Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats1
Introduction
Caffeic acid (3,4-dihydroxycinnamic acid) is one of the natural phenolic compounds widely distributed in plant materials such as vegetables, fruits, coffee and tea[1–3]. As a potent antioxidant[4,5], caffeic acid exerts anti-inflammatory effects[6]. One of caffeic acid derivatives, caffeic acid phenethyl ester (CAPE), suppressed cerebral lipid peroxidation[7] and reduced brain infarct[8] after cerebral ischemia in rats. In contrast, caffeic acid is an inhibitor of 5-lipoxygenase (5-LOX)[9,10] and can inhibit the biosynthesis of pro-inflammatory leukotrienes. We have recently found that 5-LOX is activated after oxygen-glucose deprivation (OGD)-induced in vitro ischemic injury and caffeic acid attenuated this injury in PC12 cells[11] and cultured rat cortical neurons[12]. However, whether caffeic acid affects brain injury via inhibiting 5-LOX activity after focal cerebral ischemia in vivo is unknown.
Ischemic brain injury can be separated into three serial phases: metabolic stress and excitotoxicity (acute, minutes to hours), inflammation and apoptosis (subacute, hours to days), and repair and regeneration (chronic, days to months)[13,14]. Neuron injury, including necrosis and apoptosis, is the main lesion in the acute or subacute phase; while one of the chronic changes after cerebral ischemia or other brain injuries is the formation of a glial scar that results from reactive gliosis (mainly consisting of proliferated astrocytes)[15,16]. During the chronic phase of cerebral ischemia, gliosis (astrogliosis) is detected in the infarct boundary[17], which may be a physical and biochemical barrier for the regeneration of axons[15]. Whether caffeic acid has an effect on brain ischemia in acute and subacute/chronic phases is still unclear.
In the present study we evaluate the effects of caffeic acid on injuries after focal cerebral ischemia in rats, and determined the relation to 5-LOX inhibition. We defined that injury occurs at 24 h after ischemia as the early injury (including acute and subacute phases), and at 14 d as the delayed injury (including subacute and chronic phases).
Materials and methods
Measurements of physiological variables Male Sprague-Dawley rats weighing 250–300 g (Experimental Animal Center, Zhejiang Academy of Medical Sciences) were used in this study. Rats were housed under a controlled temperature (22±1 °C), 12 h light/12 h dark cycle and allowed free access to food and water. All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Rats were anaesthetized with an intraperitoneal injection of chloral hydrate (400 mg/kg). A polyethylene tube was inserted into the right femoral artery for continuous monitoring of blood pressure using a computer-assisted system (PC-Lab, Kelong, Nanjing, China) and for measuring Pao2, Paco2, and arterial blood pH (Blood Gas Analyzer ABL 330, Leidu, Copenhagen, Denmark). Blood glucose was monitored by one touch basic blood glucose monitoring system (Lifescan, Los Angeles, CA, USA). Rectal (core) temperature was monitored and maintained at 37.0±0.5 °C with a heating pad and a heating lamp during the surgery. Percent changes in regional cerebral blood flow (rCBF) over the middle cerebral arterial (MCA) territory (2 mm in diameter; 6 mm lateral and 2 mm caudal to bregma) were recorded as in a previous study[18] using a laser Doppler flowmeter (ML191, AD Instruments, New South Wales, Australia), as the steady state baseline of rCBF value before ischemia was defined to be 100%.
Focal cerebral ischemia Transient focal cerebral ischemia was induced by a modified method of middle cerebral artery occlusion (MCAO)[19] according to a previously reported method[20]. Briefly, after anesthesia, a midline incision was made in the neck, the right external carotid artery (ECA) and the right internal carotid artery (ICA) were carefully exposed and dissected, and a 3-0 monofilament nylon suture was inserted from the ECA into the ICA to occlude the origin of the right MCA. Thirty minutes after occlusion, the suture was withdrawn to allow reperfusion, the ECA was ligated and the incision was closed. Sham-operated rats underwent identical surgery, except for inserting the intraluminal filament. Achievement of MCAO was confirmed by a reduction of 50% or more in rCBF to the baseline value[21]. After surgery, rats were kept for approximately 2 h in a warm box heated by lamps to maintain their body temperature.
