful to Dr G KARSENTY for providing mouse osteocalcin gene 2 promoter (pIIBS1.3).
Reference
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 1995;332:305-11.
- Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res 1998;13:793-802.
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 1998;102:274-82.
- Plotkin LI, Weinstein RS, Parfitt AM, Roberson RK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 1999;104:1363-74.
- Daniel S, Arvelo MB, Patel VI, Longo CR, Shrikhande G, Shukri T, et al. A20 protects endothelial cells from TNF-, Fas-, and NK-mediated cell death by inhibiting caspase 8 activation. Blood 2004;104:2376-84.
- Heyninck K, Beyaert R. A20 inhibits NF-kappaB activation by dual ubiquitin-editing function. Trends Biochem Sci 2005;30:1-4.
- Storz P, Doppler H, Ferran C, Grey ST, Toker A. Functional dichotomy of A20 apoptotic and necrotic cell death. Biochem J 2005;387:47-55.
- Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signaling. Nature 2004;430:694-9.
- Gon Y, Asai Y, Hashimoto S, Mizumura K, Jibiki I, Machino T, et al. A20 inhibits toll-like receptor 2- and 4-mediated interleukin-8 synthesis in airway epithelial cells. Am J Respir Cell Mol Biol 2004;31:330-6.
- Longo CR, Patel VI, Shrikhande GV, Scali ST, Csizmadia E, Daniel S, et al. A20 protects mice from lethal radical hepatectomy by promoting hepatocyte proliferation via a p21 waf1-dependent mechanism. Hepatology 2005;42:156-64.
- Janicke RU, Lee FH, Porter AG. Nuclear c-Myc plays an important role in the cytotoxicity of tumor necrosis factor a in tumor cells. Mol Cell Biol 1994;14:5661-70.
- Jaattela M, Mouritzen H, Elling F, Bastholm L. A20 zinc finger protein inhibits TNF and IL-1 signaling. J Immunol 1996;156:1166-73.
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, et al. Failure to regulate TNF-induced NF-kB and cell death responses in A20-deficient mice. Science 2000;289:2350-4.
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 2004;5:1052-60.
- Yu LY, Lin B, Zhang ZL, Guo LH. Direct transfer of A20 gene into pancreas protected mice from streptozotocin-induced diabetes. Acta Pharmacol Sin 2004;25:721-6.
- Miao HS, Yu LY, Hui GZ, Guo LH. Antiapoptotic effect both in vivo and in vitro of A20 gene when transfected into rat hippocampal neurons. Acta Pharmacol Sin 2005;26:33-8.
- Zhang ZL, Lin B, Yu LY, Shen SX, Zhu LH, Wang WP, et al. Gene therapy of experimental autoimmune thyroidits mice by in vivo administration of plasmid DNA coding for human interleukin-10. Acta Pharmacol Sin 2003;24:885-90.
- Opipari AW, Boguski MS, Dixit VM. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J Biol Chem 1990;265:14705-8.
- Grey ST, Arvelo MB, Hasenkamp W, Bach FH, Ferran C. A20 inhibits cytokine-induced apoptosis and nuclear factor kB-dependent gene activation in islets. J Exp Med 1999;190:1135-46.
- Laybutt DR, Kaneto H, Hasenkamp W, Grey S, Jonas JC, Sgroi DC, et al. Increased expression of antioxidant and anti-apoptotic genes in islets that may contribute to beta-cell survival during chronic hyperglycemia. Diabetes 2002;51:413-23.
- Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity 2000;12:301-11.
- Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol 1995;15:1858-69.
- Bach FH, Hancock WW, Ferran C. Protective genes expressed in endothelial cells: a regulatory response to injury. Immunol Today 1997;18:483-6.
- He KL, Ting AT. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol Cell Biol 2002;20:6034-45.
