Microtubule stability and MAP1B upregulation control neuritogenesis in CAD cells
Introduction
The dynamics of the cytoskeleton network is essential for the development of neuronal processes[1]. Microtubules are one of the major cytoskeleton elements that are particularly abundant in neurons, which not only serve as the scaffolding for neurite formation, but also provide a means for transporting organelles and macromolecules that are essential for the growth and maintenance of developing neurites. The dynamic organization of microtubules is controlled by a variety of microtubule associated proteins (MAPs), including MAP1A, MAP1B, MAP2, and tau[2]. All MAPs stabilize microtubules, which in turn govern normal cell function, growth and development. Nocodazole is an antimitotic agent that disturbs microtubules by binding to β-tubulin and preventing formation of one of the two interchain disulfide linkages. Taxel is the prototype of the taxane family of antitumor drugs. It was the first natural product shown to stabilize microtubules by formation of non-functional microtubule bundles. Drugs that disturb microtubule stability or satalilize microtubules, represented by nocodazole and taxol respectively, abrogate neurite outgrowth and axonal extension[3]. Therefore, the balance between microtubule stability and destabilization is essential for neurite development.
Accumulating evidence suggests that MAP1B is an important player that regulates microtubule stability in early brain development[4]. In mice, MAP1B is predominantly expressed in the nervous system and is the first MAP expressed in the embryonic brain[5]. During active neuritogenesis, MAP1B is vigorously upregulated in primary neuronal cultures and in neuronal cell lines upon induced differentiation[6]. However, MAP1B expression gradually declines during neonatal development and is limited in the adult brain to the regions that maintain plasticity for neural regeneration. Furthermore, a marked increase of MAP1B expression is associated with drug-induced axonal sprouting as well as lesion-induced neuronal regeneration[7]. These observations collectively suggest that upregulation of MAP1B is crucial for neurite extension. Indeed, deficient MAP1B expression, either by anti-sense mediated knockdown of MAP1B in PC12 cells or by genetic disruption of the MAP1B gene in transgenic mice[4], results in severe deficits in neurite extension and axonal formation.
Although the level of MAP1B gene expression during development has been extensively studied, the exact role of MAP1B on microtubule stability during neurite extension has not been defined. In this study, we report that treatment by either nocodazole or taxol can block neurite outgrowth of the CAD catecholaminergic neuronal cell line, indicating that both polymerization and disassembly of microtubules are necessary for neuritogenesis. Interestingly, neurite extension is more sensitive to nocodazole than taxol, suggesting that neuritogenesis requires high levels of activity in stabilizing newly assembled microtubules. MAP1B is the major MAP in CAD cells and is markedly upregulated upon induced neurite extension. Acetylated-tubulin is a marker of microtubule stability, tyrosinated-tubulin represent tubulin in activation. We focus on the consequence of MAP1B upregulated and the accompanied changes of acetylated-tubulin and tyrosinated-tubulin in neurogenesis to provide the first evidence suggesting the role of MAP1B upregulated on microtubule stability in CAD cells. Further studies on detailed mechanisms such whether MAP1B upregulation is translational or post-translational and how MAP1B enhance tubulin acetylation will be needed to strengthen our results.
Materials and methods
Chemicals and treatment Nocodazole powder (Sigma-Aldrich, St Louis, MO,USA) was reconstituted in sterile 10% DMSO- 0.01mol/L PBS at 10 mg/mL and stocked at -20 °C. Taxol (7 mg/mL) was kindly provided by Dr Paraskevi Giannakakou (Winship Cancer Institute, Emory University). All drug treatments were carried out in DMEM/F12 (Dulbecco’s Modified Eagle Medium:Nutrient Mixture F12)with final concentrations of 10 or 30 nmol/L for nocodazole, 3.3 or 10 µmol/L for Taxol at 37 °C. CAD cells were treated for 2 h or overnight (16 h) before fixation and staining.
Cell culture and cell lysate The CAD cell line was maintained for proliferation in DMEM/F12 containing 8% Fatel bovine Serum (Invitrogen Corporation,Grand Island,NY,USA). Differentiation was induced by switching proliferating cells to a chemically defined media as described in a previous study[8]. For whole cell lysate preparation, CAD cells were harvested before being subjected to sonication in 1× Laemmli buffer[9]. Primary cultured cortical neuron were dissociated from mouse embryos at 18 d of gestation and cultured in neurobasal medium with 10% FBS.
