Cytotoxic and apoptotic effects of prenylflavonoid artonin B in human acute lymphoblastic leukemia cells1
Introduction
Natural products from plants or Chinese herbs have been used as traditional remedies in Asian countries for hundreds of years. The development of compounds with antitumor effects from natural products has become a very important topic. Flavonoids are commonly found in most plants and exert a remarkable spectrum of biological activities that affect basic cell functions, and several beneficial biological activities of flavonoids including antioxidant, antitumor, and anti-inflammation properties have been identified in several studies[1,2]. Flavonoids are also dietary pharmacological agents, which may block neoplastic inception or delay disease progression[3,4]. These data indicate that certain flavonoids may be used as possible chemopreventive or chemotherapeutic agents.
The great prevalence of flavonoids in the vegetal kingdom act, not only as the colored pigments of flowers, but also as enzyme inhibitors, precursors of toxic substance, and a defense against ultraviolet radiation exposure. Flavonoids were found to act on the growth of cancer cells, which means that it possesses potential anti-tumor activity. For example, baicalein, epigallocatechin (EGC) gallate and green tea extract were reported to inhibit tumor growth[5–7]. Furthermore, epidemiological studies indicated that diets containing linseed and soy (rich in isoflavonoids and lignans) might protect against colon, breast, and prostate cancer[8]. Flavonoids are benso-r-pirone derivatives that can be grouped according to the presence of different substituents on the rings and to the degree of benso-r-pirone ring saturation. Artonin B is a prenylflavonoid that is obtained from the root bark of Artocarpus heterophyllus Lamk[9]. Artonin B is a derivative from heterophyllin and possesses the structure in C-10 position of isoprenoid moiety. However, the effects of artonin B on cancer cell growth have rarely been investigated in great detail.
Apoptosis is a cell suicide program, which is essential for the development and maintenance of tissue homeostasis and the elimination of unwanted or damaged cells from multicellular organisms[10,11]. Apoptosis is characterized by a series of morphological changes involving cell shrinkage, chromatin condensation and the formation of apoptotic bodies[12]. It can be triggered by various extracellular and intracellular stimuli that result in the coordinated activation of family proteases called caspases. The activation of caspase 3 pathways is an important downstream executioner in apopto-sis[13].
Human leukemia is a commonly diagnosed neoplasm and the major leading cause of human death. CCRF-CEM cells are acute lymphoblastic leukemia (ALL) cells. ALL represents the clonal proliferation of malignantly transformed lymphoid progenitors in the bone marrow. The treatment of patients with recurrent cancer is usually unsuccessful, and the development of new potent treatments has become the focal point for cancer treatment. Therefore, we evaluated the effects and action mechanism of artonin B on human acute lymphoblastic leukemia CCRF-CEM cells. In this present study, the cell cytotoxicity of four prenylflavonoid compounds was examined. Furthermore, morphological nuclear fragmentation, apoptotic body formation, the change of mitochondrial membrane potential, cytochrome c release, cell cycle change, Bcl-2, Bax, and Bak protein expression, and caspase 3 activity in artonin B treated human CCRF-CEM leukemia cells were investigated.
Materials and methods
Materials RPMI-1640, fetal bovine serum (FBS), and antibiotics were purchased from Hyclone. Caspase 3 assays kits and caspase 3 inhibitor (z-DEVD-fmk) were obtained from Biovision. The cytochrome c was purchased from R&D Systems. Monoclonal antibodies of Bcl-2, Bax, Bak, and anti-rabbit IgGs were obtained from Cell Signaling. Hoechst 33258 and the rest of chemicals were purchased from Sigma.
The four prenylflavonoid compounds that were extracted from Artocarpus species (Moraceae) were obtained from Dr Chun-nan LIN (School of Pharmacy, Kaohsiung Medical University, Taiwan, China). Artocarpanone (Figure 1A) was an isoprenoid-flavone[14]. Artonin A and artonin B (Figure 1B and 1C) were extracted from the root bark of Artocarpus

Cell culture Human acute lymphoblastic leukemia cell line (CCRF-CEM) was purchased from the Culture Collection and Research Center (Taiwan). CCRF-CEM cells were maintained in RPMI-1640 medium (Hyclone) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone), 100 µg/mL penicillin, 100 µg/mL streptomycin and 100 µg/mL amphotericin B (Hyclone). The cells were grown in a humidified incubator at 37 ºC under a 5% CO2/95% air atmosphere. For each experiment, 3×105 cells were seeded in each well in a 24-well plate containing 1 mL of fresh medium and incubated with or without chemical treatment for the indicated time. For toxicity study, the cells were treated with drugs during the exponential phase of cell growth.