Caffeic acid administration Caffeic acid (Aldrich-Sigma, Saint Louis, MO, USA) was dissolved in dimethyl sulphoxide (DMSO); the solution was freshly diluted with saline to final concentrations of 10 mg/mL and 50 mg/mL before use. Caffeic acid (10 and 50 mg/kg, 0.1 mL per 100 g bodyweight)[22] or 20% (v/v) DMSO-saline solution was injected intraperitoneally 30 min before MCAO and 0, 1, 2 h after reperfusion in the first day, and twice daily in the 2nd to 5th day. For evaluating the early injury 24 h after reperfusion, caffeic acid was injected only for 1 d; for evaluating the effect on rCBF, it was only injected 30 min before and immediately after MCAO.
Neurological examination Neurological deficit scores were evaluated 24 h and 14 d after MCAO, according to a reported method[23], as follows: 0, no deficit; 1, flexion of contralateral forelimb upon lifting of the whole animal by the tail; 2, decrease of thrust toward contralateral plane; 3, circling to the contralateral side. An inclined board test was performed to assess balance and coordination[24] based on modification of a previously reported method[25]. Rats were placed on a board (50 cm×30 cm); once they stayed stably the board was inclined from horizontal to vertical. The degree at which the animal fell from the board was recorded. The test was repeated three times and the average degree was used.
Histological examination Rats were anesthetized after neurological examination and perfused transcardially with 4% paraformaldehyde after pre-washing with saline. Brains were removed and photographed by a digital camera (FinePix S602 Zoom, Fuji, Japan). The surface area of ipsilateral (ischemic) or contralateral hemisphere was calculated and compared using an image analysis program (AnalyPower 1.0, Zhejiang University, Hangzhou, China). Then, six serial coronal sections (20 µm) at 2 mm intervals from the frontal to the occipital poles were cut by cryomicrotomy (CM1900, Leica, Germany) for gross photographic examination after being stained with 1% toluidine blue. The infarct area and hemisphere area were measured, and the infarct volume and ipsilateral/contralateral hemisphere ratio were calculated as reported[24].
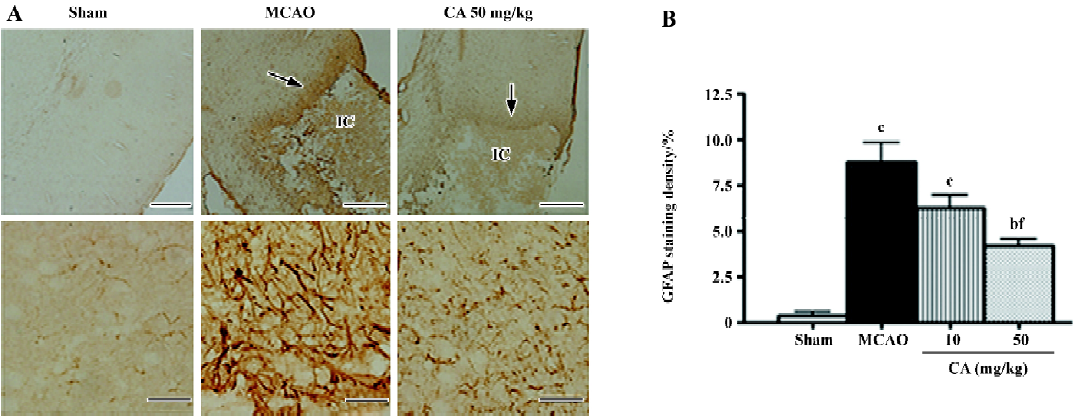
Simultaneously, 12-µm coronal sections (1.8–2.0 mm caudal from bregma) were cut for histological examination. Apparently survival neurons in similar regions of temporoparietal cortex III, IV layers were counted in five randomly selected fields of the sections stained with 1% toluidine blue by an image analysis program (AnalyPower1.0, Zhejiang University, Hangzhou, China). Astrocytes were stained with a rabbit polyclonal antibody against glial fibrillary acidic protein (GFAP), a specific marker of astrocyte (1:200, Zhongshan Biotechnology, Beijing, China). Brain sections were sequentially incubated overnight at 4 °C with the antibody against GFAP, biotinylated goat anti-rabbit IgG (1:200), and with horseradish peroxidase streptavidin (1:200, Zhongshan Biotechnology, Beijing, China) for 2 h; finally visualized using diaminobenzidine (DAB Kit, Zhongshan Biotechnology). Negative control sections were treated by identical procedure, except that the primary antibodies were omitted. The stained sections were observed by light microscopy. To evaluate astrocyte proliferation, the optic density of GFAP staining in an area of 1 mm×1 mm in the boundary zone adjacent to ischemic core was compared to that in the same location of contralateral non-ischemic cortex. The percentage increase was calculated.