Antibodies and immunoblotting SDS-PAGE immunoblot analysis was performed using the standard procedure. The primary antibodies were diluted as follows: acetylated α-tubulin, 1:1000 (Sigma); tyrosinated-tubulin, 1:400 (Sigma); eIF5, 1:5000 (Santa Cruz Biotechonology, Santa Cruz,CA,USA). Antibodies against MAP1B (1:10 000) were provided by Dr I Fisher (Drexel University, Philadelphia).
Indirect immunofluorescent staining Cells cultured in circular 13-mm diameter glass coverslips were fixed in 3% paraformaldehyde in PHEMO buffer (68 mmol/L PIPES, 25 mmol/L HEPES acid, 15 mmol/L EGTA Na2, 3 mmol/L MgCl2, 10% DMSO) at room temperature. After washing, the slides were blocked in PBS containing 5% normal goat serum for 1 h at room temperature followed by incubation with primary antibodies at room temperature for another hour. Primary antibodies were diluted as follows: MAP1B, 1:500; α-tubulin (Chemicon International Inc., Temecula, CA, USA), 1:1000; acetylated-tubulin (Sigma-Aldrich,St Louis,MO,USA), 1:400; tyrosinated-tubulin (Sigma-Aldrich, St Louis, MO,USA), 1:400. FITC or Texas Red conjugated secondary antibodies were then incubated with the slides for 30 min at room temperature. After washing, CAD cells were detected with fluorescence staining using an Olympus fluorescence microscope and the ZeissLMS510 confocal microscopic imaging system (Carl Zeiss Inc., Thornwood, NY, USA).
Results
Microtubule dynamics were essential for the outgrowth and maintenance of neurites during CAD cell differentiation CAD is a mouse CNS catecholaminergic neuronal cell line that can be induced for differentiation in a chemically defined medium[8]. As shown in Figure 1A, vigorous neurite outgrowth occurred within 16 h of induced differentiation (Figure 1A, c). These neurites rapidly extended in the next few days (3-d, 5-d differentiated CAD in Figure 1A, e and g), similar to what was observed in primary cultures of mouse cortical neurons (Figure 1A, d, f, h). The length of those differentiated CAD cell neurites (Figure 1A, c, e, g) were comparable with that in primary cortical neurons (overnight,3-d, 5-d cultured neurons). To determine the role of microtubule dynamics during induced neuritogenesis in CAD cells, we treated differentiating CAD cells with various concentrations of nocodazole and taxol to inhibit microtubule polymerization and microtubule disassembly, respectively (the drug concentration range was tested before formal experiments were performed). The drug treatments were repeated independently more than 3 times. All experimental results are stable. After 2 h or 12 h of drug treatment, the cultured cells were immediately fixed with 3% paraformaldehyde in PHEMO buffer and performed by α-tubulin fluorescence staining. As shown in Figure 1B, a, after 24 h of differentiation, most cells neurites extended longer than the diameter of the soma. While mocked-treated cells’ (without drug treatment) neurites continued to elongate for another 12 h (Figure 1B, b), treatment with either nocodazole (Figure 1B, c and e) or taxol (Figure 1B, d and f) for 12 h blocked further extension of the developing neurite in a dose-dependent manner. Furthermore, higher doses of nocodazole and taxol resulted in retraction of the extended neurite that had undergone 24 h (Figure 1C, b and c) and 48 h of differentiation (Figure 1C, e and f). Noticeably, neurites that underwent 48 h of development were less sensitive to drug-induced retraction (Figure 1C, e and f compared to b and c). These data indicated that both polymerization and depolymerization of microtubules are necessary for the outgrowth and the maintenance of the developing neurite.