Cytotoxicity assay The cytotoxic effect of drugs was determined using the MTT method[16]. In brief, 100 µL MTT solution (0.5 mg/mL in phosphate-buffer saline or PBS) was added to each well at the end of each experiment. After 1–2 h incubation at 37 ºC, 10 µL Triton X-100 (10%) was added and mixed well. Once the cells were completely dissolved, the absorbance difference at 550 nm was measured using a microplate reader, with the RPMI medium as a blank.
Microscopic observation of morphology and nuclear fragmentation After artonin B treatment, cells were harvested by centrifugation, washed with PBS, and fixed with 1% glutaraldehyde in 100 µL of PBS at room temperature for 1 h. Fixed cells were washed with PBS and then stained with 200 µmol/L Hoechst 33258 in 20 µL PBS for 30 min at room temperature. Five hundred stained cells from each treatment group were examined and counted under an Olympus fluorescence microscope.
DNA content and cell cycle analysis Human CCRF-CEM cells were collected and rinsed with PBS, after being cultured with 0, 1, 5, or 10 µmol/L artonin B for 24 h, and suspended in 75% ethanol at -20 ºC overnight. Fixed cells were centrifuged at 1200×g and washed with PBS twice. To detect DNA content, cells were contained in the dark with Propidium Iodide (PI) 50 mg/L and 0.1% RNase A in 400 µL PBS at 25 ºC for 30 min. Stained cells were analyzed on FACSort (Becton Dickinson). The percentage of apoptotic cells was determined using the CellQuest software program.
Assay of mitochondrial membrane potential Initially, 1×106 human CCRF-CEM cells/mL were incubated with 2 mmol/L rhodamine 123 for 10 min at 37 °C. After the incorporation of a fluorescent probe, the cells were incubated for up to 4 h with or without 10 µmol/L artonin B. At the end of incubation, the cells were washed twice with PBS, harvested by centrifugation, and then resuspended in 1.5 mL PBS. The fluorescent intensity of each cell suspension was measured at an excitation wavelength 480 nm and an emission wavelength 530 nm in a Perkin-Elmer Victor 3 fluorescent microplate reader. The fluorescence intensity was used as an arbitrary unit representing the mitochondrial transmembrane potential.
Cytochrome c release Human CCRF-CEM cells were seeded in 2 mL fresh medium at an initial density of 1×106 cells/mL and incubated for up to 4 h with or without 10 μmol/L artonin B. After the incubation, the cells were harvested by centrifugation and washed twice with PBS. The cells were suspended in 200 mL lysis buffer (195 mmol/L mannitol; 65 mmol/L sucrose; 2 mmol/L HEPES, pH 7.4; 0.05 mmol/L EGTA; 0.01 mmol/L MgCl2; 0.5 g/mL BSA) and lysed by the addition of 0.01% digitonin. The cytosolic fraction was obtained from 10 000×g centrifugation for 10 min and was collected for cyt c assay in 1×RD5P calibrator diluent (cyto-chrome c Immunoassay Kit; R&D Systems, MN, USA). After reacting with cyt c antibody and substrate, the absorbance was measured at 450 nm (reference wavelength is 540 nm).
Western blot analysis After being exposed to the indicated concentration of artonin B, human CCRF-CEM cells were washed with cold PBS. Whole cell extracts were prepared by incubating the cells with cold lysis buffer (20 mmol/L Tris-HCl; pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerophosphate, 1 mmol/L Na3VO4, 1 mg/mL leupeptin, and 1 mmol/L PMSF). The protein content of the lysates was determined using the DC protein assay kit (Bio-Rad). The cell lysates (25 µg protein/lane) were electro-phoresized on 12% SDS-polyacrylamide gels. The cellular proteins were then transferred to PVDF membranes by electroblotting for 2 h and Western blot analysis was carried out as previously described[17]. The protein levels were visualized with an enhanced chemiluminescence detection kit (Amersham).
