Over-expression of nm23-H1 in HeLa cells provides cells with higher resistance to oxidative stress possibly due to raising intracellular p53 and GPX11
Introduction
Oxidative stress is known for its involvement in the pathophysiology of many human diseases, including, but not restricted, to cancer. Reactive oxygen species (ROS) changes have been implicated at all stages of the carcinogenic process[1]. Oxidative DNA damage can trigger tumor initiation. In terms of cell proliferation, ROS have been shown to modulate the cell cycle through the modulation of various cell cycle proteins, including p53[2] and the ataxia telangiectasia-mutated protein[3]. Metastasis is an integral part of tumor progression, during which ROS have been documented to play a major role[4,5]. A previous study reported that the long-term exposure of mouse NMuMG mammary epithelial cells to H2O2, which mimics chronic inflammation, showed a cellular phenotypic conversion with striking similarities to malignant transformation, accompanied by the induction of genes associated with cell adhesion and migratory behavior, together with the activation of the small G protein Rac1 and mitogen-activated protein (MAP) kinases[6]. Importantly, following these changes, the epithelial cells ultimately acquire the potential to invade a reconstituted basement membrane in the presence of normal fibroblasts. In fact, various studies have shown that metastatic tumor cells produce higher levels of ROS than primary malignant cells, which greatly reduce metastasis, together with increasing levels of ROS metabolizing enzymes and antioxidant compounds[7–9].
nm23 is an antitumoral metastatic gene that was first reported in murine melanoma. In humans, 8 related genes, nm23-1 to -8, have been described[10]. The first 2 genes (nm23-H1 and nm23-H2), as well as their respective counterparts in rats (nm23-β and nm23-α) and mice (nm23-M1 and nm23-M2), have been shown to encode different isoforms of the enzyme, named nucleoside diphosphate kinase (NDPK). Extensive studies have revealed that the nm23 protein participates in many biological activities, including development, differentiation, proliferation, endocytosis, and apoptosis[11]. Recently, nm23-H1 was confirmed to have been involved in the regulation of the p53-induced signaling pathway, in which nm23-H1 acts as a positive regulator[12]. Additionally, mouse nm23-M2 can activate endogenous transcription of the nm23-M1 gene, and the over-expression of nm23-M1 and -M2 prevents the cells from oxidative stress-induced death[13].
Human p53 is a 393 amino acid nuclear phosphoprotein and transcription factor. Evidence highlights that p53 works in a more positive way, participating in the maintenance of intracellular redox homeostasis and the protection of the genome from oxidative damage. Several p53-regulated genes, such as GPX1, SOD2, and aldehyde dehydrogenase 4 family member A1 (ALDH4A1), encode products as antioxidants[14,15]. The reactivation of overoxidized Prx is mediated by 2 p53-regulated sestrins, namely PA26 and Hi95 (encoded by SESN1 and SESN2, respectively), which are required for the regeneration of Prx containing cysteine sulfuric acid[16,17]. In the absence of stress or after mild stress, a relatively low level of p53 is sufficient to upregulate several antioxidant genes that decrease ROS levels and protect cells from DNA damage[18].
To address the possibility that the antitumoral activity of nm23-H1 is related with antioxidation, a recombinant plasmid encoding the full-length human nm23-H1 gene was constructed and transiently induced into HeLa cells in this study. A remarkably low level of ROS and strong resistance to oxidative stress were observed in the cells overexpressing nm23-H1. Furthermore, we found that the levels of p53 and p53-regulated gene GPX1 were markedly increased in the cells receiving the nm23-H1-expressing plasmid. The downregulation of p53 in the cells overexpressing nm23-H1 resulted in higher cellular ROS and lower cell viability; the cellular level of GPX1 also decreased. This suggests that nm23-H1 as a metastasis suppressor may act as a protector against oxidative stress, possibly triggering the p53-related antioxidative pathway.
