High-throughput screening assay for new ligands at human melatonin receptors1
Introduction
Melatonin (N-acetyl-5-methoxytryptamine, MT) is a neurohormone synthesized and secreted primarily by the pineal gland in a circadian manner, with peak levels in all species occurring during the period of darkness. The synthesis of melatonin within the pineal gland is mainly regulated by the daily and seasonal changes in the environmental light/dark cycle. Melatonin, which is released into the blood circulation, is thought to transduce photoperiodic information to all the tissues expressing its receptors or other binding sites[1]. After its discovery and chemical characterization in 1959[2], many different physiological and behavioral responses have been ascribed to melatonin. It not only regulates the sleep/wake cycle[3], but also affects cardiovascular[4], reproductive[5], cell growth[6], and retinal processes[7].
The discovery of different melatonin receptors was facilitated by the introduction of the agonist radioligand 2-[125I]iodomelatonin[8]. Two human melatonin receptor subtypes, hMT1 and hMT2, have been cloned and shown to be G-protein-coupled receptors (GPCR)[9–11]. MT3, a third melatonin binding site, has been subsequently described in hamsters as the human homolog of the cytoplasmic quinone reductase-2[12,13]. The homology between hMT1 and hMT2 receptors at the amino acid level is 55% with approximately 70% overall identity within the transmembrane domains. The activation of these 2 receptor subtypes leads to the inhibition of adenyl cyclase, probably through the activation of a Gi-protein[9,10]. Studies of their tissue distribution in mammals have shown that these receptors are localized in different areas of the brain (suprachiasmatic nucleus, pars tuberalis, hypothalamus, cerebellum, cortex, hippocampus, and cerebral vessels), and at the peripheral level in the kidney, small intestine, and caudal arteries[9,10,14–19]. The differences in tissue distribution are in favor of different functions for each receptor subtype. Pharmacological investigations with selective agonists or antagonists are therefore necessary to determine the physiological roles of these melatonin receptor subtypes and their implications in pathological processes. This information will probably open new therapeutic perspectives for selective ligands, different from the chronobiotic properties of melatonin clearly demonstrated in humans[20,21].
Scintillation proximity assay (SPA) technology is a homogeneous approach that does not involve post-reaction liquid handling steps and is well-suited to automation and high-throughput screening (HTS). In the SPA system, membranes that express a particular receptor are attached to a microbead coated with wheat germ agglutinin. A radioisotope atom (eg [125I]) is brought very close to a scintillant-impregnated microbead by binding to the receptor. Because the emitted β particles can only travel short distances in the bulk solution, the microbeads preferentially capture electrons from the bound radiolabeled ligand. Thus, the amount of light emitted from the scintillant in the microbeads is directly proportional to the amount of bound radiolabeled ligand[22].
In this study, we screened a library of 48 240 synthetic (91.2%) and natural compounds (8.8%) made in house, collected from various research institutions across China or purchased from commercial sources, with the goal of finding novel selective and potent hMT1 and/or hMT2 receptor agonists and antagonists. Using SPA technology, binding affinities were determined with 2-[125I]iodomelatonin and their functional activities by guanosine-5’[γ-35S]triphosphate ([35S]-GTP-γS) binding assay[23]. As a result, we discovered a series of selective antagonists for hMT1 and hMT2 receptors.
Materials and methods
Reagents The radioligand 2-[125I]iodomelatonin (specific activity: 81.4 TBq/mmol) and FlashBlue GPCR beads were purchased from PerkinElmer (Boston, MA, USA). [35S]GTP-γS (specific activity: 37 TBq/mmol) was obtained from Amersham Biosciences (Buckinghamshire, England). Guanosine diphosphate (GDP), GTP-γS, saponin, bovine serum albumin (BSA), HEPES, and melatonin were from Sigma–Aldrich (St Louis, MO, USA). Fetal bovine serum (FBS) was bought from Hyclone (Logan, UT, USA), and HamF12 medium was purchased from GIBCO BRL (Rockville, ML, USA).