Leukotriene measurement Brain samples were removed from ischemic or contralateral non-ischemic cortex at 3 h and 7 d after reperfusion (which were the peak times for leukotriene production as indicated in our previous study), and prepared according to a previously reported method[26]. The tissue was freshly homogenized in 100% ethanol. After centrifugation of the homogenates at 15 000×g, 4 °C for 30 min, the supernatant was collected and applied to a 0.2-µm filtrator. The filtrate was dried under nitrogen and resuspended with an enzyme immunoassay buffer (Cayman Chemical, Ann Arbor, MI, USA). Tissue concentrations of cysteinyl leukotrienes (CysLTs, including LTC4, LTD4, and LTE4) and leukotriene B4 (LTB4) were measured using an enzyme immunoassay kit (Cayman Chemical) according to the manufacturer’s instructions. All measurements were carried out in duplicate, and calculated as pg/g wet tissue.
Statistical analysis Values are expressed as mean±SD. Statistical analyses were performed using one-way ANOVA according to the experimental design, followed by Bonferroni test (Prism 4 for windows, 2003, GraphPad Software, San Diego, CA, USA). P<0.05 was considered to be statistically significant.
Results
Physiological variables Mean blood pressure, arterial blood pH, Pao2, Paco2, and glucose were not changed before and after the surgery, and there was no differences between sham operation, ischemia and caffeic acid groups. However, in all the ischemic rats, rCBF of the MCA territory was reduced by approximately 50%–60% during 30-min MCAO, and recovered to nearly baseline levels 15 min after reperfusion. Treatment