Materials and methods
Plasmid construction The full-length human nm23-H1 (X73066) sequence was amplified using PCR. The primers used were human nm23-H1-P1 (
Cell culture and transient transfection Human cervical carcinoma cell line HeLa was maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, USA). The cells were plated into 6-, 24-, and 96-well plates (Falcon, Japan) 1 d before transfection. The recombinant plasmid pcDNA–nm23-H1, as well as vector pcDNA3.1, was transfected into the monolayer cells with Lipofectamine 2000 transfection reagent (Invitrogen, USA). Twenty-four hours after transfection 5, 10, 20, or 40 µmol/L H2O2 was added to DMEM and maintained for another 12 h. The cells were then harvested in phosphate-buffered saline (PBS), pelleted by centrifugation, and suspended in lysis buffer (10 mmol/L Tris-HCl, pH 7.8, 0.5% sodium deoxycholate, 0.5% Nonidet P-40, 100 mmol/L NaCl, and 10 mmol/L EDTA) supplemented with a complete protease inhibitor mixture. The protein content of each lysate was determined using the BCA assay kit (Beyotime Biotechnology, Haimen, Jiangsu, China).
Western blotting Various cell lysates were separated by 12% SDS-PAGE and electrotransferred onto nitrocellulose membranes. After blocking with 5% non-fat milk in PBST (PBS, pH 7.6, containing 0.05% Tween-20) overnight at 4 ºC, the membranes were incubated with 1:100 nm23-H1, 1:100 p53 antibody (Boston, China), or 1:1000 GPX1 antibody (Cell Signaling Technology, Danvers, MA, USA) for 2 h at room temperature, and then incubated with 1:2000 horseradish peroxidase-conjugated antimouse immunoglobulin G (IgG) or anti-rabbit IgG (Santa Cruz, Santa Cruz, CA, USA). The protein bands were visualized by an ECL kit (PE Applied Biosystems, Foster City, CA, USA).
Measurement of intracellular oxidative activity Determination of the level of intracellular reactive oxygen species was carried out with a ROS assay kit (Beyotime Biotechnology, Haimen, China). Briefly, the cells were treated with dihydrodichloro-fluorescein diacetate (DCF-DA) in serum-free medium at 37 ºC for 30 min and harvested in PBS. The fluorescence of each well was then measured on a flow cytometer with 480 nm excitation and 530 nm emission wavelengths.
Trypan blue and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays The cells were cultured in 24-well tissue culture plates and transiently transfected with nm23-H1-expressing plasmids or pcDNA3.1. Forty-eight hours after transfection, the cells were harvested and stained with the DNA-binding dye trypan blue (Sigma, St Louis, MO, USA) for 30 min. The number of trypan blue positive-stained cells in each well was counted under a microscope. To evaluate the cell viability, the cells were plated as normal in 96-well trays and exposed to 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma, USA) in culture medium for 4 h. After being lysed, the formazan product was dissolved in DMSO. Absorbance was measured at 540 nm in a spectrophotometer.
siRNA experiment To interfere with the expression of the p53 protein in the cultured cells, a commercial p53 siRNA kit (SignalSilence p53 siRNA kit; Cell Signaling Technology, Danvers, MA, USA) was used for generating a status of downregulated p53. In brief, the HeLa cells were plated into 6-well tissue culture plates 1 d before transfection. pcDNA-nm23-H1 was cotransfected into the cells with transfection reagent and p53 siRNA or control siRNA. Forty-eight hours after transfection, the cellular expression of p53 and other cytokines were evaluated by Western blotting.
Statistical analysis Statistical analysis was performed using the t-test. Probabilities of less than 0.05 were considered statistically significant.
