Discovering novel 3-nitroquinolines as a new class of anticancer agents1
Introduction
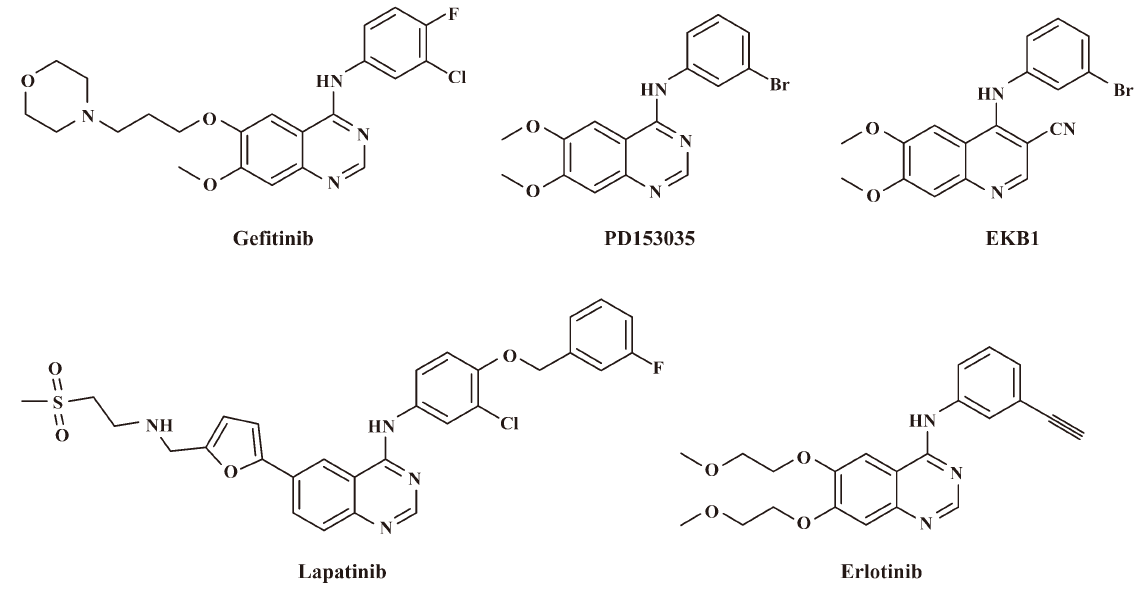
Receptor tyrosine kinases play crucial roles in signal transduction pathways that regulate cell differentiation and proliferation[1]. The overexpression of certain growth factor receptor kinases is strongly associated with carcinogenesis[2]. The epidermal growth factor receptor (EGFR/Her-1/ErbB-1), which belongs to the ErbB receptor family, is a 170 kDa glycoprotein that contains an extracellular ligand-binding domain, a transmembrane region, and an intracellular domain with kinase activity[3]. A strong correlation has been found between solid tumors with high levels of EGFR and poor prognosis[4]. Thus, EGFR is an attractive target for the design and development of compounds that can specifically bind to the receptor and inhibit its tyrosine kinase (TK) activity and its signal transduction pathway in cancer cells. A variety of approaches can be used to target EGFR family members, and the most popular 2 have been extensively explored for cancer chemotherapy against cancers that overexpress EGFR family receptors: blocking ligands binding to the extracellular domain with humanized monoclonal antibodies, and using small molecule inhibitors that interact at the ATP-binding site[5]. The most promising small molecule inhibitors of the EGFR kinase are currently several scaffolds, which include quinazolines[5–10], pyridopyrimidines[11,12], benzamides[13–15], indolinones[16], and pyrrolotriazines[17]. Of these, the 4-anilinoquinazoline derivatives exhibit IC50 values up to the subnanomolar range in enzymatic assays[18,19]. Figure 1 includes some representative small molecule inhibitors in the 4-anilinoquinazoline series that are potent inhibitors of the EGFR kinase, in which gefitinib[20], erlotinib[21], and lapatinib[22] are currently used in the market.
The crystal structure of OSI-774/EGFR-TK indicates that the nitrogen atom located at the 3-position of these quinazoline inhibitors is an important feature for good activity[23]. This nitrogen atom could be interacting with a water molecule, and that this water molecule could then serve as a bridge between the drug and enzyme. Replacing this atom with a carbon leads to a significant loss in the ability of the compound to inhibit the enzyme. According to this, Wissner[24] et al removed and replaced this nitrogen atom with a carbon atom that had an attached cyano group. A series of 4-anilinoquinoline-3-carbonitriles was then synthesized, and some of them exhibited significant ability in inhibiting the EGFR kinase.
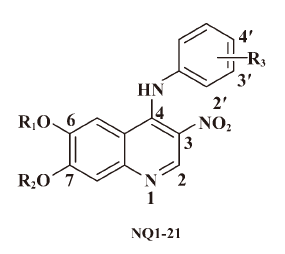
Chen et al noted that when there was an indirect, water-mediated hydrogen bond from an inhibitor to the protein, there is good reason to attempt to build into the space occupied by the water molecule[25]. Wissner et al indicated that the space due to removal of the water molecule bound to Thr830 could accommodate a small group. On the basis of these considerations, we planned to design novel nitroquinolines related to Ekb1, in which the cyano group at the 3-position is replaced by the nitro group. The aim of the study was to find new structural types of EGFR kinase inhibitors. In this present work, we report on the synthesis and biological activity of a series of novel 3-nitroquinoline derivatives represented by the general formula of nitroquinolines in Figure 2. The cellular activity in relevant tumor cell lines will be discussed to develop the structure-activity relationship of this new series. This work was the first to explore the effect of 3-nitro group substitution on the EGFR kinase activity of the 4-anilinoquinoline series. Significantly, several of these compounds have shown promising antiproliferative effects against EGFR-overexpressing tumor cell lines.
Materials and methods
Synthetic procedures An efficient and facile syn-thesis approach was developed to prepare a variety of 3-nitroquinoline derivatives with various C-4, C-6, and C-7 substituents. As depicted in Figure 3, the straightforward 8-step synthetic route allowed us to diversify position 4 of the quinoline moiety via the key intermediate 8 at a later stage.