with caffeic acid (50 mg/kg) did not alter rCBF reduction and recovery (Table 1). No rats died of ischemic injury in all groups 24 h after MCAO, whereas 20% of rats in each ischemic group died (2/10 rats) within 14 d after MCAO but no sham-operated rats died.
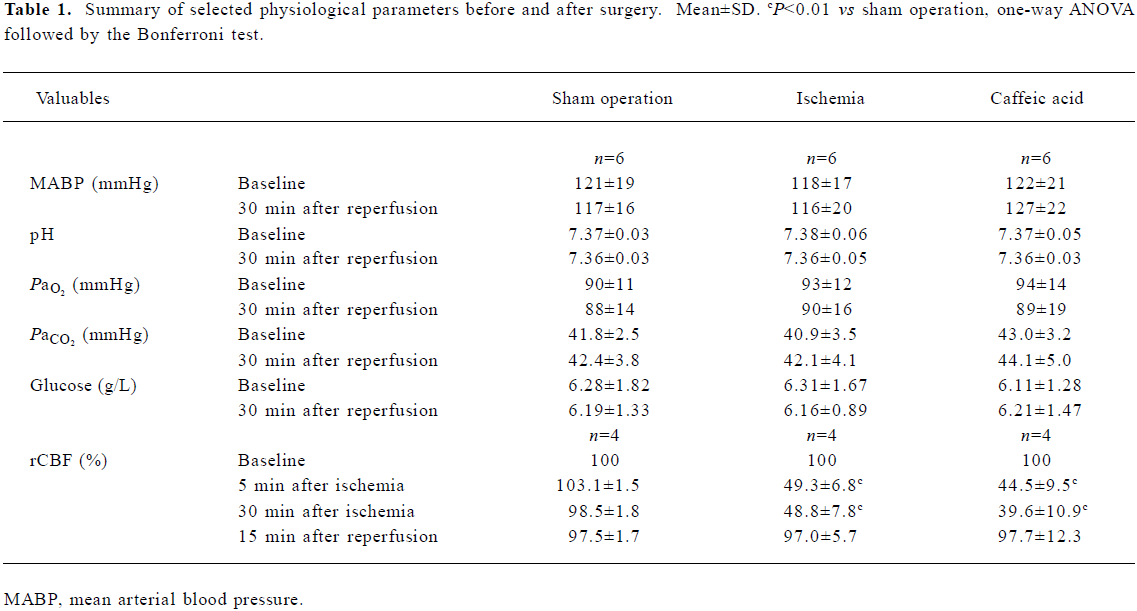
Full table
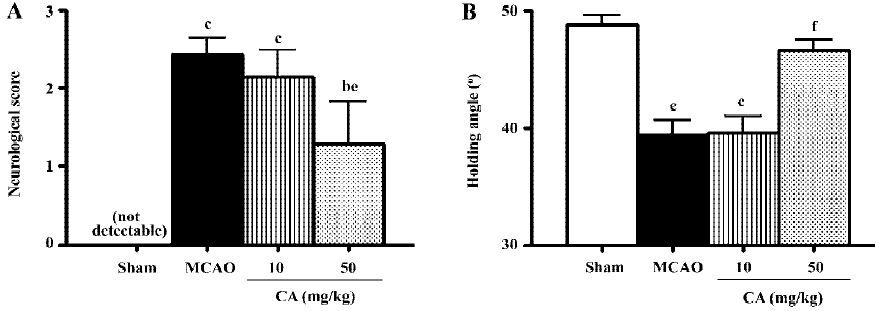
Ischemic injury Neurological deficit scores were maximal at 24 h, and disappeared 14 d after ischemia. Similarly, holding angle in the inclined board test significantly decreased 24 h then recovered 14 d after ischemia. Treatment with caffeic acid 50 mg/kg, not 10 mg/kg, for 5 d significantly reduced neurological deficit scores and increased holding angle 24 h after ischemia (Figure 1).
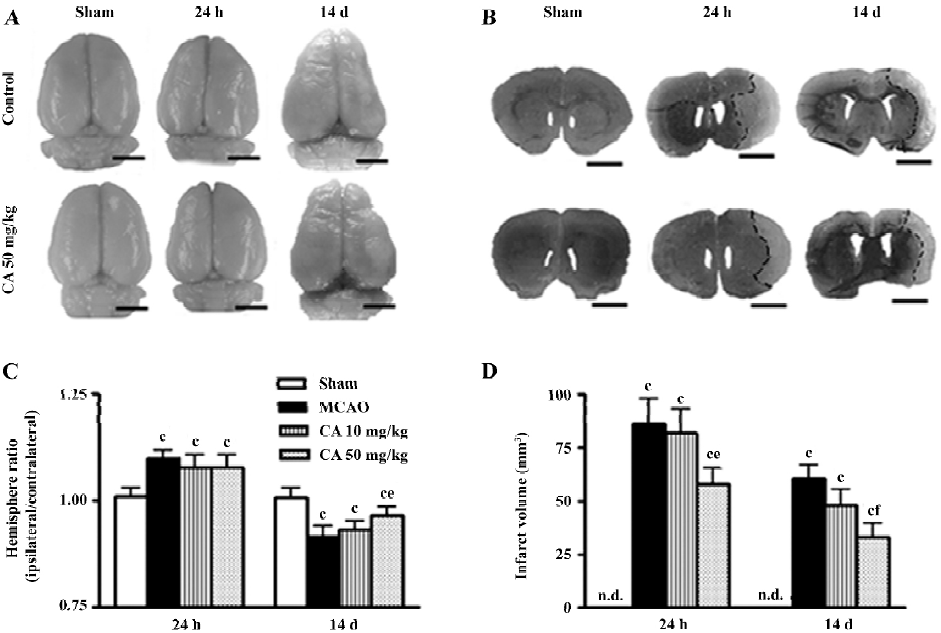
The gross photographs of whole brains showed the changes in the surface area (Figure 2A), and the coronal slices stained with toluidine blue showed the infarct tissue (Figure 2B) in the ischemic hemispheres 24 h and 14 d after ischemia. The area of brain surface was significantly increased 24 h indicating brain edema, and reduced in the ischemic hemisphere 14 d after ischemia indicating brain atrophy. The brain atrophy was primarily localized in cortical and subcortical regions of the ipsilateral hemisphere as indicated in brain sections (Figure 2B). Infarct volume was obviously increased 24 h and 14 d after reperfusion. Caffeic acid (50 mg/kg) did not significantly inhibit the changes in hemisphere ratio but reduced infarct volume 24 h after reperfusion, whereas it ameliorated both the changes 14 d after reperfusion (Figure 2C and 2D).