Results
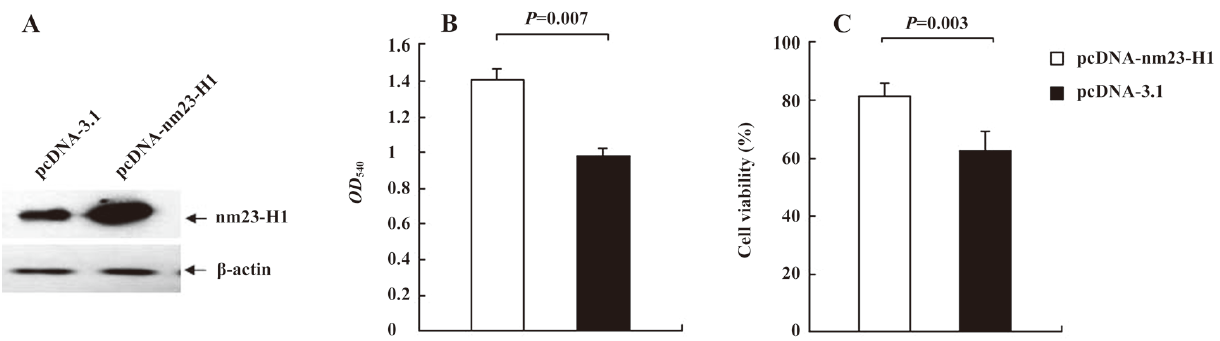
Cells overexpressing nm23-H1 showed more active growth capacity Recombinant plasmid pcDNA-nm23-H1 was transiently transfected into the HeLa cells, and the expression of nm23-H1 was evaluated by nm23-H1-specific Western blotting. Figure 1A shows that the cells receiving pcDNA-nm23-H1 produced a remarkably higher level of nm23-H1 signals, compared with the cells receiving pcDNA3.1. To observe the influence of the over-expression of the nm23-H1 protein on cell growth activities, the viabilities of the transfected cells were measured 48 h after transfection with trypan blue and MTT assays. The MTT analyses showed that more dead cells in the cells with pcDNA3.1 than those with pcDNA-nm23-H1 (Figure 1B). The mean optical density (OD) value of the cells overexpressing nm23-H1 was 1.411, while that of the cells transfected with pcDNA3.1 was 0.978, with statistical differences (P=0.007). The trypan blue assays revealed that the viabilities of the HeLa cells overexpressing nm23-H1 were repeatedly higher than that of the cells transfected with plasmid pcDNA3.1 (Figure 1C), showing statistical differences (P=0.003). The results suggest that the over-expression of nm23-H1 helps the cells to maintain more active growth ability.
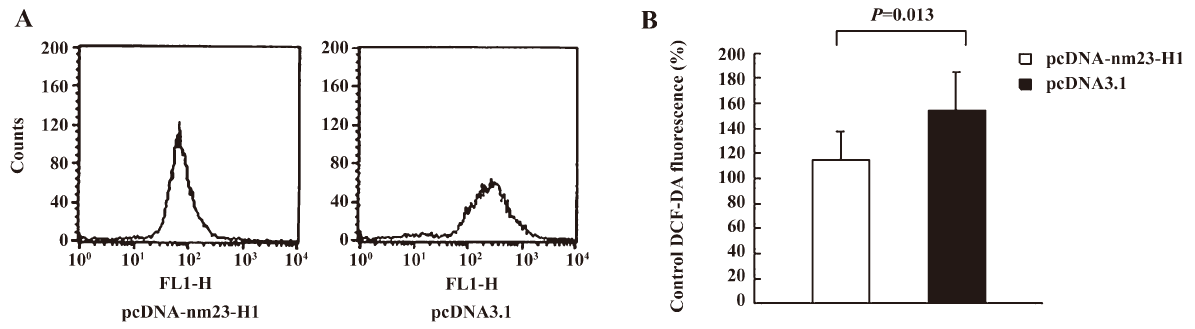
Cells overexpressing nm23-H1 possessed low levels of intracellular free radicals To address the influence of nm23-H1 on the intracellular oxidation process, the generation of free radicals in the cells receiving pcDNA-nm23-H1 and pcDNA3.1 was measured by staining with fluorescent dye DCF-DA. Compared with the preparation transfected with pcDNA3.1, the ROS level in the cells overexpressing nm23-H1 was obviously lower (Figure 2A). The average ROS level in the pcDNA3.1 groups was 1.33-fold higher than that of nm23-H1 (Figure 2B), revealing statistical difference (P=0.013).
To test the antioxidant role of nm23-H1 under oxidative stress, the mock and nm23-H1 overexpressed cells were challenged with 10 μmol/L H2O2 for 12 h and the ROS levels were measured. An obviously high level of intracellular free radicals was seen in all preparations after treatment with 10 μmol/L H2O2, compared with those without H2O2 challenge. As expected, a stronger decrease of intracellular free radicals was identified in the cells expressing nm23-H1, showing statistical difference compared with the cells with pcDNA3.1 (P<0.05, data not shown). The results presented suggest that nm23-H1 over-expression in the HeLa cells may provide a protective function by participating in antioxidant defense.