Membrane preparation Chinese hamster ovary (CHO) cells stably expressing hMT1 or hMT2 receptors were provided by Servier (Neuilly-sur-Seine, France)[24]. The cells were cultured in HamF12 medium supplemented with 10% FBS, 2 mmol/L glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. They were grown at confluence, harvested in phosphate-buffered saline, and centrifuged at 1000×g for 5 min (4 °C). The resulting pellet was suspended in isotonic buffer (5 mmol/L Tris/HCl, 0.2 mmol/L MgCl2, and 0.25 mol/L sucrose, pH 7.4), and homogenized using the BioNeb Cell Disruption System (Terre Haute, IN, USA). The homogenate was then centrifuged (20 000×g for 30 min at 4 °C), and the resulting pellet was suspended in binding buffer 1 (50 mmol/L Tris/HCl, pH 7.4, 5 mmol/L MgCl2, 1 mmol/L EDTA). The protein content was determined using the Bradford assay[25]. Aliquots of membrane preparations were stored at –80 °C until use.
Homogenous binding assay The membranes were incubated overnight in binding buffer 1 containing 18 pmol/L 2-[125I]iodomelatonin, 0.75 mg/mL FlashBlue GPCR beads, various titrations of melatonin from a stock solution of 40 μmol/L, and library compounds with an average concentration of 6.7 μmol/L (final volume: 100 μL)Non-specific binding was defined with 1 μmol/L melatonin. Data were analyzed with GraphPad PRISM (GraphPad Software, San Diego, CA, USA). The competitive inhibition constant (Ki) was calculated according to the Cheng–Prusoff equation: Ki=IC50/(1+L/Kd]), where IC50 is the concentration that produced 50% inhibition, Kd is the dissociation constant of inhibitor-binder reaction, and L is the concentration of the radiolabeled ligand used[26].
[35S]GTP-γS binding assay The membranes and test compounds were diluted in binding buffer 2 (20 mmol/L HEPES, pH 7.4, 100 mmol/L NaCl, 3 mmol/L MgCl2, 12 μmol/L GDP, and 0.1% BSA) in the presence of 20 μg/mL saponin in order to enhance the agonist-induced stimulation level[27], and pre-mixed with 1 mg/mL FlashBlue GPCR beads. For the agonist test, incubation was started by the addition of 0.2 nmol/L [35S]GTP-γS to the membranes and test compounds, and continued for 4 h at room temperature in a final volume of 100 μL. To study the antagonist activity, the membranes were pre-incubated for 2 h with melatonin (30 nmol/L) in conjunction with a given concentration of a test compound. Reaction begun with the addition of 0.2 nmol/L [35S]GTP-γS followed by 4 h incubation at room temperature. Non-specific binding was assessed using non-radiolabeled GTP-γS (10 μmol/L). Data were analyzed with PRISM (GraphPad, USA) to calculate the 50% effective concentration (EC50) and the maximal effect expressed as a percentage of that observed with 1 μmol/L melatonin, 100% (Emax) for agonists. KB was used to describe antagonist potency: KB=IC50/(1+[agonist]/EC50), where IC50 is the antagonist concentration that gives 50% inhibition of [35S]GTP-γS binding in the presence of a fixed concentration of agonist, and EC50 is the 50% effective concentration of an agonist when tested alone[26]. The maximal inhibitory effect (Imax) was expressed as a percentage of that observed with 30 nmol/L melatonin for hMT1 or hMT2 receptors.