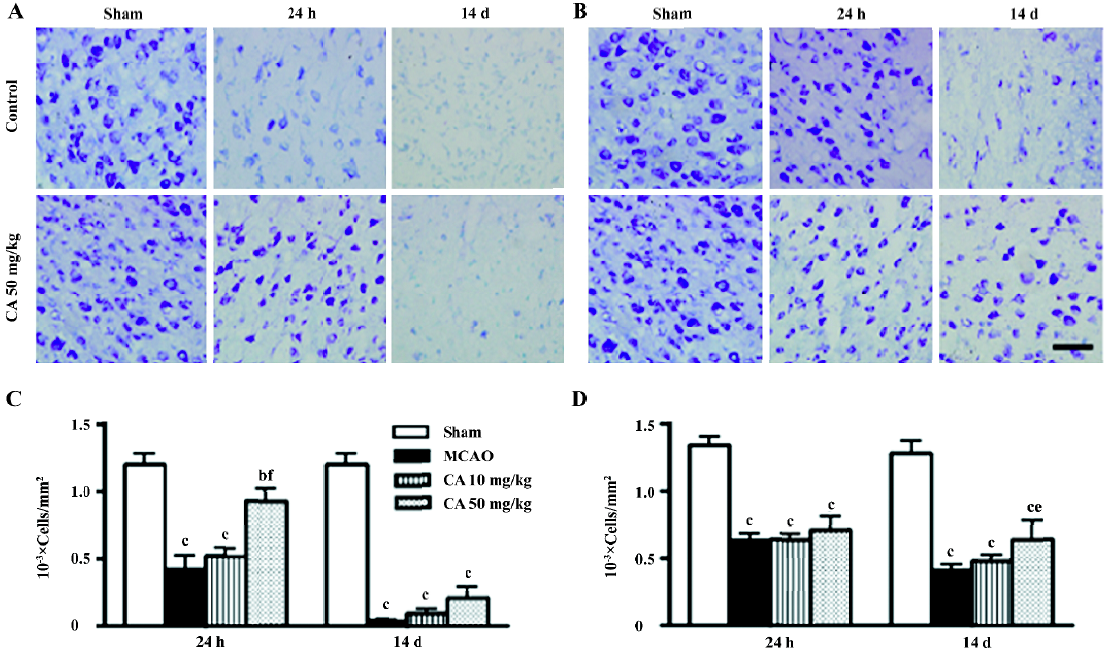
In the ischemic core, the density of the apparently surviving neurons was significantly reduced 24 h, and almost disappeared 14 d after ischemia (Figure 3A). In the boundary zone adjacent to the ischemic core, the neurons were reduced by approximately 60%–70% 24 h and 14 d after reperfusion (Figure 3B). Caffeic acid (50 mg/kg) attenuated neuron loss in the ischemic core, but not in the boundary zone, 24 h after ischemia; however, it did not reverse neuron loss in the ischemic core but attenuated neuron loss in the boundary zone 14 d after ischemia (Figure 3C and 3D). On the other hand, in the ischemic core, GFAP-positive astrocytes were increased at 24 h, but almost disappeared 14 d after reperfusion (data not shown). However, in the boundary zone, the number of GFAP-positive astrocytes was greatly increased and the intense astrocytic fibers surrounded the ischemic core 14 d after ischemia (Figure 4A). The astrocyte proliferation was partially inhibited by caffeic acid (50 mg/kg, Figure 4B).