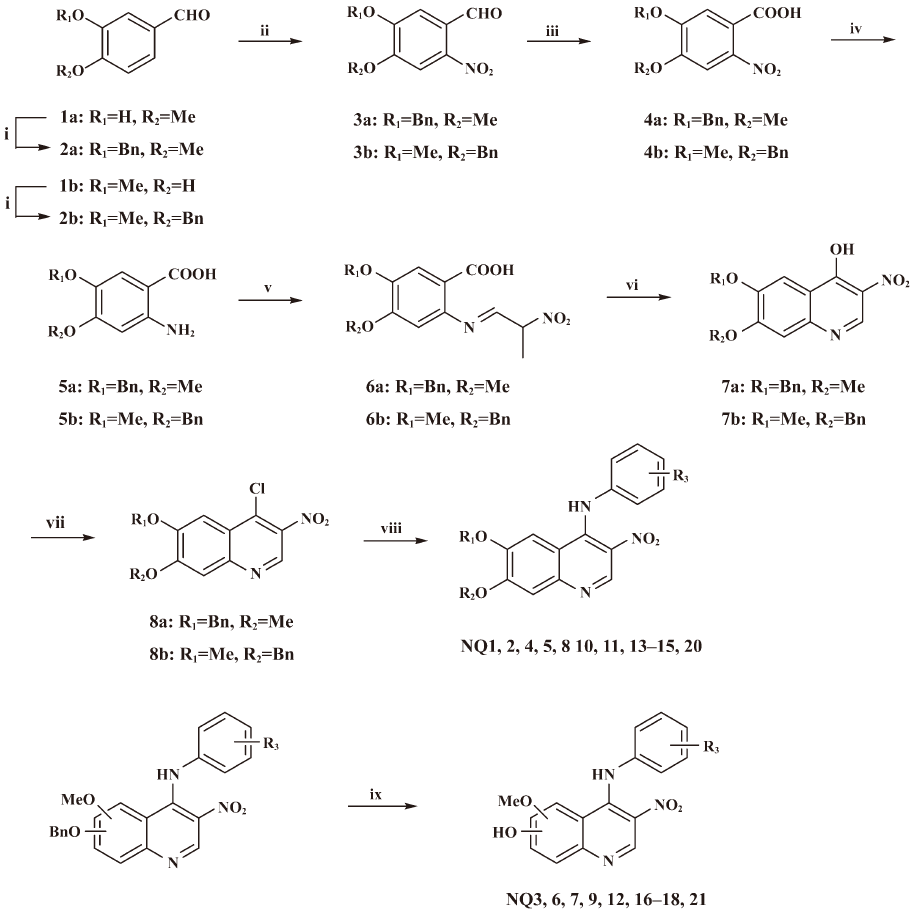
Beginning with the commercially-available isovanillin or vanillin, benzylation with benzyl bromide gave aldehyde 2 in good yield. The treatment of 2 with fuming nitric acid furnished selective nitration product 3. Compound 3 was converted to the corresponding o-nitrobenzoic acid 4 by refluxing with 10% KMnO4. Afterwards, the nitro group was reduced and was then condensed with nitromethane followed by thermal cyclization in refluxing aceticanhydride, giving key intermediate 7. The quinoline was converted in good yield to the corresponding chloroquinoline 8 by refluxing in an excess of POCl3. Then, refluxing a solution of a chloroquinoline and a substituted aniline derivative in DMF generated the desired final products, 3-nitro-4-anilino-6,7-dialkoxyquinolines (NQ1, 2, 4, 5, 8, 10, 11, 13–15, 20) in good yield. Debenzylation of these benzyl group-containing compounds through hydrogenolysis catalyzed by 10% Pd/C afforded NQ3, 6, 7, 9, 12, 16–18, and NQ21 in excellent yields. The nucleophilic displacement of ethyl bromoacetate with 4-(4-ethoxyphenylamino)-7-methoxy-3-nitroquinolin-6-ol in basic refluxing DMF solution generated NQ19.
Cell growth inhibition assay Human breast adenocarcinoma cancer cell MDA-MB-468 and epidermoid carcinoma cancer cell A431 were obtained from ATCC (Manassas, VA, USA) and used for the cell proliferation assay. Both cell lines were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, Grand Island, NY, USA) in a highly-humidified atmosphere of 95% air with 5% CO2 at 37 ºC. The cytotoxity of the compounds was analyzed by the sulforhodamine B (SRB; Sigma, St Louis, MO, USA) assay. Briefly, the cells were seeded at 6000 cells/well in 96-well plates (Falcon, San Mateo, CA, USA) and allowed to attach overnight. The cells were treated in triplicate with graded concentrations of compounds at 37 ºC for 72 h. After being fixed with 10% trichloroacetic acid at 4 ºC for 1 h, the cells were stained with 100 μL SRB solution (0.4% w/v in 1% acetic acid) for 15 min and washed with 1% acetic acid to remove any unbound dye. Bound dye was solubilized with 10 mmol/L Tris base (pH 10.5). The absorbance values of the plates were measured using a multiwell spectrophotometer (VERSAmax; Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 515 nm. The growth inhibitory rate of treated cells was calculated by the following formula: (1–[A515 treated/A515 control])×100%. The results were also expressed as IC50 (the compound concentration required for 50% growth inhibition of tumor cells), which was calculated by the Logit method.
Molecular docking To explore the interaction mechanism between the novel 3-nitroquinoline derivatives and the EGFR kinase, molecular docking was carried out with the AutoDock 3.0.5 program[26,27]. The 3-D structures of the target proteins of human EGFR are from the Protein Data Bank (entry N
Results
Analog design and synthesis On the basis of the enzyme-binding features of Ekb1, introducing a nitro group at the 3-position of the quinoline core, a series of novel 3-nitroquinolines were designed and synthesized; their chemical structures are shown in Table 1. These compounds were synthesized through the route outlined in Scheme 1, and the details for the synthetic procedures have been previously described.