Preparation of cytosolic extract and measurement of caspase 3 activity After the treatment with indicated agents, cells were harvested and washed with PBS by centrifugation at 750× g for 5 min at 4 ºC. The cell pellets were resuspended in lysis buffer (caspase colorimetric assay kits; Biovision) and left on ice for 30 min. The lysates were centrifuged at 10 000×g for 10 min and the supernatant (20 µL) was used for caspase-3 activity assay in the lysis buffer containing DEVD-pNA, a specific substrate to caspase-3. The concentration of pNA, as the product from enzymatic converting of DEVD-pNA by caspase-3, was measured at 405 nm and used as an indication of caspase-3 activity.
Assessment of cell necrosis The necrotic cell death was measured by the release of lactate dehydrogenase (LDH) into the culture medium, which indicates the loss of membrane integrity and cell necrosis. LDH activity was measured using a commercial assay kit (Cytotoxicity assay kit, Promega), where the released LDH in culture supernatants is measured with a coupled enzymatic assay, which results in the conversion of a tetrazolium salt into a red formazan product. The necrotic percentage was expressed as (sample value/maximal release)×100%, where the maximal release was obtained following the treatment of control cells with 0.5% Triton X-100 for 10 min at room temperature.
Statistic analysis For each experiment involving assessment of cell survival, apoptotic cell, and caspase-3 activity are presented as the mean and standard error (SEM) for four to five experiments. The statistical analysis of data was performed by one-way ANOVA, followed by the Schefft test, and P-values less than 0.05 were considered significant.
Results
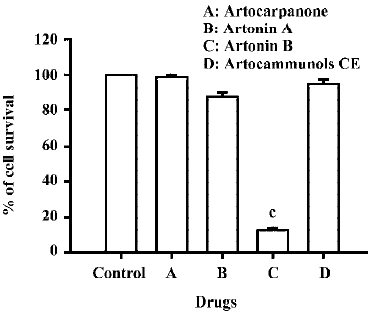
Effects of prenylflavonoid compounds on cytotoxicity in human CCRF-CEM leukemia cells The cytotoxic effects of four prenylflavonoid compounds were examined in human CCRF-CEM leukemia cells. When CCRF-CEM leukemia cells were incubated with 10 µmol/L of four prenylflavonoids, the data showed that artonin B had a more potent cytotoxicity than the other compounds (Figure 2). In addition, the cell survival rate decreased in a dose-dependent manner (Figure 3A). Under the same treatment, artonin B did not cause any cell loss in HaCa cells (Figure 3B). Artonin B elicited a significant decrease of cell survival rate in human CCRF-CEM leukemia cells, which also exhibited a time-dependent manner (Figure 4). After an exposure time of 6 h, the cytotoxic effects were noticed at a concentration of 5 µmol/L and 10 µmol/L of artonin B, with a the survival rate decreasing to 64% at 5 µmol/L and 22% at 10 µmol/L. Accordingly, after 24 h exposure cytotoxic effects started to become apparent at a concentration of approximately 3–10 µmol/L, cell viabi-lity declined considerably at 3 μmol/L and was approximately 10% at 10 µmol/L of artonin B (Figure 4). The IC50 value of artonin B was 3.45±0.50 µmol/L.


Assessment of artonin B-induced cell apoptosis and intracellular events To determine whether the artonin B-induced cytotoxicity was to undergo the apoptotic cell pathway, human CCRF-CEM leukemia cells were incubated in presence of 1–10 µmol/L artonin B for 24 h. The morphological examination reveled that artonin B-treated cells showed typical apoptotic morphological changes, such as cell shrinkage, nuclear fragmentation, and apoptotic body formation (Figure 5). Artonin B-treatment significantly increased the number of cells with apoptotic body formation (Figure 5D,5E), while the control cells and 1–3 µmol/L of artonin B-treated cells showed seldom apoptotic body formation (Figure 5A–5C). The number of apoptotic cells, which carry fragmented nuclear particles, were significantly increased in artonin B-treated cells in a dose-dependent manner (Figure 5F).