Results
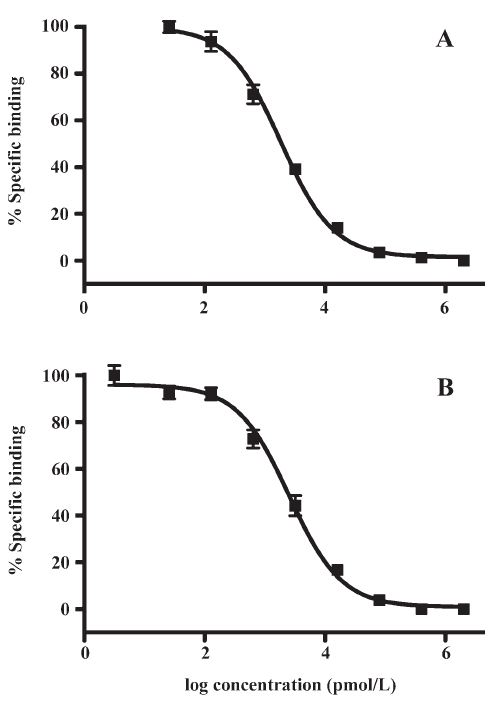
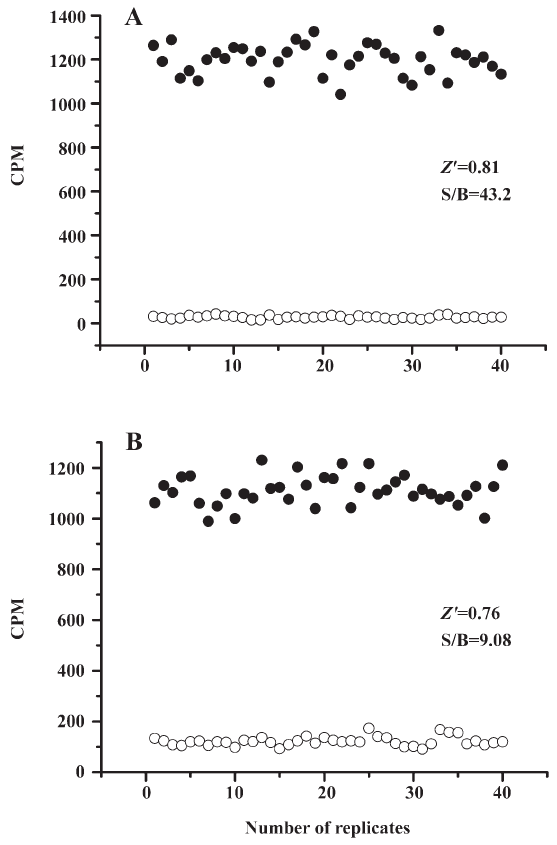
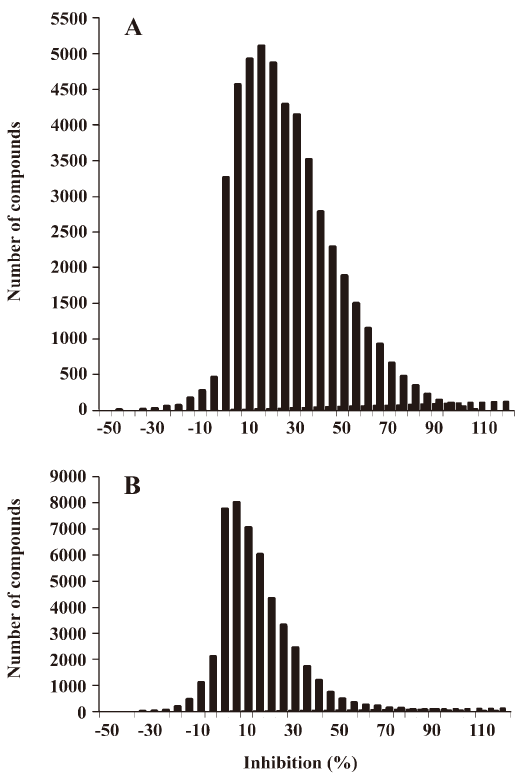
HTS campaign In the validation process, various assay parameters were studied. The optimal concentrations of radioligand and microbeads were 18 pmol/L and 0.75 mg/mL, respectively, for both receptor subtypes, while protein concentrations were optimized to 2.6 μg/mL for the hMT1 receptor and 0.9 μg/mL for the hMT2 receptor, respectively (data not shown). The control compound, melatonin, was tested at both receptors to evaluate the system. As shown in Figure 1, the Ki values of melatonin were 0.87 nmol/L for the hMT1 receptor and 2.07 nmol/L for the hMT2 receptor, respectively, compatible to that reported in the literature[24]. In order to apply the assay to a HTS format, the Z´ value and the signal/background (S/B; ie specific vs non-specific binding) were studied. The Z´ factor, a screening window coefficient, is reflective of both the assay signal dynamic range and the data variation associated with the signal measurements. Therefore, it is suitable for the assay quality assessment[28]. As shown in Figure 2, the Z´ value for the hMT1 receptor was 0.81 with a S/B ratio of 43.2 and a coefficient of variation value of 