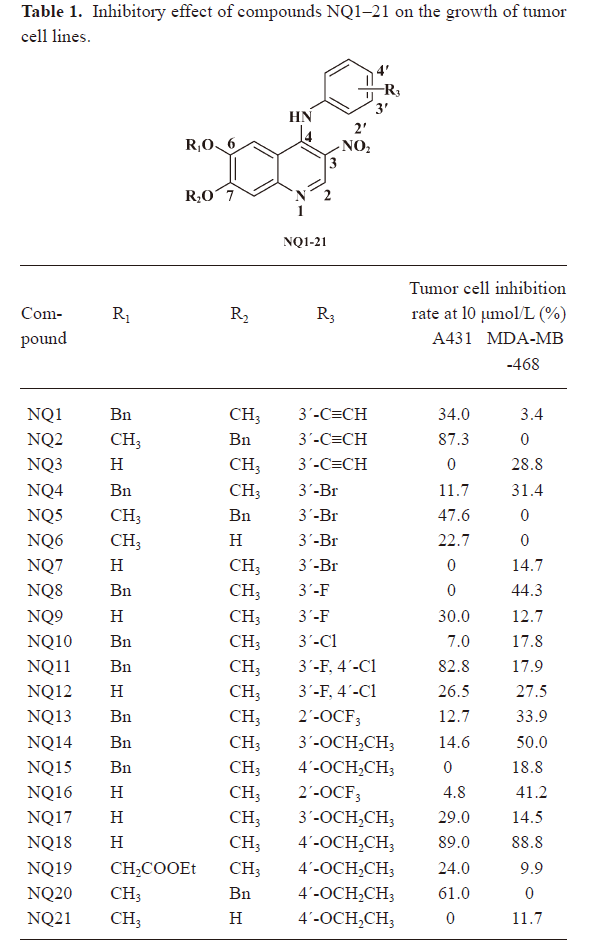
Full table
Biology assay The inhibition of EGFR activities by NQ1–21 was evaluated and analyzed by SRB assay for their inhibitory activities toward human epidermoid carcinoma (A431) cells and breast cancer (MDA-MB-468) cells. These cells are known to overexpress EGFR, which leads to the continuous activation of the EGFR pathway involved in cell proliferation. For the primary assay, the percentage of inhibition of the compounds at the 10 μmol/L concentration against A431 and MDA-MB-468 was measured. The biological results for the 3-nitro-4-anilino-6,7-dialkoxyquinolines inhibitors are shown in Table 1.
Discussion
Due to earlier work by some research groups, who found that the 4-anilinoquinazoline-based inhibitors of EGFR established that a meta-substituted electro-withdraw group in the aniline moiety is compatible with good activity, we decided to retain this feature in our initial compounds. As shown in Table 1, the initial compound NQ2 with an attached 3´-ethynyl group exhibited potential inhibitory activities towards the A431 cell line, with an 87.3% inhibition at the 10 μmol/L concentration, while replacing the ethynyl group with the bromo atom did not improve the inhibitory activities. Substitution of the bromo atom with the chloro atom or fluoro atom resulted in a sharp loss of inhibitory activities to the A431 cell line. The 3-fluoro-4-chlorobenzenamine substituted nitroquinoline derivative NQ11 presented potent inhibitory effects against the A431 cell line, but changing the substituted groups at position 6 resulted in a clear decrease in the ability to inhibit EGFR. These findings indicated that both the aniline moiety and the 6,7-dialkoxy substitution play important roles in the inhibitory activities. Thus, compound NQ11 was chosen as the benchmark compound for subsequent optimization studies. Compounds NQ13–15, which retained the 6,7-dialkoxy substitution of NQ11, were first investigated. Among them, compound NQ14 was a little more active than the initial compounds, with 50% inhibition against MDA-MB-468 at 10 μmol/L. However, their inhibitory activities toward A431 were decreased. Surprisingly, when the benzyl group was removed from NQ15, compound NQ18 exhibited high inhibitory activity toward both A431 and MDA-MB-468, with 89% and 88.8% inhibition at 10 μmol/L, respectively. Subsequently, derivatives NQ19–21 were synthesized, which were designed based on potent inhibitor NQ18. Disappointingly, all of these compounds showed decreased inhibitory activities toward both A431 and MDA-MB-468, and a few proved to have completely lost inhibitory activity. To some extent, NQ20 exhibited a better ability to inhibit A431 than the other compounds, whereas all were poor inhibitors of MDA-MB-468.
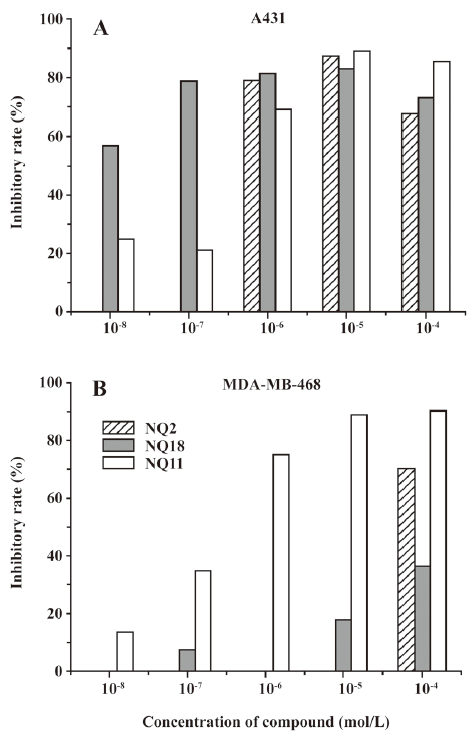
To determine the potency of the compounds that exhibited significant inhibition toward A431 or MDA-MB-468 at 10 μmol/L, 3 compounds (NQ2, NQ11, and NQ18) were further investigated in concentration-response studies, and the results are summarized in Figure 4. Compound NQ2 displayed good activity for the cell line A431 (IC50=0.49 μmol/L), but was much less effective in inhibiting the MDA-MB-468 cell line. Encouragingly, compound NQ11 showed a remarkably positive response on the both cell lines (IC50=0.40 and =0.22 μmol/L, for A431 and MDA-MB-468, respectively). Even more remarkable is that compound NQ18 showed prominent inhibitory activities against the A431 cell line with IC50 values up in the nanomolar range. Compound NQ18 exhibits inhibitory activity as high as 56.9% against A431, even at 10 nmol/L.
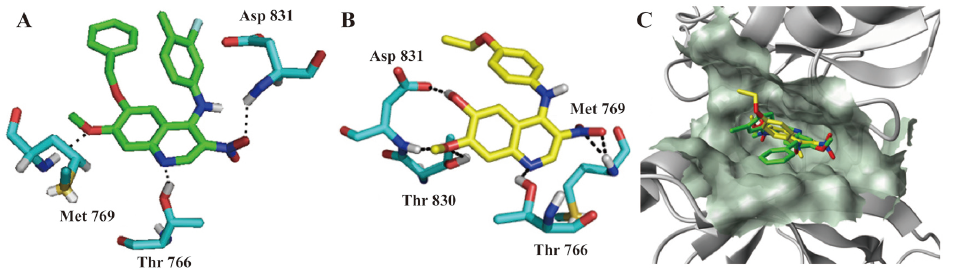
Molecular modeling experiments were carried out to investigate the binding interactions between this series of compounds and the active site of EGFR. The conformation with the lowest predicted binding free energy of the most occurring binding modes in the EGFR active pocket was selected. In the final model with compound NQ11 (Figure 5), the N1 atom of the quinoline forms a hydrogen bond with the hydroxyl group of Thr766, and the 3-nitro group, extending deep into the cleft, forms a hydrogen bond interaction with the backbone NH of Asp831. As for the 6,7-dialkoxy moiety, the 6-benzyloxy group points to the entrance of the active pocket. Interestingly, the oxygen atom at the 7-methoxy group forms a hydrogen bond with the NH of Met769. For the orientation of NQ18, its interactions with the protein are not similar to that observed in the NQ11 model. The hydrogen bond of the N1 atom to the hydroxyl group of Thr766 is retained. In this model, the 3-nitro group displaces the 7-methoxy group of NQ11 that was previously hydrogen bonded to the protein, and instead forms 2 hydrogen bonds with the backbone NH of Met769. The significance of this interaction was particularly reinforced by the performance of the 6-hydroxy group and the 7-methoxy group which form 3 hydrogen bonds with Asp831 and Thr830, respectively. From the binding modes of NQ11 and NQ18 with EGFR, we found that although different conformations were adopted for the 2 compounds in the EGFR active pocket, both formed favorable hydrogen bonds with the hydroxyl group of Thr766 and the backbone NH of Met769. As reported previously, the interaction with the backbone NH of the Met769 is important for binding to the ATP site, both for ATP and inhibitors, which can explain why NQ11 and NQ18 are potent with respect to their ability to inhibit the growth of EGFR-overexpressing cell lines. This model will be helpful for our further structural elaboration of the novel nitroquinolin series to improve kinase activity.