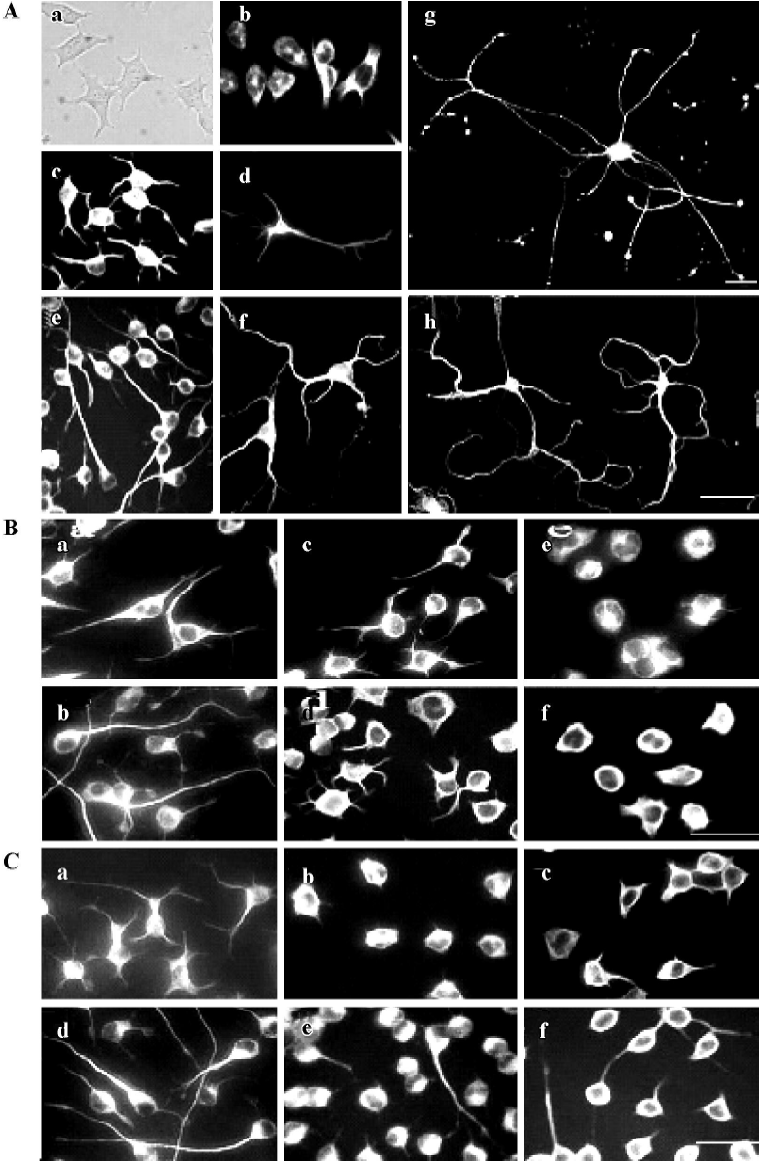
Neuritogenesis of CAD cells is highly sensitive to the disturbance of microtubule polymerization As shown in Figure 1 B and 1C, the molar concentration of nocodazole, which effectively blocked neurite extension of CAD cells, was approximately 300-fold lower than that of taxol. To further investigate the effects of nocodazole and taxol on the microtubule network, we performed indirect immunofluorescence staining of α-tubulin by confocal microscopy (Figure 2). Under proliferation (Figure 2A and 2C) or differentiation (Figure 2D and 2F) conditions, exposure to nocodazole (Figure 2C and 2E) or taxol (Figure 2B and 2F) resulted in retraction of growth cones and neurite shafts, and reduction of soma volume. Taxol treatment resulted in marked bundling of microtubule fibers in the soma and in the residual neurites (arrows in Figure 2B and 2F). In contrast to taxol, the effective dose of nocodazole that blocked neuritogenesis resulted in negligible disturbance of the microtubule network at the global level including soma and neurites (Figure 2C and 2E).