5.7%. In the case of the hMT2 receptor, the corresponding values were 0.76, 9.08, and 5.4%, respectively. These characteristics suggest that the 2 assay systems are of high quality and suitable for HTS[28]. The number of hits at different inhibition rates discovered from a parallel HTS campaign directed towards hMT1 and hMT2 receptors are shown in Figure 3. Of the 48 240 samples screened (which did not include 4 P-PDOT and other 17 ligands known to react with hMT1 and hMT2 receptors[24]), 205 and 199 initial hits were identified for hMT1 and hMT2 receptors (≥85% binding activities), respectively. Among them, 63 (hMT1) and 54 (hMT2) hits were subsequently confirmed and subjected to IC50 determination, and the values of these confirmed hits were within the range of dozens of nmol/L to a few μmol/L for both hMT1 and hMT2 receptors (Table 1 and data not shown).
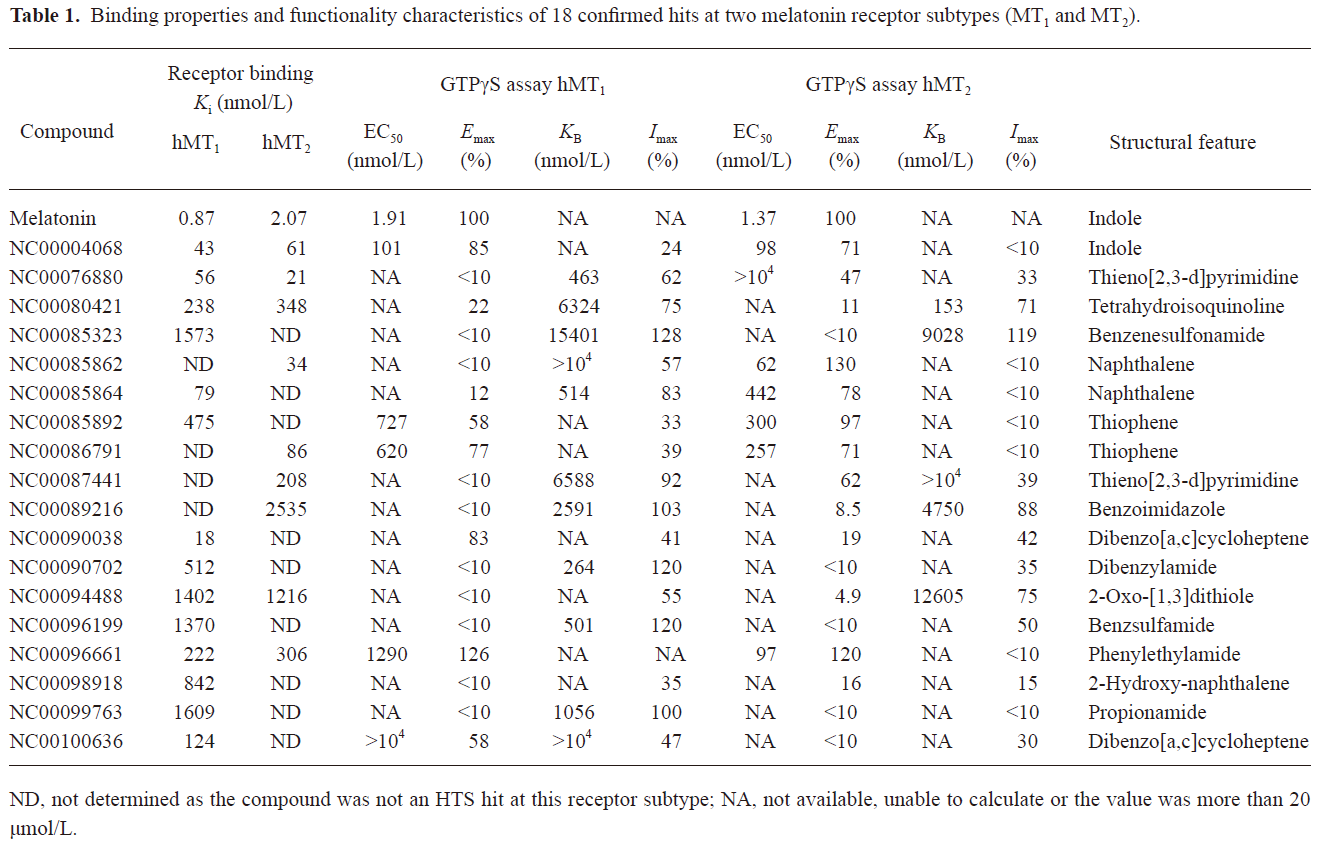
Full table
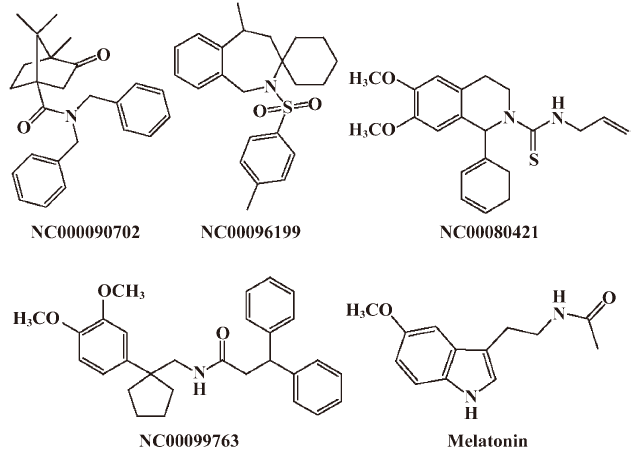
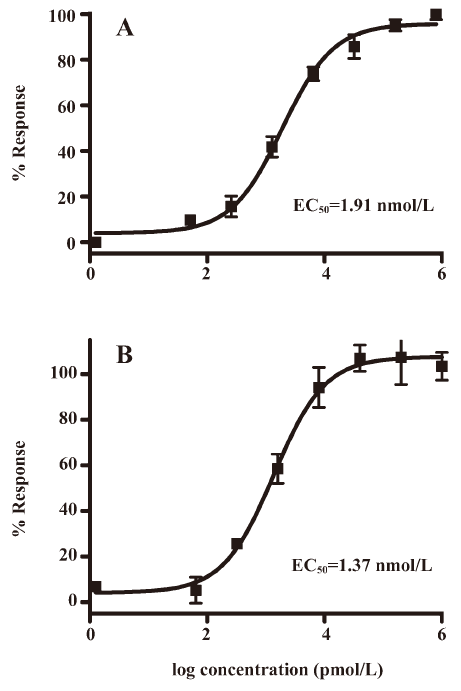
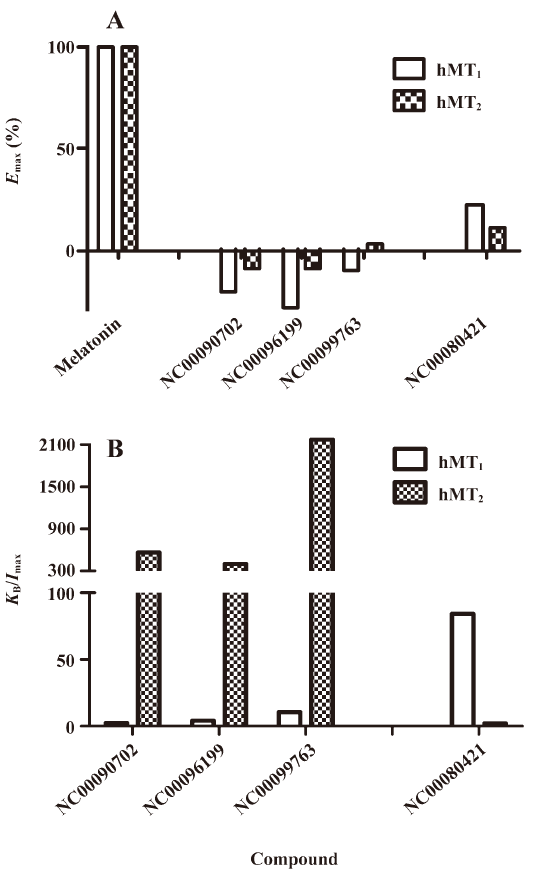
Functionality characterization In order to examine the pharmacological properties (agonist or antagonist) of these compounds at each melatonin receptor subtype, [35S]GTP-γS binding assays were performed. For both