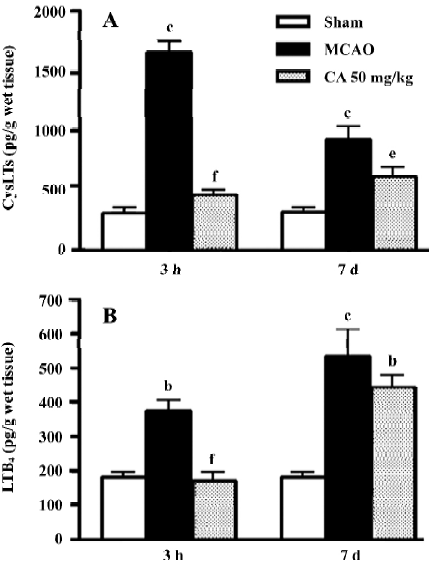
5-LOX enzymatic activity To evaluate whether the effects of caffeic acid is mediated by inhibiting 5-LOX enzymatic activity, we measured the levels of CysLTs and LTB4 in the ischemic cortex 3 h and 7 d after reperfusion, the peak time points for leukotriene production. Caffeic acid administered for 5 d reduced the levels of both CysLTs and LTB4 at 3 h, but only reduced the level of CysLTs at 7 d after reperfusion (Figure 5).
Discussion
In the present study, we found that caffeic acid attenuated both early and delayed ischemic injuries, and also inhibited enzymatic activation of 5-LOX as reducing the production of the metabolites, CysLTs and LTB4, 3 h and 7 d after ischemia. Its neuroprotective effect on early injury is consistent with previously published reports[7,8,27], but our findings further indicate its effect on the delayed ischemic injury. The effects of caffeic acid on 5-LOX activation confirms such an effect in PC12 cells or neurons after in vitro ischemic injury[11,12].
In the early injury after focal cerebral ischemia, caffeic acid attenuated neurological symptoms, the neuron loss in the ischemic core and the infarct volume, but not the brain edema. Because inflammation is one of the events occurring in early ischemic injury, pro-inflammatory mediators play an important role[13]. Among the mediators, the production of 5-LOX metabolites, CysLTs and LTB4, is increased after cerebral ischemia[28–31]. Our present result showed that caffeic acid inhibited the production of both CysLTs and LTB4 in the brain 3 h after ischemia (a peak time as indicated in our recent study), which indicated the inhibition of the enzymatic activity of 5-LOX by caffeic acid. This finding suggests that 5-LOX inhibition may be one of the mechanisms of its neuro-protective effect on early ischemic injury. However, the potent antioxidant effect of caffeic acid may be another explanation for its effect against acute ischemic injury. Caffeic acid and its derivative CAPE can scavenge oxygen radicals in vitro[5] and inhibit the reactive oxygen species (ROS) production in rats[32]. However, in the present study caffeic acid did not inhibit the brain edema in early injury although most ischemia injuries were ameliorated. This incomplete protection may be because post-ischemic brain edema is determined by a complex system and caffeic acid only inhibits parts of the system; for example, caffeic acid does not inhibit prostaglandin production even at higher concentrations[9].