The necrotic indication of cellular lactate dehydrogenase (LDH) release was also examined after artonin B treatment (Figure 6). The data showed that 5 μmol/L and 10 μmol/L artonin B induced an increase of LDH release in only 20.59%±2.12% and 28.23%±0.81%, respectively, while the cell survival rates were 35.33%±0.9% and 14.76%±3.56%, respectively. These data indicated necrotic cytotoxicity was less involved in artonin B action. Thus, artonin B induces leukemia cell death employing an apoptotic pathway.

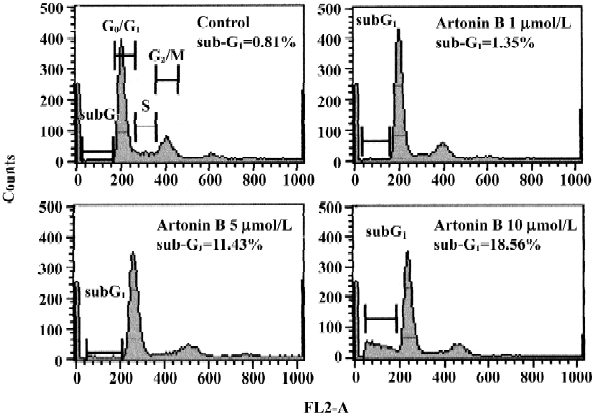
Cell cycle analysis Figure 7 illustrates the changes of DNA content distribution treated with artonin B 0, 1, 5, or 10 µmol/L for 24 h. We examined these cells for DNA degradation characteristic of apoptosis, indicated by hypoploid DNA content using hypotonic PI staining. Exposure of human CCRF-CEM leukemia cells to 1 µmol/L artonin B promoted approximately the same percentage of hypoploid cells observed in the DMSO treated control. As the treatment dose increased the percentage of cells in the hypoploid (sub-G1) phase increased accordingly. Treated with 5 or 10 µmol/L artonin B for 24 h, the rate of sub-G1 phase cells were increased by 11.43% or 18.56%, respectively.
Changes of mitochondrial membrane potential and release of cytochrome c from mitochondria In the present study, the mitochondrial membrane potential and cytochrome c release were analyzed spectrophotometrically. As shown in Figure 8, artonin B-induced a time-dependent mitochondrial transmembrane depolarization, represented as the decrease of mitochondrial membrane potential (Figure 8A). Concomitantly, a time-dependent artonin B-induced cytochrome c release was also observed in human leukemia CCRF-CEM cells, representing a significant increase of cytosolic cytochrome c concentration (Figure 8B). These data suggest that loss of mitochondrial membrane potential may be required for artonin B-induced cytochrome c release into cytosol, that later triggered the cleavage and activation of mitochondrial downstream caspases and onset of apoptosis.

Regulation of Bcl-2 family proteins in artonin B-treated human leukemia CCRF-CEM cells To determine whether Bcl-2 family proteins were modulated in artonin B-induced apoptosis in human leukemia CCRF-CEM cells, the expression of several members of Bcl-2 family proteins was examined by Western blot analysis. As shown in Figure 9, the exposure of human leukemia CCRF-CEM cells to 1–10 µmol/L artonin B resulted in a marked decrease of Bcl-2 protein expression, but a drastic increase of Bax and Bak protein expression.