subtypes, the optimal concentrations of saponin, MgCl2,, and GDP were 20 μg/mL, 3 mmol/L and 12 μmol/L, respectively (data not shown), similar to that reported previously[24]. The agonist activities of melatonin at hMT1 (EC50=1.91 nmol/L) and hMT2 receptors (EC50=1.37 nmol/L) are shown in Figure 4. Based on the novelty in molecular structures and potency in binding activities, we selected 18 confirmed hits identified from the HTS campaign for simultaneous functionality studies with both receptor subtypes. As shown in Table 1, melatonin acted as a full agonist for both hMT1 and hMT2 receptors. Of the 18 compounds, NC00090702, NC00096199, and NC00099763 displayed selective hMT1 receptor antagonist activities with KB values of 0.26, 0.50, and 1.06 μmol/L, respectively; NC00080421 exhibited selective hMT2 receptor antagonist activities with a relatively more potent KB value of 0.15 μmol/L. The chemical structures of the 4 selective antagonists, all obtained from a commercial source (Specs, Delft, the Netherlands), are presented in Figure 5. Figure 6 depicts both the agonist and antagonist features of these compounds, as defined by Emax and KB/Imax. Apart from NC00080421, which demonstrated some partial agonist activities (at the hMT1 receptor in particular), these antagonists are highly selective for a particular receptor subtype.
Discussion
SPA is an innovative approach to assay development and biochemical screening that allows the rapid and sensitive measurement of a wide variety of molecular interactions in a homogeneous system. In the present study, we employed this technology in the HTS of 48 240 samples against 2 melatonin receptor subtypes: hMT1 and hMT2. Not only did it show strong reliability in terms of consistent EC50 values measured for the control ligand (melatonin) and assay parameters exemplified by the high Z´ values (0.81 for the hMT1 receptor and 0.76 for the hMT2 receptor), this affinity detection-based HTS method successfully identified a series of active compounds with a confirmed hit rate of 0.13% and 0.11% for the hMT1 and hMT2 receptors, respectively. Subsequent IC50 determination and functionality studies with GTP-γS assays verified the results obtained from the HTS campaign. It was noted that the S/B window of our GTP-γS assay was somehow reduced (data not shown), probably due to the lack of wash step, as compared to that reported elsewhere[24]. Nevertheless, the system was robust enough for the eventual identification of several novel melatonin receptor modulators.