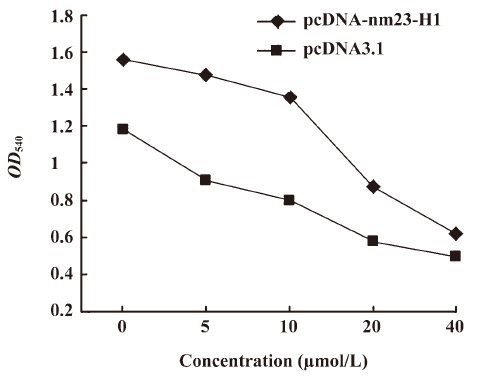
Cells overexpressing nm23-H1 were more resistant to the challenge of H2O2 To observe the possible effectiveness of the antioxidation capacity produced by the expression of exogenous nm23-H1 on cell growth, HeLa cells transfected with pcDNA-nm23-H1 and pcDNA3.1 were challenged with different amounts of H2O2 for 12 h and the cell viabilities were evaluated by MTT assays. It showed that the number of living cells was reduced, along with increases in the amount of H2O2 in all preparations. Compared with the mock cells, more living cells were observed in that of nm23-H1 (Figure 3). To address the difference in the reduction extent of living cells, the relative cell viability of each sample was calculated by dividing the OD value at various concentrations of H2O2 with that of the same preparation without treatment of H2O2. More alive cells were observed in the groups of pcDNA-nm23-H1 at 5, 10, and 20 µmol/L (P<0.05 data not shown), but not at 40 µmol/L. These results indicate that the over-expression of nm23-H1 in the monolayer cells protects the cells against oxidative damage to a certain extent.
Over-expression of nm23-H1 upregulated the expressions of p53 and p53-regulated gene GPX1 A previous study reported that the nm23-H1 tumor suppressor could activate p53 function. To observe the possible influence of nm23-H1 on the expression of p53, recombinant plasmid pcDNA-nm23-H1 was transfected into the cultured HeLa cells. p53-specific Western blotting found that the level of cellular p53 was clearly increased in the cells receiving pcDNA-nm23-H1, but remained unchanged in the cells receiving pcDNA3.1 compared with the mock cells (Figure 4A). As with the expression of p53, the level of GPX1, a p53-regulated gene product, was markedly increased in the cells transfected with pcDNA-nm23-H1 (Figure 4A). This indicates that the expression of nm23-H1 increases the intracellular levels of p53 and p53-regulated GPX1.
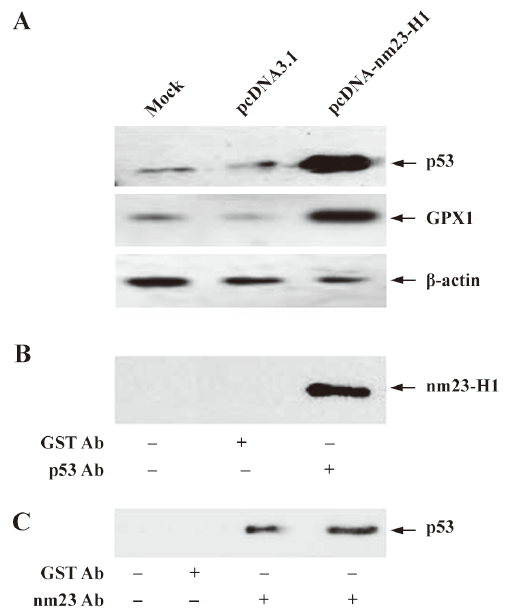
To observe whether nm23-H1 formed a molecular complex with p53, total HeLa extracts were prepared and co-immunoprecipitation experiments were carried out. Using the p53 antibody or gutathione S-transferase (GST) antibody as the capturing antibody and the nm23-H1 anti body as the detecting antibody, obvious nm23-H1 signaling was detected in the preparations precipitated with the p53 antibody, but not in those with the GST antibody (Figure 4B). In contrast, the HeLa extracts were precipitated with nm23-H1 or GST antibodies, and subsequently detected by p53-specific Western blotting. As expected, specific p53 signals were observed in the preparations precipitated with the nm23-H1 antibody, but not in those with the GST antibody (Figure 4C). These results suggest that nm23-H1 may form a physical protein complex with p53.