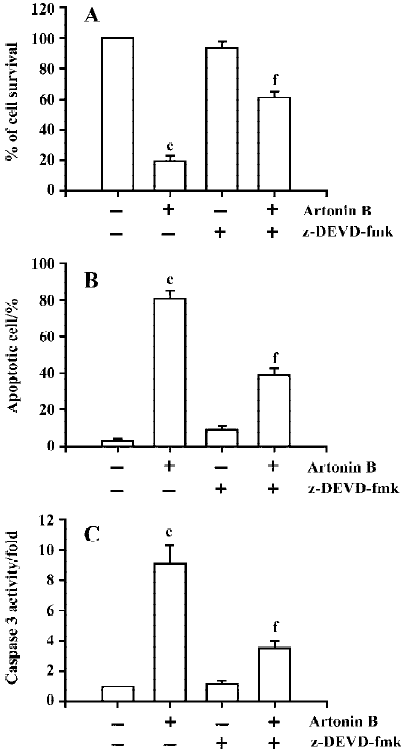
Determination of the involvement of caspase 3 activation Artonin B-induced nuclear fragmentation may thus be an apoptotic event provoked along with endonuclease activation via caspase 3 protein. We also examined the caspase 3 activity under the 1–10 µmol/L artonin B treatment. The present study has demonstrated that artonin B treatment increased caspase 3 activities in human CCRF-CEM leukemia cells in a dose-dependent manner (Figure 10). Human leukemia CCRF-CEM cells were pretreated with 50 µmol/L caspase 3 inhibitor (z-DEVD-fmk) for 2 h, and then induced to undergo apoptosis by treatment with artonin B. The results clearly showed that the administration of caspase 3 inhibitor alone did not affect the caspase 3 activation, apoptotic cell formation, and cell viability (Figure 11). How-ever, z-DEVD-fmk (a specific caspase 3 inhibitor) significantly inhibited artonin B-induced caspase 3 activation, apoptotic cells formation, and cell death in human acute lymphoblastic leukemia cells.

Discussion
In the present work, we demonstrated that artonin B, one of prenylflavonoids, strongly inhibited the growth of human acute lymphoblastic leukemia CCRF-CEM cells, whereas artonin A, artocarpanone, and artocammunols prenylfla-vonoid compounds had no effect on the growth of CCRF-CEM cells. This is a pioneer study of artonin B-induced cell cytotoxicity in human leukemia CCRF-CEM cells. Prenylfla-vonoids exist extensively in plants; however, the structure-activity relationship of their effect is still unknown. These results indicate that heterophyllin structure would be needed in the growth inhibitory effect of prenylflavonoid compounds. Importantly, we observed that artonin B had no effect on the growth of HaCa cells; this is in agreement with data showing that artonin B had a growth inhibitory effect on cancer cells, but not on normal cells. Therefore, artonin B could be a good candidate for acute lymphoblastic leukemia cells therapy without toxicity for normal cells.
Our results revealed that human CCRF-CEM cells treated with artonin B exhibited characteristic morphological features of apoptosis, such as membrane shrinkage chromosomal condensation. The notion that artonin-B treated cells undergo apoptosis rather than necrosis is further supported by the results from cell cycle analysis. The proportion of hypoploid cells (sub-G1) was dramatically increased after artonin-B treatment. These results support the finding that artonin-B induces cell death through apoptotic pathway.
The several mechanisms of activation of apoptosis in different physiological or pathological conditions in cells have been proposed and studied intensively[18]. Numerous factors, such as cytosolic cytochrome c release, the expression of Bcl-2 family proteins, and caspase 3 activation have been suggested to play an essential role in the apoptotic process in cancer cells. Our study demonstrated that a progressive decrease of the mitochondrial membrane potential and release of cytochrome c into the cytosol were observed in artonin-B treated human CCRF-CEM leukemia cells. It has been noticed in many in vitro systems that apoptosis was associated with a loss of mitochondrial membrane potential, which may correspond to the opening of an outer membrane permeability transition pore. Thus, this event has been suggested to be responsible for cytochrome c release into cytosol from mitochondria[19]. In our present study, the cytosolic cytochrome c accumulation in artonin B-induced human CCRF-CEM cells is probably the consequence of the loss of mitochondrial membrane potential, which finally leads to cell death.