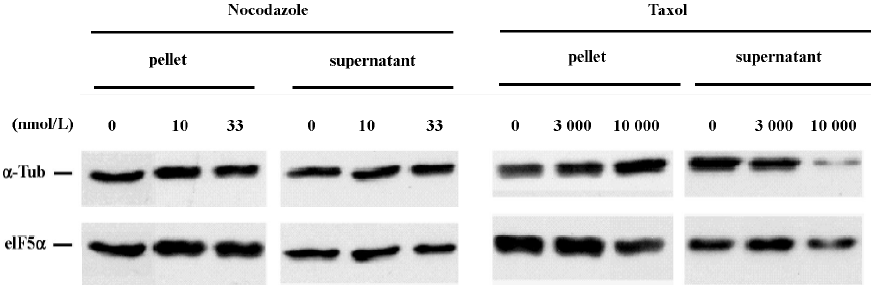
We next performed SDS-PAGE immunoblot analysis to examine the effect of nocodazole and taxol on microtubule polymer mass. Analysis of α-tubulin distribution in pellet microtubule polymers (P) and in cytosolic supernatant (S) revealed that the effective dose for nocodazole to block neuritogenesis did not significantly alter the amount of microtubule polymers (Figure 3). In contrast, the effective dose for taxol to block neuritogenesis caused a marked increase of α-tubulin in the microtubule pellet with a concomitant reduction of α-tubulin in the cytosolic supernatant. The immunoblot experiments were repeated more than 3 times. These results suggest that taxol may block neuritogenesis by sequestering tubulin monomers into stable but non-functional microtubule bundles, whereas nocodazole may abrogate neurite extension by increasing microtubule dynamic instability, perhaps in the extending neurites without altering microtubule polymer mass. The fact that neuritogenesis is more sensitive to nocodazole than taxol (Figures 1 and 2) suggests that high levels of activity for new microtubule assembly may be a driving force for neurite extension in CAD cells.
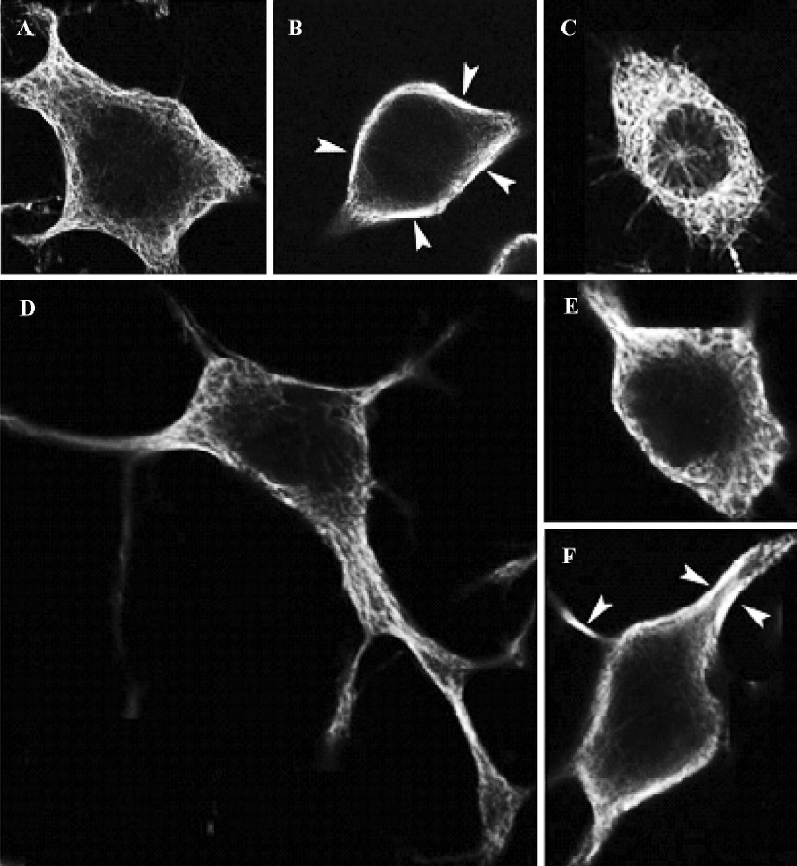
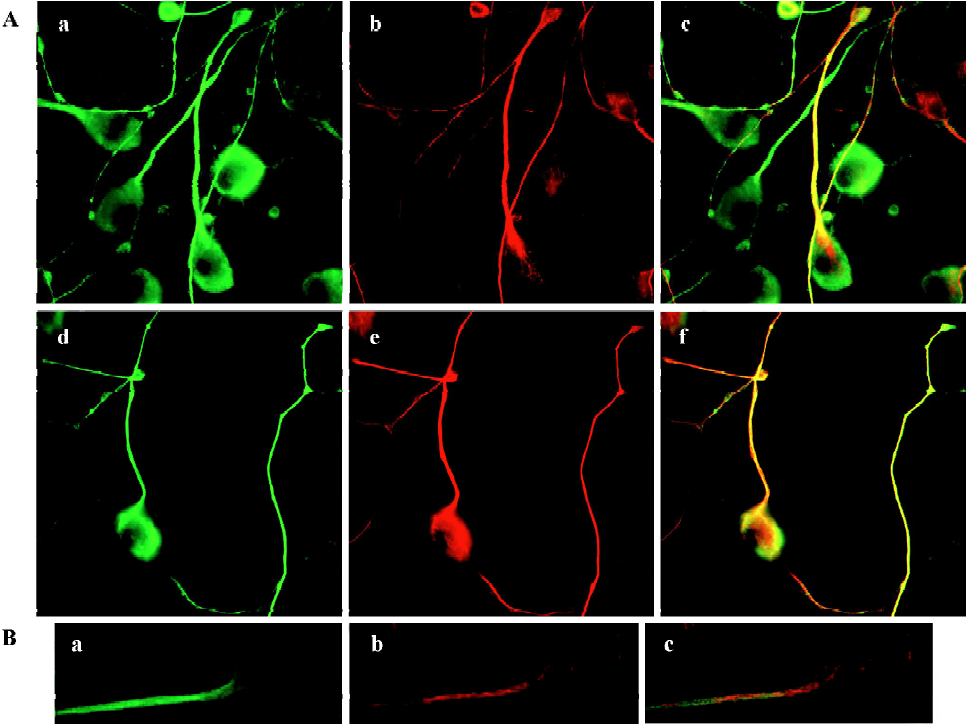
MAP1B expression was upregulated in CAD cells upon induced neurite extension, accompanied with increased microtubule stability Several lines of evidence indicated that MAP1B plays critical roles in regulating microtubule dynamics during early neural development[4]. Thus, we next questioned whether MAP1B may be involved in the neurito-genesis of CAD cells. Immunofluorescent staining detected MAP1B protein in both the soma and the neurite in differentiated CAD cells (Figure 4). The signal of MAP1B in neurites appeared more intense as compared to that in the soma (Figure 4A, a and d), suggesting that MAP1B may exert more influence on microtubule stability in the developing neurite. Acetylated-tubulin, a marker for stable microtubules, was