Of the 4 functionally-selective compounds, 1 was characterized as a hMT2 antagonist and 3 as hMT1 receptor antagonists. NC00080421, with a similar structure to known selective hMT2 receptor antagonist 4P-PDOT[29], showed approximately 40-fold selectivity at the hMT2 receptor. However, NC00090702, NC00096199, and NC00099763, with selective hMT1 receptor antagonist activities, exhibited little structural similarity to melatonin. Because hMT1 receptor modulators are scarcely available, our findings certainly provide some new knowledge for continued pursuit in this direction using the dual melatonin receptor subtype assay system[30]. Unfortunately, we have not found selective hMT1 or hMT2 receptor agonists in this study, but further structure–activity relationship analyses on these leads and relevant structural modifications may dial in agonist activity.
In humans, it has been suggested that melatonin has a variety of clinical applications, such as the treatment of delayed sleep phase syndrome, jet lag, work shift disturbances[31], seasonal affective disorders[32], and aging[33]. Melatonin was also implicated to have immuno-modulating properties[34]. Recent studies demonstrated that melatonin may play a role in Alzheimer’s disease[35], anesthesia[36], depression[37], and obesity[38]. However, the very short biological half-life (20–30 min), extensive metabolism, and lack of selectivity of melatonin at its target sites prompt the search for metabolically-stable agonists and antagonists. Although studies involving animal models relevant to human pathologies and appropriate clinical trials have indicated that circadian sleep disorders and insomnia are probably the sole therapeutic indications today for selective hMT1 or hMT2 receptor ligands[39], the selective antagonists described in this study may provide important tools in further understanding the physiological functions of the different receptor subtypes (hMT1 or hMT2) in vivo.
Acknowledgements
We are indebted to Mr Xin HUI, Ms Hong YU, and Mr Qiang SHEN for their technical assistance, and to Dr Dale E MAIS for valuable discussions.
References
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev 1991;12:151-80.
- Lerner AB, Case JD. Structure of melatonin. J Am Chem Soc 1959;81:6084-5.
- Cassone VM. Melatonin's role in vertebrate circadian rhythms. Chronobiol Int 1998;15:457-73.
- Krause DN, Geary GG, Doolen S, Duckles SP. Melatonin and cardiovascular function. Adv Exp Med Biol 1999;460:299-310.
- Reiter RJ. Melatonin and human reproduction. Ann Med 1998;30:103-8.
- Jones MP, Melan MA, Witt-Enderby PA. Melatonin decreases cell proliferation and transformation in a melatonin receptor-dependent manner. Cancer Lett 2000;151:133-43.
- Dubocovich ML. Characterization of a retinal melatonin receptor. J Pharmacol Exp Ther 1985;234:395-401.
- Dubocovich ML, Takahashi JS. Use of 2-[125I]iodomelatonin to characterize melatonin binding sites in chicken retina. Proc Natl Acad Sci USA 1987;84:3916-20.
- Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994;13:1177-85.
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci USA 1995;92:8734-8.
- Hunt AE, Ghoul WM, Dubocovich ML. Activation of melatonin MT2 receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol 2001;280:C110-C8.
- Nosjean O, Ferro M, Cogé F, Beauverger P, Henlin JM, Lefoulon F, . Identification of the melatonin binding site MT as the quinone reductase 2. J Biol Chem 2000; 257: 31 311–7.
- Nosjean O, Nicolas JP, Klupsch F, Delagrange P, Canet E, Boutin JA. Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. Biochem Pharmacol 2001;61:1369-79.
- Dubocovich ML, Cardinali DP, Delagrange P, Krause DN, Strosberg D, Sugden D, . The IUPHAR compendium of receptor characterization and classification. IUPHAR Media, London 2000: 270–7.
- Al-Ghoul WM, Herman MD, Dubocovich ML. Melatonin receptor subtype expression in human cerebellum. Neuroreport 1998;9:4063-8.