In summary, a series of novel 3-nitroquinoline derivatives was synthesized. All of the compounds were evaluated for their antiproliferative effect against the EGFR-overexpressing tumor cell lines. Several compounds for concentration–response studies showed prominent inhibitory activities with IC50 values in the micromolar or nanomolar range. The SAR was discussed in terms of the inhibitory activity against the proliferation of the 2 human carcinoma cell lines. The results suggest that both the aniline portion and the 6,7-dialkoxy substituents may play strong roles in determining the potency of the 3-nitroquinolin series as kinase inhibitors. It is noteworthy that the substitution in the aniline moiety need not be an electro-withdraw group at the meta position. This study was the first to identify new structural types of EGFR kinase inhibitors by the incorporation of the nitro group at position 3 of the quinoline core structure, providing promising new templates for the further development of potent inhibitors targeting EGFR kinase. There is an urgent need to discover small molecule EGFR inhibitors, so the new chemical structures produced in this study are of significance.
Appendix
The reagents (chemicals) were purchased from commercial sources (Alfa, Acros, Sigma-Aldrich and Shanghai Chemical Reagent Company), and used without further purification. Analytical-thin layer chromatography was HSGF254 (0.15–0.2 mm thickness; Yantai Huiyou Company, Yantai, China). Yields were not optimized. Melting points were measured in a capillary tube on a SGW X-4 melting point apparatus (Shanghai Precision & Scientific Instrument Co, Ltd) without correction. Nuclear magnetic resonance (NMR) spectra were given on a Brucker AMX-400 and AMX-300 (Brucker, Fällanden, Switzerland; internal standard as tetramethylsilane). Chemical shifts were reported in parts per million (ppm, δ) downfield from tetramethylsilane. Proton coupling patterns were described as singlet, doublet, triplet, quartet, multiplet, and broad. Low- and high-resolution mass spectra were given with an electric ionization (EI) and electrospray and a LCQ-DECA spectrometer produced by Finnigan MAT-95 (Finnigan, Santa Clara, CA, USA).
3-(Benzyloxy)-4-methoxybenzaldehyde (2a) A mixture of 3-hydroxy-4-methoxybenzaldehyde (9 g, 59 mmol), Na2CO3 (8.1 g), benzylchloride (11.4 g, 90 mmol), and 40 mL of ethanol was stirred at reflux for 5 h. The resulting mixture was filtered and the filtrate was evaporated. The crude product was recrystallized from EtOH to give 10.3 g (76%) of a white solid. Mp 59 ºC (lit[28], mp 63.5 ºC). 1H NMR (CDCl3): δ 9.8 (s, 1H, CHO), 7.3–7.5 (m, 5H, Ph-H), 7.25–7.28 (m, 2H, Ph-H), 7.0 (s, 1H, Ph-H), 5.25 (s, 2H, PhCH2O), 4.0 (s, 3H, CH3O).
4-(Benzyloxy)-3-methoxybenzaldehyde (2b) The compound was prepared in 69.4% yield according to the procedure for 2a using 4-hydroxy-3-methoxybenzaldehyde. Mp 55 ºC (lit[28], mp 53.5 ºC). 1H NMR (CDCl3): δ 9.8 (s, 1H, CHO), 7.3–7.5 (m, 5H, Ph-H), 7.25–7.28 (m, 2H, Ph-H), 7.0 (s, 1H, Ph-H), 5.25 (s, 2H, PhCH2O), 4.0 (s, 3H, CH3O).
5-(Benzyloxy)-4-methoxy-2-nitrobenzaldehyde (3a) 3-(Benzyloxy)-4-methoxybenzaldehyde (10 g, 41 mmol) was added cautiously to 40 mL of concentrated nitric acid at 0 ºC. The mixture was then stirred at 15 °C for 40 min. On pouring the reaction mixture into ice water, the precipitate was filtrated to afford 5-(benzyloxy)-4-methoxy-2-nitrobenzaldehyde (10.4 g, 93%) as a yellow solid. Mp 131 ºC (lit[28], mp 133 ºC). 1H NMR (CDCl3): δ 10.4 (s, 1H, CHO), 7.6 (s, 1H, Ph-H), 7.3–7.5 (m, 5H, Ph-H), 7.20 (s, 1H, Ph-H), 5.25 (s, 2H, PhCH2O), 4.0 (s, 3H, CH3O).
4-(Benzyloxy)-5-methoxy-2-nitrobenzaldehyde (3b) The compound was prepared in 91% yield according to the procedure for 3a using 4-(benzyloxy)-3-methoxybenzaldehyde. Mp 131 ºC (lit[28], mp 133 ºC). 1H NMR (CDCl3): δ 10.4 (s, 1H, CHO), 7.60 (s, 1H, Ph-H), 7.3–7.5 (m, 5H, Ph-H), 7.2 (s, 1H, Ph-H), 5.25 (s, 2H, PhCH2O), 4.0 (s, 3H, CH3O).