The most important finding in the present study is that caffeic acid primarily inhibits astrocyte proliferation in addition to other delayed injuries (14 d after ischemia). We found that caffeic acid inhibited brain atrophy, brain infarct volume and neuron loss in the boundary zone. Whereas caffeic acid did not recover the neuron loss in the ischemic core, indicating that it only postponed neuron death in this region because an amelioration of neuron loss was observed here in the early injury. Because neurological dysfunction (neurological deficit scores and holding angles in the inclined board test) was already recovered 3–5 days after transient ischemia in the present study, no change was observed 14 d after ischemia. Furthermore, caffeic acid significantly inhibited the proliferated astrocytes in the boundary zone, suggesting a beneficial effect on delayed injury after cerebral ischemia. Because 5-d administration of caffeic acid also inhibited the production of 5-LOX metabolites, CysLTs but not LTB4, 7 d after ischemia (a second peak time as indicated in our recent study), the effects of caffeic acid might also relate to 5-LOX activation. As supporting evidence, we found that 5-LOX expression was increased in the boundary zone accompanied with astrocyte proliferation 7–14 d after focal cerebral ischemia (Zhou Y, et al 2006; unpublished observations). Moreover, the 5-LOX metabolites, CysLTs, mediate astrocyte proliferation via activating CysLT1 receptor (one of the receptors for CysLTs) as shown in the cultured astrocytes[26]. We recently found that CysLT1 receptor antagonist pranlukast inhibits glial scar formation in the chronic phase of cerebral ischemia[24], supporting the role of 5-LOX in astrocyte proliferation. However, the antioxidant effect of caffeic acid may be another mechanism. Since cerebral ischemic injury is a temporally continuous process[13], inhibition of oxidant stress in early injury may reasonably attenuate the secondarily delayed injuries like astrocyte proliferation and glial scar formation. Until now, few effective agents on post-ischemic astrocyte proliferation are reported, although heparin oligosaccharides or phosphodiesterase inhibitor rolipram inhibited glial scar formation after traumatic brain or spinal cord injury[33,34], and A2A adenosine receptor antagonists, dexamethasone and isoproterenol inhibited astrocyte proliferation in vitro[35,36]. Therefore, the effect of caffeic acid may represent a novel approach for ameliorating astrocyte proliferation and glial scar formation in the ischemic brain.
In conclusion, the present study shows that caffeic acid ameliorates both early and delayed injuries, especially the astrocyte proliferation, after focal cerebral ischemia in rats; one mechanism of the neuroprotection may be the inhibition of 5-LOX. Our findings indicate that caffeic acid may be a prototype compound of neuroprotective agents against cerebral ischemia injury; however its effective dose is relatively larger so further investigations will be necessary to search its derivatives and fully clarify the properties and mechanisms.
References
- Czok G. Coffee and health. Z Ernahrungswiss 1977;16:248-55.
- Cui CB, Tezuka Y, Kikuchi T, Nakano H, Tamaoki T, Park JH. Constituents of a fern, Davallia mariesii Moore. I. Isolation and structures of davallialactone and a new flavanone glucuronide. Chem Pharm Bull (Tokyo) 1990;38:3218-25.
- Sondheimer E. On the distribution of caffeic acid and the chlorogenic acid isomers in plants. Arch Biochem Biophys 1958;74:131-8.
- Vieira O, Laranjinha J, Madeira V, Almeida L. Cholesteryl ester hydroperoxide formation in myoglobin-catalyzed low density lipoprotein oxidation: concerted antioxidant activity of caffeic and p-coumaric acids with ascorbate. Biochem Pharmacol 1998;55:333-40.
- Bors W, Michel C, Stettmaier K, Lu Y, Foo LY. Antioxidant mechanisms of polyphenolic caffeic acid oligomers, constituents of Salvia officinalis. Biol Res 2004;37:301-11.
- da Cunha FM, Duma D, Assreuy J, Buzzi FC, Niero R, Campos MM, et al. Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties. Free Radic Res 2004;38:1241-53.
- Irmak MK, Fadillioglu E, Sogut S, Erdogan H, Gulec M, Ozer M, et al. Effects of caffeic acid phenethyl ester and alpha-tocopherol on reperfusion injury in rat brain. Cell Biochem Funct 2003;21:283-9.
- Tsai SK, Lin MJ, Liao PH, Yang CY, Lin SM, Liu SM, et al. Caffeic acid phenethyl ester ameliorates cerebral infarction in rats subjected to focal cerebral ischemia. Life Sci 2006;78:2758-62.
- Koshihara Y, Neichi T, Murota S, Lao A, Fujimoto Y, Tatsuno T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim Biophys Acta 1984;792:92-7.
- Maccarrone M, Battista N, Meloni M, Bari M, Galleri G, Pippia P, et al. Creating conditions similar to those that occur during exposure of cells to microgravity induces apoptosis in human lymphocytes by 5-lipoxygenase-mediated mitochondrial uncoupling and cytochrome c release. J Leukoc Biol 2003;73:472-81.