predominantly localized in the developing neurite shafts (Figure 4A, b), whereas tyrosinated-tubulin, which is present in non-polymer tubulin as well as in newly assembled unstable microtubules, was detected throughout entire neurons (Figure 4A, e). MAP1B was colocalized with tyrosinated-tubulin in most of neurite shafts and the extremities of neurite (Figure 4A, d and f).MAP1B was also located in most of the smaller bulb-like growth cones along neurite shafts (Figure 4A, a), where there was no obvious acetylated-tubulin staining (Figure 4A, b and c), they may be temporary or immature during the neuritogenesis. Furthermore, confocal microscopy confirmed the preferential localization of MAP1B in the developing neurites (Figure 4B, a). Acetylated-tubulin located in differentiated CAD cells (Figure 4B, b) show intense staining in the main neurite shafts and the portion of soma where the neurite was given. So, not all of MAP1B overlapped with acetylated-tubulin. The different location between MAP1-B and acetylated-tubulin was clearly indicated in the merge image (Figure 4B, c). These staining patterns are consistent with the view that differentiation of CAD cells is associated with stabilization of microtubules in the developing neurites.
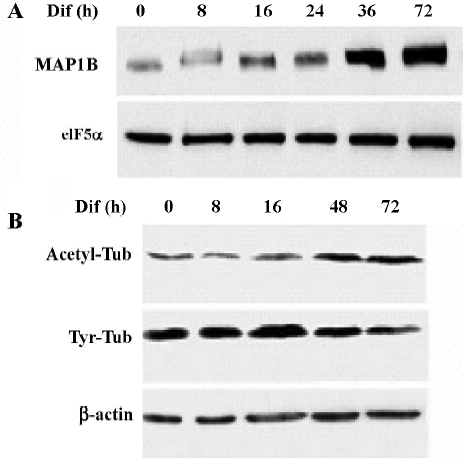
To determine whether MAP1B expression was regulated during neuritogenesis of CAD cells, we analyzed the level of MAP1B protein. MAP1B protein was vigorously upregulated when cells were induced to differentiate for 36–72 h, suggesting that MAP1B may play a critical role in promoting neurite extension (Figure 5A). In addition, a reduction of tyrosinated-tubulin and a concomitant increase of acetylated-tubulin were associated with the increase of MAP1B protein during neurite extension (Figure 5B). This result indicates that microtubule stability was increased during CAD cell differentiation. The elevated MAP1B expression in differentiated CAD cells most likely contributes to the stabilization of the newly formed microtubule polymers.