The Bcl-2 family is composed of a number of genes that play critical roles in the control of mitochondrial integrity. Several studies have shown that overexpression of Bcl-2 prevents the mitochondrial release of cytochrome c, thereby inhibiting the activation of caspases cascade and apopto-sis[20–23]. In the present study, artonin B-induced apoptosis in human CCRF-CEM leukemia cells was accompanied by upregulation of Bax and Bak and downregulation of Bcl-2. Other studies have demonstrated that Bcl-2, Bax, and Bak can act as channel proteins within the mitochondrial membrane[21,24,25]. It is conceivable that the channel property of Bax and Bak may control the mitochondrial permeability transition and other early mitochondrial perturbation. Thus, Bax and Bak may facilitate the passage of some important proteins, such as cytochrome c or other apoptosis-inducing factors that trigger the activation of caspases cascade and apoptosis. Previous reports have also documented that the ratio of pro-and anti-apoptotic proteins determines, at least in part, the susceptibility of cells to a death signal[21,26,27]. Our results showed that expression of Bcl-2 family proteins Bcl-2, Bax, and Bak can be regulated differently by artonin B, suggesting that the artonin B-induced apoptosis is controlled by a balanced expression between those apoptosis-inducing and apoptosis-suppressing molecules.
Apoptosis is a type of cell death, and agents with the ability to induce apoptosis in tumors have the potential to be used for antitumor therapy. The apoptotic mechanism has been extensively studied, and activation of caspase 3 has been shown to occur in the common apoptotic pathway[28]. The activation of caspases plays a pivotal role in the execution of cell apoptosis[29]. Recent studies have demonstrated that the caspase 3 is a major caspase, which is activated in response to distinct stimuli [30–33]. Moreover, human acute lymphoblastic leukemia CCRF-CEM cells were preincubated with specific caspase 3 inhibitor (z-DEVD-fmk) before treatment of artonin B, and the caspase 3 activity, apoptotic cells and cell viability were analyzed by spectrophotometry analysis, Hoechst 33258 staining and MTT assay, respec-tively. Results showed that pre-incubation of cells with z-DEVD-fmk effectively inhibited artonin B-induced caspase 3 activity, apoptotic cell formation and cell death. Our data reveled that artonin B-induced nuclear fragmentation may be an apoptotic event provoked along with endonuclease activation via a caspase protein[34,35]. We also examined the possibility that caspase 3 was involved in the morphological changes in artonin B-treated cells by measuring the caspase 3 activity with or without its inhibitor, z-DEVD-fmk. Theses data demonstrated that artonin B activated caspase 3 activity and consequent cell death.
However, the development of effective chemopreventive approaches must take into consideration the selective and differential effects manifested by different bioactive substances. Target specific agents that are capable of inducing selective apoptosis of cancer cells, but are harmless to normal cells are receiving considerable attention in the fields of cancer prevention and therapy[36]. Artonin B-induced cell cytotoxicity on human leukemia CCRF-CEM cells, but not on normal cells. Therefore, artonin B is a candidate for development as a chemopreventive agent.
In summary, our results demonstrate that cell cytotoxicity induced by artonin B in human CCRF-CEM leukemia cells is possibly mediated through apoptotic cell formation, mitochondrial pathways, Bcl-2 family protein expression, and the activation of caspase 3. However, artonin A, artocarpanone, and artocammunols CE have no effect on the cell survival rate of human CCRF-CEM leukemia cells. By analyzing the structure, more effective compounds might be reconstruc-tured and new strategies for cancer therapy can be explored.
Acknowledgements
We would like to thank Dr C N LIN (School of Pharmacy, Kaohsiung Medical University, Taiwan, China) for his generosity in providing the drugs.
References
- Lin HY, Uan SH, Shen SC, Hsu FL, Chen YC. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem Pharmacol 2003;66:1821-32.
- Wang YH, Hou AJ, Chen L, Chen DF, Sun HD, Zhao QS, et al. New isoprenylated flavones, artochmins A-E, and cytotoxic principles from Artocarpus chama. J Nat Prod 2004;67:757-61.
- Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta 1999;1472:643-50.
- Wang WS, McLean AE. Effects of phenolic antioxidants and flavonoids on DNA synthesis in rat liver, spleen, and testis in vitro. Toxicology 1999;139:243-53.
- Lea MA, Xiao Q, Sadhukhan AK, Cottle S, Wang ZY, Yang CS. Inhibitory effects of tea extracts and (-)-epigallocatechin gallate on DNA synthesis and proliferation of hepatoma and erythroleukemia cells. Cancer Lett 1993;68:231-6.