5-(Benzyloxy)-4-methoxy-2-nitrobenzoic acid (4a) In total, 10% KMnO4 was added to a stirred mixture of 5-(benzyloxy)-4-methoxy-2-nitrobenzaldehyde (10 g, 47 mmol) and 120 mL of acetone in hot water (100 mL). The resulting mixture was stirred for 1 h, and the reaction mixture was filtered. The filtrate was concentrated to remove the acetone. Then 4 mol/L HCl was added slowly with cooling until the insoluble material precipitated. The product was collected to give 10.2 g (58%) of a white solid. Mp 192 ºC (lit[28], mp 195 ºC). 1H NMR (DMCO-d6): δ 7.60 (s, 1H, Ph-H), 7.52 (s, 1H, Ph-H), 7.30–7.41 (m, 5H, Ph-H), 5.35 (s, 2H, PhCH2O), 4.0 (s, 3H, CH3O).
4-(Benzyloxy)-5-methoxy-2-nitrobenzoic acid (4b) The compound was prepared in 60% yield according to the procedure for 4a using 4-(benzyloxy)-5-methoxy-2-nitrobenzaldehyde. Mp 192 ºC (lit[28], mp 195 ºC). 1H NMR (DMCO-d6): δ 7.59 (s, 1H, Ph-H), 7.52 (s, 1H, Ph-H), 7.30–7.41 (m, 5H, Ph-H), 5.35 (s, 2H, PhCH2O), 4.0 (s, 3H, CH3O).
2-Amino-5-(benzyloxy)-4-methoxybenzoic acid (5a) Iron powder (3 g, 188 mmol) was partially added to a stirred mixture of 5-(benzyloxy)-4-methoxy-2-nitrobenzoic acid (10 g, 33.3 mmol) and 300 mL of acetic acid at 90 °C in 20 min. The resulting mixture was stirred for 45 min, and the reaction mixture was filtered. The filtrate was poured into 10% aqueous hydrochloric acid solution (500 mL), and the insoluble material precipitated. The residue was dissolved in hot water, and 15% sodium hydroxide solution was carefully added until the pH was 12. After cooling to room temperature, the insoluble material precipitated. The product was filtered, recrystallized from isopropanol, and dried to give 6.1 g (70%) of a white solid. Mp 108 ºC. 1H NMR (DMCO-d6): δ 7.30–7.41 (m, 5H, Ph-H), 7.02 (s, 1H, Ph-H), 6.82 (s, 1H, Ph-H), 5.35 (s, 2H, PhCH2O), 4.0 (s, 3H, CH3O).
2-Amino-4-(benzyloxy)-5-methoxybenzoic acid (5b) The compound was prepared in 68% yield according to the procedure for 5a using 4-(benzyloxy)-5-methoxy-2-nitrobenzoic acid. Mp 102 ºC. 1H NMR (DMCO-d6): δ 7.30–7.41 (m, 5H, Ph-H), 7.10 (s, 1H, Ph-H), 6.79 (s, 1H, Ph-H), 5.35 (s, 2H, PhCH2O), 4.0 (s, 3H, CH3O).
5-(Benzyloxy)-4-methoxy-2-(2-nitropropylideneamino)benzoic acid (6a) Nitromethane (1.34 g, 22 mmol) was added to a stirred mixture of NaOH (2.68 g, 67 mmol) and 10 mL of water at 0 ºC. The mixture was stirred at 40 ºC until the solid dissolved. Then nitromethane (1.34 g, 22 mmol) was added, and the mixture was stirred at 50 ºC for 15 min. The reaction mixture was poured into ice water, and concentrated hydrochloric acid was carefully added until the pH was 2. The brown mixture was added to a solution of 2-amino-5-(benzyloxy)-4-methoxybenzoic acid (5.48 g, 20 mmol) and concentrated hydrochloric acid (1 mL) in water (50 mL). The reaction mixture was stirred at room temperature for 24 h, and insoluble material precipitated. The product was filtered and dried to give 6.3 g (90%) of a yellow solid. Mp 159 ºC. EI-MS m/z 358 [M]+.
4-(Benzyloxy)-5-methoxy-2-(2-nitropropylideneamino)benzoic acid (6b) The compound was prepared in 85% yield according to the procedure for 6a using 2-amino-4-(benzyloxy)-5-methoxybenzoic acid. Mp 165 ºC. EI-MS m/z 358 [M]+.
7-(Benzyloxy)-6-methoxy-2-nitronaphthalen-1-ol (7a) A stirred mixture of 5-(benzyloxy)-4-methoxy-2-(2-nitropropylideneamino)benzoic acid (3.58 g, 10 mmol) and 10 mL of acetic anhydride was heated at 110 oC until the solid dissolved. After cooling to room temperature, NaOH (400 mg, 10 mmol) was added cautiously. Then the reaction mixture was stirred at 100 ºC for 5 h, allowed to cool to room temperature, and filtered. The residue was filtered off and dried to give 1.3 g (40%) of solid. Mp >300 ºC. EI-MS m/z 326 [M]+.
6-(Benzyloxy)-7-methoxy-2-nitronaphthalen-1-ol (7b) The compound was prepared in 37% yield according to the procedure for 7a using 4-(benzyloxy)-5-methoxy-2-(2-nitropropylideneamino)benzoic acid. Mp>300 ºC. EI-MS m/z 326 [M]+.
7-(Benzyloxy)-1-chloro-6-methoxy-2-nitronaphthalene (8a) A stirred mixture of 7-(benzyloxy)-6-methoxy-2-nitronaphthalen-1-ol (1 g, 3 mmol) and 15 mL of fresh POCl3 was heated at reflux for 18 h. After cooling to room temperature, the POCl3 was removed under vacuum. The product was recrystallized from ethanol, and dried to give 1.1 g (97%) of a brown solid. Mp >300 ºC. EI-MS m/z 344 [M]+.
6-(Benzyloxy)-1-chloro-7-methoxy-2-nitronaphthalene (8b) The compound was prepared in 90% yield according to the procedure for 8a using 6-(benzyloxy)-7-methoxy-2-nitronaphthalen-1-ol. Mp >300 ºC. EI-MS m/z 344 [M]+.
6-(Benzyloxy)-N-(3-ethynylphenyl)-7-methoxy-3-nitroquinolin-4-amine (NQ1) A mixture of 8a (1 g, 2.9 mmol), 3-ethynylbenzenamine (340 mg, 2.9 mmol), and 20 mL of DMF was stirred at 100 ºC for 24 h. The solvent was removed under vacuum. The crude product was recrystallized from methanol to give 1.1 g (89%) of a yellow solid. Mp 222 ºC. 1H NMR (DMSO-d6): δ 9.87 (s, 1H, quinoline-2-H), 7.19–7.68 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.0 (s, 3H, OCH3), 3.8 (s, 1H, C≡CH). EI-MS m/z 425[M]+. HR-MS Calcd. For C25H19N3O4: 425.1376; found: 425.1225.