Downregulation of cellular p53 level elevated intracellular ROS and decreased viability in the cells overexpressing nm23-H1 In order to assess the detailed contribution of p53 to the antioxidation of nm23-H1, the cultured HeLa cells were cotransfected with pcDNA-nm23-H1 and p53 siRNA. In contrast, cells were cotransfected with pcDNA-nm23-H1, and unrelated control siRNA was supplied as the negative control. Forty-eight hours after transfection, the cells were harvested and the expressions of p53 and other agents were evaluated by Western blotting. Figure 5 shows that the p53 protein level was markedly decreased in the cells receiving p53 siRNA and pcDNA-nm23-H1, compared with the mock cells transfected with pcDNA-nm23-H1 alone and the cells transfected with a combination of pcDNA-nm23-H1 and control siRNA. In contrast to p53, the levels of nm23 and p42 MAP kinase, which were used as a control in various preparations, were almost comparable. This indicates that the introduction of p53 siRNA successfully reduces the expression of p53, while the expression of nm23-H1 after the transfection of pcDNA-nm23-H1 is not affected. Moreover, the level of GPX1 in the cells receiving p53 siRNA was notably decreased (Figure 5), highlighting that the expression of GPX1 was highly repressed after p53 knockdown, even in the cells overexpressing nm23-H1.
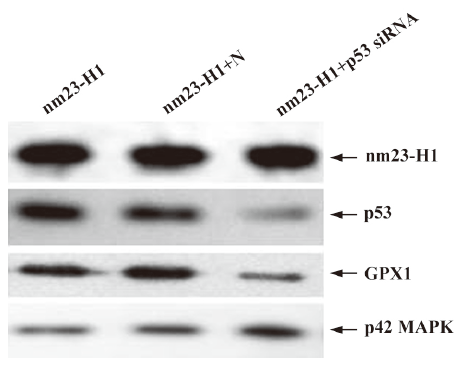
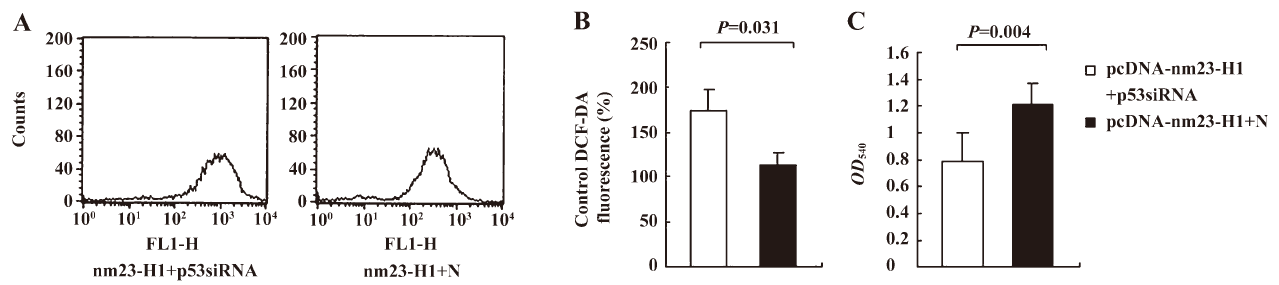
To observe the influences of p53 knockout on the features of antioxidation and growth of the cells, the over-expression of nm23-H1, cellular ROS level, and viability were measured 48 h after transfection. Compared with the cells receiving pcDNA-nm23-H1 and control siRNA, the ROS level in the cells receiving pcDNA-nm23-H1 and p53 siRNA were markedly increased (Figure 6A), 1.52-fold higher than that of the control (Figure 6B), revealing statistical difference (P=0.031). The MTT assays revealed that the viability of the cells transfected with pcDNA-nm23-H1 and p53 siRNA was obviously lower than that of the cells transfected with a combination of pcDNA-nm23-H1 and control siRNA (Figure 6C), with statistical differences (P=0.004). These data suggest that the increased resistance to oxidative stress in the cells after the over-expression of nm23-H1 is due to the p53-related antioxidant pathway.
Discussion
In this report, we provide evidence that the transient expression of human nm23-H1 in HeLa cells endows the cells not only higher viability, but also a lower level of ROS and stronger resistance against oxidative stress in vitro. As a potential tumor repressor, nm23 family products are involved in many cellular biological processes. The expression of nm23 products in malignant tumors is believed to be a marker, representing relatively good prognosis[17]. It has been reported that cells overexpressing either mouse nm23-M1 or –M2 products, NDPKA or NDPKB, could protect the cells from oxidative stress-induced death[13]. Transferring the orthologous NDPK2 gene into Arabidopsis thaliana has been shown to induce plant tolerance against several environmental stresses, such as cold conditions, salt, and H2O2 by decreasing the level of ROS. The excessive production of ROS as a result of oxidative stress may cause cell death, or sometimes induce chronic inflammation, which could be due to severely damaged cell DNA[18]. Both NDPKA and NDPKB may act as DNA glycosylase lyases[19], which are involved in the mechanisms of oxidative DNA damage repair[20]. Therefore, the nm23-reduced intracellular ROS level may be involved in cell protective activity.