- Larocca LM, Giustacchini M, Maggiano N, Ranelletti FO, Piantelli M, Alcini E, et al. Growth-inhibitory effect of quercetin and presence of type II estrogen binding sites in primary human transitional cell carcinomas. J Urol 1994;152:1029-33.
- Komori A, Yatsunami J, Okabe S, Abe S, Hara K, Sganuma M, et al. Anticarcinogenic activity of green tea polyphenols. Jpn J Clin Oncol 1993;23:186-90.
- Ren S, Lien EJ. Natural products and their derivatives as cancer chemopreventive agents. Prog Drug Res 1997;48:147-71.
- Hano Y, Aida M, Shiina M, Nomura T, Kwai T, Hiroshi O, et al. Artonin A and Artonin B, two new prenylflavones from the root vark of Artocarpus Heterophyllus Lamk. Heterocycles 1989;29:1447-53.
- Wyllie AH. Apoptosis. Br J Cancer 1993;67:205-8.
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995;267:1456-62.
- Borner C. The bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 2003;39:615-47.
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Ann Rev Biochem 1999;68:383-424.
- Venkataraman K. Wood phenolics in the chemotaxonomy of the moraceae. Phytochemistry 1972;11:1571-86.
- Chan SC, Ko HH, Lin CN. New prenyulflavonoids from Artocarpus communis. J Nat Prod 2003;66:427-430.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Lin HI, Lee YJ, Chen BF, Tsai MC, Lu JL, Chou CJ, et al. Involvement of Bcl-2 fmaily, cytochrome c release and caspase 3 in induction of apoptosis by beauvericin in human non-small cell lung cancer cells. Cancer Lett 2005;230:248-59.
- Vaux DL, Korsmeyer SJ. Cell death in development. Cell 1999;96:245-54.
- Kantrow SP, Piantasdosi CA. Release of cytochrome c from liver mitochondria during permeability transition. Biochem Biophys Res Commun 1997;232:669-71.
- Solange D, Martinou JC. Mitochondria as the central control point of apoptosis. Trends cell Biol 2000;10:369-77.
- Gross A, McDonnell JM, Korsmeyer SJ. Bcl-2 family members and mitochondrial in apoptosis. Genes Dev 1999;13:1899-911.
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999;16:269-90.
- Salvesen G, Dixit V. Caspase activation: the induced proximity model. Proc Natl Acad Sci USA 1999;96:10964-7.
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 2000;20:929-35.
- Wood DE, Newcomb EW. Cleavage of Bax enhances its cell death function. Exp Cell Res 2000;256:375-82.
- Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis. Nat Cell Biol 1999;1:E209-16.
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science 2000;290:989-92.
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Ann Rev Biochem 1999;68:383-424.
- Polverino AJ, Patterson SD. Selective activation of caspases during apoptotic induction in HL-60 cells. J Biol Chem 1997;272:7013-21.
- Springer JE, Nottingham SA, McEwen ML, Azbil RDL, Jin Y. Caspase-3 apoptotic signaling following injury to the central nervous system. Clin Chem Lab Med 2001;39:299-307.
- Wang J, Lenardo MJ. Roles of caspases in apoptosis, development, and cytokine maturation revealed by homozygous gene defici-encies. J Cell Sci 2000;113:753-7.
- Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 1998;391:496-9.
- Amos CL, Woetmann A, Nielsen M, Geisler N, Brown BL, Dobson PR. The role of caspase 3 and BclxL in the action of interleukin 7 (IL-7): A survival factor in activated human T cells. Cytokine 1998;10:662-8.
- Masuda Y, Nakaya M, Nakajo S, Nakaya K. Geranulgeraniol potently induces caspase-3-like activity during apoptosis in human leukemia U937 cells. Biochem Biophys Res Commun 1997;234:641-5.
- Arita K, Utsumi T, Kato A, Kanno T, Kobuchi H, Inoue B, et al. Mechanism of dibucaine-induced apoptosis in promyelocytic leukemia cells (HL-60). Biochem Pharmacol 2000;60:905-15.
- Mukherjee AK, Basu S, Sarkar N, Ghosh AC. Advances in cancer therapy with plant-based natural products. Curr Med Chem 2001;8:1467-87.