7-(Benzyloxy)-N-(3-ethynylphenyl)-6-methoxy-3-nitroquinolin-4-amine (NQ2) Using the procedure described earlier for NQ1 using 8b, the title compound was obtained in 87% yield. Mp 223 ºC. 1H NMR (DMSO-d6): δ 9.87 (s, 1H, quinoline-2-H), 7.19–7.68 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.0 (s, 3H, OCH3), 3.8 (s, 1H, C≡CH). EI-MS m/z 425 [M]+. HR-MS Calcd. For C25H19N3O4: 425.1376; found: 425.1325.
4-(3-Ethynylphenylamino)-7-methoxy-3-nitroquinolin-6-ol (NQ3) A mixture of NQ1 (1 g, 2.35 mmol) and 15 mL trifluoroacetic acid was stirred at reflux for 1 h. The solvent was removed under vacuum. Then ammonium hydroxide was carefully added until the pH was 7, and the solvent was removed under vacuum again. The crude product was washed by water and recrystallized from methanol to give the title compound (95%). Mp 156 ºC. 1H NMR (DMCO-d6): δ 9.27 (s, 1H, quinoline-2-H), 7.19–7.68 (m, 6H, Ph-H), 4.0 (s, 3H, OCH3), 3.8 (s, 1H, C≡CH). EI-MS m/z 425 [M]+. Element analysis: Calcd. For C23H18BrN3O4: C, 57.51; H, 3.78; N, 8.75; found: C, 57.01; H, 3.58; N, 8.55. HR-MS Calcd. For C18H13N3O4: 335.0906; found: 335.0902.
6-(Benzyloxy)-N-(3-bromophenyl)-7-methoxy-3-nitroquinolin-4-amine (NQ4) Using the procedure described earlier for NQ1 with 3-bromobenzenamine, the title compound was obtained in 91% yield. Mp 268 ºC. 1H NMR (DMSO-d6): δ 9.77 (s, 1H, quinoline-2-H), 7.19–7.68 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.0 (s, 3H, OCH3). EI-MS m/z 480 [M+1]+. Element analysis: Calcd. For C23H18BrN3O4: C, 57.51; H, 3.78; N, 8.75; found: C, 57.01; H, 3.58; N, 8.55.
7-(Benzyloxy)-N-(3-bromophenyl)-6-methoxy-3-nitroquinolin-4-amine (NQ5) Using the procedure described earlier for NQ2 with 3-bromobenzenamine, the title compound was obtained in 91% yield. Mp 201 ºC. 1H NMR (DMSO-d6): δ 9.87 (s, 1H, quinoline-2-H), 7.19–7.68 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.0 (s, 3H, OCH3). EI-MS m/z 480 [M+1]+. Element analysis: Calcd. For C23H18BrN3O4: C, 57.51; H, 3.78; N, 8.75; found: C, 57.41; H, 3.28; N, 8.65.
4-(3-Bromophenylamino)-6-methoxy-3-nitroquinolin-7-ol (NQ6) Using the procedure described earlier for NQ3 with NQ5, the title compound was obtained in 95% yield. Mp 254 ºC. 1H NMR (DMCO-d6): δ 9.27 (s, 1H, quinoline-2-H), 7.12-7.42 (m, 6H, Ph-H), 4.0 (s, 3H, OCH3). EI-MS m/z 390 [M+1]+. HR-MS Calcd. For C16H12BrN3O4: 355.0011; found: 355.0005.
4-(3-Bromophenylamino)-7-methoxy-3-nitroquinolin-6-ol (NQ7) Using the procedure described earlier for NQ3 with NQ4, the title compound was obtained in 95% yield. Mp 254 ºC. 1H NMR (DMCO-d6): δ 9.27 (s, 1H, quinoline-2-H), 7.12–7.42 (m, 6H, Ph-H), 4.0 (s, 3H, OCH3). EI-MS m/z 389 [M]+. HR-MS Calcd. For C16H12BrN3O4: 355.0011; found: 355.0005.
6-(Benzyloxy)-N-(3-fluorophenyl)-7-methoxy-3-nitroquinolin-4-amine (NQ8) Using the procedure described earlier for NQ1 with 3-fluorobenzenamine, the title compound was obtained in 91% yield. Mp 256 ºC. 1H NMR (DMSO-d6): δ 9.0 (s, 1H, quinoline-2-H), 6.60–7.62 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.0 (s, 3H, OCH3). EI-MS m/z 419 [M]+. HR-MS Calcd. For C23H18FN3O4: 419.1281; found: 419.1236.
4-(3-Fluorophenylamino)-7-methoxy-3-nitroquinolin-6-ol (NQ9) Using the procedure described earlier for NQ3 with NQ8, the title compound was obtained in 95% yield. Mp 164 ºC. 1H NMR (DMCO-d6): δ 9.17 (s, 1H, quinoline-2-H), 7.00–7.42 (m, 6H, Ph-H), 4.0 (s, 3H, OCH3). EI-MS m/z 329 [M]+. HR-MS Calcd. For C16H12FN3O4: 329.0812; found: 329.0803.
6-(Benzyloxy)-N-(3-chlorophenyl)-7-methoxy-3-nitroquinolin-4-amine (NQ10) Using the procedure described earlier for NQ1 with 3-chlorobenzenamine, the title compound was obtained in 90% yield. Mp 286 ºC. 1H NMR (DMSO-d6): δ 9.89 (s, 1H, quinoline-2-H), 7.19–7.68 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.0 (s, 3H, OCH3). EI-MS m/z 435 [M]+. HR-MS Calcd. For C23H18ClN3O4: 435.0986; found: 435.0956.
6-(Benzyloxy)-N-(4-chloro-3-fluorophenyl)-7-methoxy-3-nitroquinolin-4-amine (NQ11) Using the procedure described earlier for NQ1 with 4-chloro-3-fluorobenzenamine, the title compound was obtained in 87% yield. Mp 284 ºC. 1H NMR (DMSO-d6): δ 9.0 (s, 1H, quinoline-2-H), 6.60–7.62 (m, 9H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.0 (s, 3H, OCH3). EI-MS m/z 453 [M]+. Element analysis: Calcd. For C23H17ClFN3O4: C, 52.83; H, 3.05; N, 11.55; found: C, 52.40; H, 3.97; N, 11.52.
4-(3-Chloro-4-fluorophenylamino)-7-methoxy-3-nitroquinolin-6-ol (NQ12) Using the procedure described earlier for NQ3 with NQ11, the title compound was obtained in 95% yield. Mp 178 ºC. 1H NMR (DMCO-d6): δ 9.17 (s, 1H, quinoline-2-H), 7.00–7.42 (m, 4H, Ph-H), 4.0 (s, 3H, OCH3). EI-MS m/z 363 [M]+. Element analysis: Calcd. For C16H11ClFN3O4: C, 52.83; H, 3.05; N, 11.55; found: C, 52.43; H, 3.00; N, 11.05.