- Ting KN, Blaylock NA, Sugden D, Delagrange P, Scalbert E, Wilson VG. Molecular and pharmacological evidence for MT1 melatonin receptor subtype in tail artery of juvenile Wistar rats. Br J Pharmacol 1999;127:987-95.
- Mazzucchelli C, Pannacci M, Nonno R, Lucini V, Fraschini F, Stankov BM. The melatonin receptor in the human brain: cloning experiments and distribution studies. Brain Res Mol Brain Res 1996;39:117-26.
- Weaver DR, Reppert SM. The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. Neuroreport 1996;8:109-12.
- Song Y, Chan CW, Brown GM, Pang SF, Silverman M. Studies of the renal action of melatonin: evidence that the effects are mediated by 37 kDa receptors of the Mel1a subtype localized primarily to the basolateral membrane of the proximal tubule. FASEB J 1997;11:93-100.
- Delagrange P, Guardiola-Lemaitre B. Melatonin, its receptors and relationships with biological rhythms disorders. Clin Neuropharmacol 1997;20:482-510.
- Mahle CD, Takaki KS, Watson AJ. Melatonin receptor ligands and their potential clinical applications. Annu Rep Med Chem 1997.31-40.
- Wu B, Gao J, Wang MW. Development of a complex scintillation proximity assay for high-throughput screening of PPARγ modulators. Acta Pharmacol Sin 2005;26:339-44.
- Harrison C, Traynor JR. The [35S]GTPγS binding assay: approaches and applications in pharmacology. Life Sci 2003;74:489-508.
- Audinot V, Mailliet F, Lahaye-Brasseur C, Bonnaud A, Gall AL, Amossé C, et al. New selective ligands of human cloned melatonin MT1 and MT2 receptors. Naunyn Schmiedeberg's Arch Pharmacol 2003;367:553-61.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.
- Cheng YC, Prussoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol 1973;22:3099-108.
- Cohen FR, Lazareno S, Birdsall NJM. The effects of saponin on the binding and functional properties of the human adenosine A1 receptor. Br J Pharmacol 1996;117:1521-9.
- Zhang J-H, Chung TDY, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 1999;4:67-73.
- Dubocovich ML, Masana MI, Iacob S, Sauri DM. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn-Schmiedeberg’s Arch Pharmacol 1997;355:365-75.
- Silvia R, Simone L, Marco M, Pier VP, Gilberto S, Annalida B, et al. Analysis of structure-activity relationships for MT2 selective antagonists by melatonin MT1 and MT2 receptor models. J Med Chem 2005;48:4049-60.
- Lewy AJ, Emens J, Jackman A, Yuhas K. Circadian uses of melatonin in humans. Chronobiol Int 2006;23:403-12.
- Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi-Perumal SR, et al. Melatonin in mood disorders. World J Biol Psychiatry 2006;7:138-51.
- Poeggeler B. Melatonin, aging, and age-related diseases: perspectives for prevention, intervention, and therapy. Endocrine 2005;27:201-12.
- Srinivasan V, Maestroni G, Cardinali D, Esquifino A, Perumal SP, Miller S. Melatonin, immune function and aging. Immun Ageing 2005;2:17.
- Brunner P, Sozer-Topcular N, Jockers R, Ravid R, Angeloni D, Fraschini F, et al. Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer's disease. Eur J Histochem 2006;50:311-6.
- Naguib M, Gottumukkala V, Goldstein PA. Melatonin and anesthesia: a clinical perspective. J Pineal Res 2007;42:12-21.
- Banki MC. Agomelatin: the first “melatoninergic” antidepressant. Neuropsychopharmacol Hung 2006;8:105-12.
- Hussein MR, Ahmed OG, Hassan AF, Ahmed MA. Intake of melatonin is associated with amelioration of physiological changes, both metabolic and morphological pathologies associated with obesity: an animal model. Int J Exp Pathol 2007;88:19-29.
- Delagrange P, Boutin JA. Therapeutic potential of melatonin ligands. Chronobiol Int 2006;23:413-8.