Discussion
The above results suggest that neuritogenesis during the differentiation of CAD cells requires well-balanced polymerization and depolymerization of microtubule polymers. Active microtubule assembly appears to be the dominant force for neurite growth, since the effective molar concentration of nocodazole that blocks neurite extension is much lower than that of taxol. Furthermore, elevated MAP1B expression provides a possible mechanism for stabilizing the newly formed microtubule polymers in the developing neurite, which in turn promotes neurite extension.
At steady state, the developing neurites of CAD cells harbor abundant microtubule arrays[8]. Treatment by either nocodazole or taxol abrogated neuritogenesis, suggesting that both polymerization and disassembly of microtubules are crucial for the extension and maintenance of the developing neurite (Figure 1B and 1C). This is consistent with the recent report that both nocodazole and taxol interfered with neurite elongation in primary cultures of neurons[10]. However, the molar concentration that effectively blocks neuritogenesis of CAD cells by nocodazole was 300-fold lower than that by taxol (Figures 1 and 2). Furthermore, the effective concentration for nocodazole to block neuritogene-sis in CAD cells was much lower than that employed for primary cultures of cortical neurons[10]. This result indicates that neuritogenesis in CAD cells is highly sensitive to the disturbance of microtubule polymerization and the consequential microtubule disassembly. Nevertheless, the increase of acetylated-tubulin and the concomitant decrease of tyrosinated-tubulin during induced differentiation of CAD cells (Figure 5B) suggest that neurite extension is associated with a net increase of microtubule stability, most likely resulting from stabilization of the newly assembled microtubule network. The elevated MAP1B expression during neurite extension in CAD cells supports the hypothesis that MAP1B is involved in the developmentally-programmed stabilization of microtubules. One interesting point to mention is that CAD cells express abundant levels of MAP1B but low levels of other MAPs that are expressed in mature neurons, such as MAP1A, MAP2 and tau (data not shown). This suggests that CAD cells most likely represent neurons at an early stage of development when MAP1B is the major MAP that stabilizes microtubules. The fact that MAP2 and tau harbor stronger activity in the stabilization of microtubules than MAP1B[11] may explain why neuritogenesis in CAD cells is highly sensitive to microtubule destabilization caused by nocodazole.
Interestingly, the effect of taxol and nocodazole on the microtubule network is more noticeable in the proximal protrusion of neurites than that in the soma of differentiating CAD cells (Figure 2), suggesting that microtubules in the developing neurites are more dynamic than that in the cell body. In particular, the effective dose for taxol that blocks neuritogenesis resulted in marked sequestration of free tubulin into non-functional microtubule bundles (Figures 2 and 3). This observation suggests that taxol-dependent abrogation of neuritogenesis may result from a lack of sufficient free tubulin to support ongoing microtubule polymerization. However, abrogation of neuritogenesis by low concentrations of nocodazole without disturbing microtubule polymer in the soma (Figures 1 and 2), and the predominant localization of acetylated-tubulin in the developing neurite shafts (Figure 4), further suggest that stabilization of newly assembled microtubules in the developing neurites may serve as a driving force for neuritogenesis.
The low level expression of other mature MAPs, such as MAP1A, in CAD cells resembles neurons at the early stage of development (data not shown). The vigorous upregulation of MAP1B protein during neurite elongation (Figure 5A) supports the hypothesis that MAP1B is a positive factor for promoting neurite extension. Our results show that MAP1B was colocalized with tyrosinated-tubulin (unstable microtubules), mainly in the neuritis and the growth cone (Figure 4A). Consistent with this idea, the upregulation of MAP1B was also observed during neuritogenesis of PC12 cells[12]. Furthermore, blocking MAP1B expression abrogated neurite outgrowth in PC12 cells[13], demonstrating that MAP1B is indeed essential for neuritogenesis. The more abundant localization of MAP1B in the developing neurite as compared to that in the soma (Figure 4) suggests that MAP1B may primarily function in the developing neurite.