We have addressed the molecular interaction between nm23-H1 and p53 in HeLa cells. nm23 products are active proteins that interact with other cellular biological products. It has been shown that nm23-H1 interacts with STRAP, a transforming growth factor (TGF) receptor-interacting protein that inhibits TGF-signaling[21]. nm23-H2 has been shown to interact with the unique A/B domain of estrogen receptor (ER)-β[22]. Jung et al found that nm23-H1 and its interacting partner STRAP were directly involved in p53 activation through a physical interaction with p53, and the over-expression of wild-type nm23-H1 or STRAP results in the upregulation of p53, as well as its targets, including p21 and Bax[12]. Our data also confirm that nm23 can form a protein complex with p53 and up-regulate p53 in cells.
Our data reveal that not only does the level of p53 increase when overexpressing nm23-H1 in HeLa cells, but also that of GPX1. In intracellular glutathione peroxidase, GPX is one of the primary antioxidant enzymes that scavenge hydrogen peroxide and organic hydroperoxides with glutathione as the hydrogen donor. There are 3 other members of the selenium-dependent GPX family, in which cytosolic GPX (GPX1) is the predominant form[23]. Because GPX decomposes hydrogen peroxide and organic hydroperoxides produced during normal metabolism and after oxidative insults, GPX prevents peroxide-induced DNA damage, lipid peroxidation, and protein degradation Even very low levels of p53 present in normal tissues in the absence of stress are sufficient enough to drive the expression of several antioxidant genes[16]. Comparative analyses of the p53 protein levels among human papillo mavirus (HPV)-positive cervical carcinoma cell lines have revealed that the SiHa cell line has the highest steady-state level of p53, exhibits the highest expressions of GPX1, SESN1 and SESN2, while possessing a higher resistance to H2O2 damage. When the expression of p53 is knocked down, the expressions of GPX1, SESN1, and SESN2 are also inhibited, and furthermore, the levels of ROS were raised[24]. In fact, direct evidence of a lower GPX1 level, higher ROS level, and lower viability were obtained in the cells overexpressing nm23-H1 after the downregulation of p53 in this study. Although the exact molecular mechanism of how overexpressed nm23-H1 reduces the intracellular ROS level remains unknown, together with these data, we can speculate that a low level of ROS and higher resistance to oxidative stress by the over-expression of nm23-H1 in HeLa cells is due to the subsequently raised expressions of p53 and GPX1.
Interestingly, the influence of p53 on the metabolism seems to be bidirectional, in that it regulates the expression of antioxidant enzymes resulting in a reduced ROS level, while occasionally inducing ROS accumulation[25]. Sablina et al proposed that in the absence of stress or after mild stress, p53 induced the expression of antioxidant genes that decrease ROS levels and protected cells from DNA damage, while severe or extended stress led to stronger activation of p53, and thus, induced the pro-oxidant genes, resulting in elevated ROS levels and cell death[16]. It lays out a cellular self-protective pathway that helps cells survive under mild stress, for example, a lower amount of H2O2, but promotes cell death when encountering severe stress in order to avoid the accumulation of malformed cells[2,26]. The transient expression of nm23-H1, which increases cell survival, has been observed only with lower concentrations of H2O2 (<40 µmol/L) in this study. Certainly, the effectiveness of the H2O2 treatment on the cultured cells involves many components, besides changes of p53, but the increases of p53 and GPX should be key factors in the protective activity of nm23-H1.
Reduced nm23 expression has been correlated with poor survival or higher frequency for metastasis in human breast, gastric, cervical, ovarian, and hepatocellular carcinoma and melanoma, although it does not represent an independent prognostic factor[17]. The over-expression of transfected nm23 reduced the in vivo tumor metastatic potential of melanoma and breast carcinoma cell lines. The pathogenesis of cancer has not yet been clarified, but substantial evidence suggests that free radicals, particularly oxygen radicals, play an important role in the complex course of multistep carcinogenesis. Much of the evidence has come from the fact that antioxidants that scavenge free radicals directly or that interfere with the generation of free radical-mediated events inhibit the neoplastic process[27]. Thereby decreasing ROS and higher resistance against oxidative stress due to the over-expression of nm23 might possibly play some role during tumor formation and metastasis.