6-(Benzyloxy)-7-methoxy-3-nitro-N-(2-(trifluoromethoxy)phenyl)quinolin-4-amine (NQ13) Using the procedure described earlier for NQ1 using 2-(trifluoromethoxy)benzenamine, the title compound was obtained in 92% yield. Mp 201 ºC. 1H NMR (DMSO-d6): δ 9.0 (s, 1H, quinoline-2-H), 6.60–7.62 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.0 (s, 3H, OCH3). EI-MS m/z 485 [M]+. Element analysis: Calcd. For C24H18F3N3O5: C, 59.38; H, 3.74; N, 8.66; found: C, 59.18; H, 3.61; N, 8.12.
6-(Benzyloxy)-N-(3-ethoxyphenyl)-7-methoxy-3-nitroquinolin-4-amine (NQ14) Using the procedure described earlier for NQ1 with 3-ethoxybenzenamine, the title compound was obtained in 93% yield. Mp 219 ºC. 1H NMR (DMSO-d6): δ 9.77 (s, 1H, quinoline-2-H), 7.19–7.88 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.31 (q, 2H, CO2CH2CH3), 4.0 (s, 3H, OCH3), 2.1 (t, 3H, CO2CH2CH3). EI-MS m/z 445 [M]+. Element analysis: Calcd. For C25H23N3O5: C, 67.41; H, 5.20; N, 9.43; found: C, 67.31; H, 5.10; N, 9.01.
6-(Benzyloxy)-N-(4-ethoxyphenyl)-7-methoxy-3-nitroquinolin-4-amine (NQ15) Using the procedure described earlier for NQ1 with 4-ethoxybenzenamine, the title compound was obtained in 94% yield. Mp 211 ºC. 1H NMR (DMSO-d6): δ 9.77 (s, 1H, quinoline-2-H), 7.19–7.88 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.31 (q, 2H, CO2CH2CH3), 4.0 (s, 3H, OCH3), 2.1 (t, 3H, CO2CH2CH3). EI–MS m/z 445 [M]+. HR-MS Calcd. For C25H23N3O5: 445.1638; found: 445.1614.
7-Methoxy-3-nitro-4-(2-(trifluoromethoxy)phenylamino)quinolin-6-ol (NQ16) Using the procedure described earlier for NQ3 with NQ13, the title compound was obtained in 96% yield. Mp 143 ºC. 1H NMR (DMCO-d6): δ 9.17 (s, 1H, quinoline-2-H), 7.00–7.42 (m, 6H, Ph-H), 4.00 (s, 3H, OCH3). EI-MS m/z 395 [M]+. Element analysis: Calcd. For C17H12F3N3O5: C, 51.65; H, 3.06; N, 10.63; found: C, 51.32; H, 3.02; N, 9.84.
4-(3-Ethoxyphenylamino)-7-methoxy-3-nitroquinolin-6-ol (NQ17) Using the procedure described earlier for NQ3 with NQ14, the title compound was obtained in 95% yield. Mp 189 ºC. 1H NMR (DMCO-d6): δ 9.27 (s, 1H, quinoline-2-H), 7.10–7.48 (m, 6H, Ph-H), 4.0 (s, 3H, OCH3). EI-MS m/z 355 [M]+. Element analysis: Calcd. For C18H17N3O5: C, 60.84; H, 4.82; N, 11.83; found: C, 60.81; H, 4.56; N, 11.12.
4-(4-Ethoxyphenylamino)-7-methoxy-3-nitroquinolin-6-ol (NQ18) Using the procedure described earlier for NQ3 with NQ15, the title compound was obtained in 95% yield. Mp 181 ºC. 1H NMR (DMCO-d6): δ 9.17(s, 1H, quinoline-2-H), 7.09–7.38 (m, 6H, Ph-H), 4.31 (q, 2H, CO2CH2CH3), 4.0 (s, 3H, OCH3), 2.1 (t, 3H, CO2CH2CH3). EI-MS m/z 355 [M]+. HR-MS Calcd. For C18H17N3O5: 355.1168; found: 355.1125.
Ethyl 2-(4-(4-ethoxyphenylamino)-7-methoxy-3-nitroquinolin-6-yloxy)acetate (NQ19) A mixture of NQ8 (700 mg, 1.97 mmol), K2CO3 (500 mg, 3.62 mmol), and 10 mL DMF was stirred at 80 ºC for 1 h. Then ethyl 2-bromoacetate (400 mg, 2.22 mmol) was added, and the mixture was stirred for another 1 h. The solvent was removed under vacuum. The crude product was recrystallized from ethyl acetate to give the title compound (95%). Mp 195 ºC. 1H NMR (DMCO-d6): δ 9.27(s, 1H, quinoline-2-H), 7.19–7.88 (m, 6H, Ph-H), 4.90 (s, 2H, COCH2O), 4.31 (q, 2H, CO2CH2CH3), 4.20 (q, 2H, CO2CH2CH3), 4.0 (s, 3H, OCH3), 2.3 (t, 3H, CO2CH2CH3), 2.0 (t, 3H, CO2CH2CH3). EI-MS m/z 441 [M]+. Element analysis: Calcd. For C22H23N3O7: C, 59.86; H, 5.25; N, 9.52; found: C, 59.63; H, 5.55; N, 9.55.
7-(Benzyloxy)-N-(4-ethoxyphenyl)-6-methoxy-3-nitroquinolin-4-amine (NQ20) Using the procedure described earlier for NQ2 with 4-ethoxybenzenamine, the title compound was obtained in 93% yield. Mp 274 ºC. 1H NMR (DMSO-d6): δ 9.77 (s, 1H, quinoline-2-H), 7.19–7.88 (m, 11H, Ph-H), 5.20 (s, 2H, CH2Ph), 4.31 (q, 2H, CO2CH2CH3), 4.0 (s, 3H, OCH3), 2.1 (t, 3H, CO2CH2CH3). EI-MS m/z 445 [M]+. HR-MS Calcd. For C25H23N3O5: 445.1638; found: 445.1614.
4-(4-Ethoxyphenylamino)-6-methoxy-3-nitroquinolin-7-ol (NQ21) Using the procedure described earlier for NQ3 with NQ20, the title compound was obtained in 94% yield. Mp 185 ºC. 1H NMR (DMCO-d6): δ 9.17 (s, 1H, quinoline-2-H), 7.09–7.38 (m, 6H, Ph-H), 4.31 (q, 2H, CO2CH2CH3), 4.0 (s, 3H, OCH3), 2.1 (t, 3H, CO2CH2CH3). EI-MS m/z 355 [M]+. HR-MS Calcd. For C18H17N3O5: 355.1168; found: 355.1125.