However, the exact role of MAP1B in promoting neurite development and in regulating microtubule dynamics remains unclear. Over-expression of MAP1B has been shown to moderately stabilize microtubules against nocodazole[14,15]. However, an isoform of phosphorylated MAP1B has been reported to colocalize with unstable microtubules, particularly in the neuronal axons and the growth cone[12]. Hence, phosphorylated MAP1B may also play a role in maintaining the dynamic instability of microtubules in a localized manner during neurite development and synapse formation/re-construction. Nonetheless, the upregulation of MAP1B during neuritogenesis of CAD cells associated with increased acetylated-tubulin and decreased tyrosinated-tubulin suggests a net effect on enhanced microtubule stabilization in this model system. Together with the fact that the neuritogenesis of CAD cells is highly sensitive to nocodazole-mediated microtubule depolymerization (Figures 1 and 2), our results support the hypothesis that the upregulation of MAP1B promotes neurite extension by stabilizing the newly assembled microtubule network in the developing neurite.
References
- Lu R, Wang H, Liang Z, Ku L, O’donnell WT, Li W, et al. The Fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA 2004;101:15201-6.
- Noiges R, Eichinger R, Kutschera W, Fischer I, Nemeth Z, Wiche G, et al. Microtubule-associated protein 1A (MAP1A) and MAP1B: light chains determine distinct functional properties. J Neurosci 2002;22:2106-14.
- Chuckowree JA, Vickers JC. Cytoskeletal and morphological alterations underlying axonal sprouting after localized transection of cortical neuron axons in vitro. J Neurosci 2003;23:3715-25.
- Gonzalez-Billault C, Avila J, Caceres A. Evidence for the role of MAP1B in axon formation. Mol Biol Cell 2001;12:2087-98.
- Ma D, Nothias F, Boyne LJ, Fischer I. Differential regulation of microtubule-associated protein 1B (MAP1B) in rat CNS and PNS during development. J Neurosci Res 1997;49:319-32.
- Gonzalez-Billault C, Owen R, Gordon-Weeks PR, Avila J. Microtubule-associated protein 1B is involved in the initial stages of axonogenesis in peripheral nervous system cultured neurons. Brain Res 2002;943:56-67.
- Gonzalez-Billault C, Avila J. Molecular genetic approaches to microtubule-associated protein function. Histol Histopathol 2000;15:1177-83.
- Qi Y, Wang JK, McMillian M, Chikaraishi DM. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J Neurosci 1997;17:1217-25.
- Li Z, Zhang Y, Li D, Feng Y. Destabilization and mislocalization of myelin basic protein mRNAs in quaking dysmyelination lacking the QKI RNA-binding proteins. J Neurosci 2000;20:4944-53.
- Chuckowree JA, Vickers JC. ytoskeletal and morphological alterations underlying axonal sprouting after localized transection of cortical neuron axons in vitro. J Neurosci 2003;23:3715-25.
- Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J Cell Sci 1992;103:953-64.
- Goold RG, Gordon-Weeks PR. Microtubule-associated protein 1B phosphorylation by glycogen synthase kinase 3beta is induced during PC12 cell differentiation. J Cell Sci 2001;114:4273-84.
- Brugg B, Reddy D, Matus A. Attenuation of microtubule-associated protein 1B expression by antisense oligodeoxynucleotides inhibits initiation of neurite outgrowth. Neuroscience 1993;52:489-96.
- Noiges R, Eichinger R, Kutschera W, Fischer I, Nemeth Z, Wiche G, et al. Microtubule-associated protein 1A (MAP1A) and MAP1B: light chains determine distinct functional properties. J Neurosci 2002;22:2106-14.
- Marcus AI, Zhou J, O’Brate A, Hamel E, Wong J, Nivens M, et al. The synergistic combination of farnesyl transferase inhibitor lonafarnib and paclitaxel enhances tubulin acetylation and requires a functional tubulin deacetylase. Cancer Res 2005;65:3883-93.