References
- Klauning JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 2004;44:239-67.
- Bensaad K, Vousden KH. Savior and slayer: the two faces of p53. Nat Med 2005;11:1278-9.
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 2006;12:446-51.
- Storz P. Reactive oxygen species in tumor progression. Front Biosci 2005;10:1881-96.
- Okada F, Kobayashi M, Tanaka H, Kobayashi T, Tazawa H, Luchi Y, et al. The role of nicotinamide adenine dinucleotide phosphate oxidase-derived reactive oxygen species in the acquisition of metastatic ability of tumor cells. Am J Pathol 2006;169:294-302.
- Mori K, Shibanuma M, Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res 2004;64:7464-72.
- Hyoudou K, Nishikawa M, Kobayashi Y, Kuramoto Y, Yamashita F, Hashida M. Inhibition of adhesion and proliferation of peritoneally disseminated tumor cells by pegylated catalase. Clin Exp Metastasis 2006;23:269-78.
- Heirman I, Ginneberge D, Brigelius-Flohé R, Hendrickx N, Agostinis P, Brouckaert P, et al. Blocking tumor cell eicosanoid synthesis by GPX4 impedes tumor growth and malignancy. Free Radic Biol Med 2006;40:285-94.
- Okada F, Shionoya H, Kobayashi M, Kobayashi T, Tazawa H, Onuma K, et al. Prevention of inflammation-mediated acquisition of metastatic properties of benign mouse fibrosarcoma cells by administration of an orally available superoxide dismutase. Br J Cancer 2006;94:854-62.
- Lacombe ML, Milon L, Munier A, Mehus JG, Lambeth OD. The human Nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr 2000;32:247-58.
- Kimura N, Shimada N, Fukuda M. Regulation of cellular functions by nucleoside diphosphate kinases in mammals. J Bioenerg Biomembr 2000;32:309-15.
- Jung H, Seong HA, Ha H. NM23-H1 tumor suppressor and its interacting partner STRAP activate p53 function. J Biol Chem 2007 30; 282: 35 293−307.
- Arnaud-Dabernat S, Masse K, Smani M, Peuchant E, Landry M, Bourbon PM, et al. Nm23-M2/NDP kinase B induces endogenous c-myc and nm23-M1/NDP kinase A over-expression in BAF3 cells. Both NDP kinases protect the cells from oxidative stress-induced death. Exp Cell Res 2004;301:293-304.
- Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res 2004;64:2350-6.
- Yoon KA, Nakamura Y, Arakawa H. Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J Hum Genet 2004;49:134-40.
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med 2005;11:1306-13.
- de la Rosa A, Williams RL, Steeg PS. Nm23/nucleoside diphosphate kinase: toward a structural and biochemical understanding of its biological functions. Bioessays 1995;17:53-62.
- Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep 2002;3:420-5.
- Postel EH. Multiple biochemical activities of NM23/NDP kinase in gene regulation. J Bioenerg Biomembr 2003;35:31-40.
- Fan Z, Beresford PJ, Oh DY, Zhang D, Lieberman J. Tumor suppressor NM23-H1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor. Cell 2003;112:659-72.
- Seong HA, Jung H, Ha H. NM23-H1 tumor suppressor physically interacts with serine-threonine kinase receptor-associated protein, a transforming growth factor-beta (TGF-beta) receptor-interacting protein, and negatively regulates TGF-beta signaling. J Biol Chem 2007; 282: 12 075−96.
- Rayner K, Chen YX, Hibbert B, White D, Miller H, Postel EH, et al. Discovery of NM23-H2 as an estrogen receptor beta-associated protein: role in estrogen-induced gene transcription and cell migration. J Steroid Biochem Mol Biol 2008;108:72-81.
- Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem 1993;268:2571-6.
- Ding B, Chi SG, Kim SH, Kang S, Cho JH, Kim DS, et al. Role of p53 in antioxidant defense of HPV-positive cervical carcinoma cells following H2O2 exposure. J Cell Sci 2007;120:2284-94.
- Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaronson SA. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol 2003;23:8576-85.
- Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol 2005;6:44-55.
- Nicco C, Laurent A, Chereau C, Weill B, Batteux F. Differential modulation of normal and tumor cell proliferation by reactive oxygen species. Biomed Pharmacother 2005;59:169-74.