- Song Y, Wei EQ, Zhang WP, Zhang L, Liu JR, Chen Z. Minocycline protects PC12 cells from ischemic-like injury and inhibits 5-lipoxygenase activation. Neuroreport 2004;15:2181-4.
- Ge QF, Wei EQ, Zhang WP, Hu X, Huang XJ, Zhang L, et al. Activation of 5-lipoxygenase after ischemic injury is partly mediated via NMDA receptor in rat cortical neurons. J Neurochem 2006;97:992-1004.
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 2003;26:248-54.
- Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke 2004;35:2220-5.
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull 1999;49:377-91.
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci 2004;5:146-56.
- Persson L, Hardemark HG, Bolander HG, Hillered L, Olsson Y. Neurologic and neuropathologic outcome after middle cerebral artery occlusion in rats. Stroke 1989;20:641-5.
- Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology 2003;98:465-73.
- Zhang WP, Wei EQ, Mei RH, Zhu CY, Zhao MH. Neuroprotec-tive effect of ONO-1078, a leukotriene receptor antagonist, on focal cerebral ischemia in rats. Acta Pharmacol Sin 2002;23:871-7.
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84-91.
- Prestigiacomo CJ, Kim SC, Connolly ES Jr, Liao H, Yan SF, Pinsky DJ. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke 1999;30:1110-7.
- Uz T, Pesold C, Longone P, Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. FASEB J 1998;12:439-49.
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 1986;17:472-6.
- Yu GL, Wei EQ, Wang ML, Zhang WP, Zhang SH, Weng JQ, et al. Pranlukast, a cysteinyl leukotriene receptor-1 antagonist, protects against chronic ischemic brain injury and inhibits the glial scar formation in mice. Brain Res 2005;1053:116-25.
- Yonemori F, Yamaguchi T, Yamada H, Tamura A. Evaluation of a motor deficit after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1998;18:1099-106.
- Ciccarelli R, D’Alimonte I, Santavenere C, D’Auro M, Ballerini P, Nargi E, et al. Cysteinyl-leukotrienes are released from astrocytes and increase astrocyte proliferation and glial fibrillary acidic protein via cys-LT1 receptors and mitogen-activated protein kinase pathway. Eur J Neurosci 2004;20:1514-24.
- Wei X, Zhao L, Ma Z, Holtzman DM, Yan C, Dodel RC, et al. Caffeic acid phenethyl ester prevents neonatal hypoxic-ischaemic brain injury. Brain 2004;127:2629-35.
- Moskowitz MA, Kiwak KJ, Hekimian K, Levine L. Synthesis of compounds with properties of leukotrienes C4 and D4 in gerbil brains after ischemia and reperfusion. Science 1984;224:886-9.
- Ciceri P, Rabuffetti M, Monopoli A, Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br J Pharmacol 2001;133:1323-9.
- Rao AM, Hatcher JF, Kindy MS, Dempsey RJ. Arachidonic acid and leukotriene C4: role in transient cerebral ischemia of gerbils. Neurochem Res 1999;24:1225-32.
- Namura Y, Shio H, Kimura J. LTC4/LTB4 alterations in rat forebrain ischemia and reperfusion and effects of AA-861, CV-3988. Acta Neurochir Suppl (Wien) 1994;60:296-9.
- Ilhan A, Akyol O, Gurel A, Armutcu F, Iraz M, Oztas E. Protective effects of caffeic acid phenethyl ester against experimental allergic encephalomyelitis-induced oxidative stress in rats. Free Radic Biol Med 2004;37:386-94.
- Hayashi N, Miyata S, Kariya Y, Takano R, Hara S, Kamei K. Attenuation of glial scar formation in the injured rat brain by heparin oligosaccharides. Neurosci Res 2004;49:19-27.
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci USA 2004;101:8786-90.
- Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia 2003;43:190-4.
- Riva MA, Molteni R, Racagni G. Differential regulation of FGF-2 and FGFR-1 in rat cortical astrocytes by dexamethasone and isoproterenol. Brain Res Mol Brain Res 1998;57:38-45.