References
- David WF. Inhibition of the epidermal growth factor receptor family of tyrosine kinases as an approach to cancer chemotherapy: progression from reversible to irreversible inhibitors. Pharmacol Ther 1999;82:207-18.
- Hickey K, Grehan D, Reid IM, O’Briain S, Walsh TN, Hennessy TPJ. Expression of epidermal growth factor receptor and proliferating cell nuclear antigen predicts response of esophageal squamous cell carcinoma to chemoradiotherapy. Cancer 1994;74:1693-8.
- Wells A. EGF receptor. Int J Biochem Cell Biol 1999;31:637-43.
- Delarue JC, Terrier P, Terrier-Lacombe MJ, Mouriesse H, Gotteland M, May-Levin F. Combined overexpression of c-erbB-2 protein and epidermal growth factor receptor (EGFR) could be predictive of early and long-term outcome in human breast cancer: a pilot study. Bull Cancer 1994;81:1067-77.
- Antonello A, Tarozzi A, Morroni F, Cavalli A, Rosini M, Hrelia P, et al. Multitarget-directed drug design strategy: a novel molecule designed to block epidermal growth factor receptor (EGFR) and to exert proapoptotic effects. J Med Chem 2006;49:6642-5.
- Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, et al. Specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 1994;265:1093-5.
- Ward WHJ, Cook PN, Slater AM, Davies DH, Holdgate GA, Green LR. Epidermal growth factor receptor tyrosine kinase. Investigation of catalytic mechanism, structure based searching and discovery of a potent inhibitor. Biochem Pharmacol 1994;48:659-66.
- Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, et al. The effects of the novel, reversible epidermal growth factor receptor/erbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 2001;1:85-94.
- Domarkas J, Dudouit F, Williams C, Qiyu Q, Banerjee R, Brahimi F, et al. The combi-targeting concept: synthesis of stable nitrosoureas designed to inhibit the epidermal growth factor receptor (EGFR). J Med Chem 2006;49:3544-52.
- Mishani E, Abourbeh G, Jacobson O, Dissoki S, Daniel RB, Rozen Y, et al. High-affinity epidermal growth factor receptor (EGFR) irreversible inhibitors with diminished chemical reactivities as positron emission tomography (PET)-imaging agent candidates of EGFR overexpressing tumors. J Med Chem 2005;48:5337-48.
- Rewcastle GW, Palmer BD, Thompson AM, Bridges AJ, Cody DR, Zhou H, et al. Tyrosine kinase inhibitors. 10. Isomeric 4-[(3-bromophenyl)amino]pyrido[d]-pyrimidines are potent ATP binding site inhibitors of the tyrosine kinase function of the epidermal growth factor receptor. J Med Chem 1996;39:1823-35.
- Rewcastle GW, Bridges AJ, Fry DW, Rubin JR, Denny WA. Tyrosine kinase inhibitors. 12. Synthesis and structure-activity relationships for 6-substituted 4-(phenylamino)pyrimido[5,4-d]pyrimidines designed as inhibitors of the epidermal growth factor receptor. J Med Chem 1997;40:1820-6.
- Norman MH, Kelly JL, Hollingsworth EB. Conformationally restricted analogues of remoxipride as potential antipsychotic agents. J Med Chem 1993;36:3417-23.
- Hodge CN, Pierce J. A diazine heterocycle replaces a six-membered hydrogen-bonded array in the active site of scytalone dehydratase. Bioorg Med Chem Lett 1993;3:1605-8.
- Asano T, Yoshikawa T, Usui T, Yamamoto H, Yamamoto Y, Uehara Y, et al. Benzamides and benzamidines as specific inhibitors of epidermal growth factor receptor and v-Src protein tyrosine kinases. Bioorg Med Chem 2004;12:3529-42.
- Sun L, Cui J, Liang C, Zhou Y, Nematalla A, Wang X, et al. Rational design of 4,5-disubstituted-5,7-dihydro-pyrrolo[2,3-d]pyrimidin-6-ones as a novel class of inhibitors of epidermal growth factor receptor (EGF-R) and HER-2 (p185erbB) tyrosine kinases. Bioorg Med Chem Lett 2002;12:2153-7.
- Hunt JT, Mitt T, Borzilleri R, Gullo-Brown J, Fargnoli J, Fink B, et al. Discovery of the pyrrolo[2,1-f][1,2,4]triazine nucleus as a new kinase inhibitor template. J Med Chem 2004;47:4054-9.
- Thompson AM, Bridges AJ, Fry DW, Kraker AJ, Denny WA. Tyrosine kinase inhibitors 7: 7-amino-4-(phenylamino)- and 7-amino-4-[(phenylmethyl)amin-o]-pyrido[4,3-d]pyrimidines: a new class of inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor. J Med Chem 1995;38:3780-8.
- Rewcastle GW, Palmer BD, Bridges AJ, Showalter HDH, Sun L, Nelson J, et al. Tyrosine kinase inhibitors 9: Synthesis and evaluation of fused tricyclic quinazoline analogues as ATP site inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor. J Med Chem 1996;39:918-28.
- Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res 2004;10:1212-8.
- Johnson JR, Cohen M, Sridhara R, Chen YF, Williams GM, Duan J, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res 2005;11:6414-21.
- Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol 2007;25:4057-65.
- Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem 2002;277:265-72.
- Wissner A, Berger DM, Boschelli DH, Floyd MBJ, Greenberger LM, Gruber BC, et al. 4-Anilino-6,7-dialkoxyquinoline-3-carbonitrile inhibitors of epidermal growth factor receptor kinase and their bioisosteric relationship to the 4-anilino-6,7-dialkoxyquinazoline inhibitors. J Med Chem 2000;43:3244-56.
- Shewchuk L, Hassell A, Wisely B, Rocque W, Holmes W, Veal J, et al. Binding mode of the 4-anilinoquinazoline class of protein kinase inhibitor: X-ray crystallographic studies of 4-anilinoquinazolines bound to cyclin-dependent kinase 2 and p38 kinase. J Med Chem 2000;43:133-8.
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 1998;19:1639-62.
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, . Autodock. Version 3.0.3. La Jolla, CA: The Scripps Research Institute; 1999.
- Althuis TH, Hess HJ. Synthesis and identification of the major metabolites of prazosin formed in dog and rat. J Med Chem 1977;20:146-9.