Endothelium-derived hyperpolarizing factor mediated relaxations in pig coronary arteries do not involve Gi/o proteins1,2
Introduction
Endothelium-dependent relaxations can be mediated through the release of nitric oxide (NO), prostacyclin, or endothelium-derived hyperpolarizing factor (EDHF)[1]. Such relaxations can be initiated by the binding of different agonists to endothelial cell membrane receptors. The relaxations produced by certain (eg serotonin, thrombin, and α2-adrenergic agonists) but not all (eg bradykinin) neurohumoral substances are mediated by pertussis toxin-sensitive Gi/o proteins[2,3]. However, whether or not the EDHF-mediated component of these responses also depends on pertussis toxin-sensitive Gi/o proteins is not known.
EDHF-mediated responses include a number of different mechanisms that may exist in combination or in isolation[1,4]. Upon activation of different endothelial receptors, there is an increase in the intracellular Ca2+ concentration and the opening of endothelial iKCa and sKCa channels. The combination of inhibitors of the iKCa and sKCa channels apamin and charybdotoxin abolishes these responses[5].
The hyperpolarization of endothelial cells is a result of the opening of these potassium channels. It then spreads to the underlying vascular smooth muscle cells by means of gap junctions, changes in interstitial K+ concentrations, release of epoxyeicosatrienoic acids (EET), lipoxygenase derivatives, hydrogen peroxide, endocannabinoids, or c-type natriuretic peptide (CNP). Theoretically, Gi/o proteins may be involved in the initial signal transduction upon endothelial receptor activation, or alternately, in the spread of hyperpolarization from the endothelium to the smooth muscle, particularly if this involves secretion of diffusible mediators.
The present experiments were designed to determine whether or not relaxations of pig coronary arteries that can be attributed to endothelium-dependent hyperpolarization involve Gi/o proteins and can be prevented or inhibited by pertussis toxin.
Materials and methods
Tissue preparation Pig hearts were collected from the local abattoir, where the animals were killed according to the regulations of the Food and Environmental Hygiene Department of the Hong Kong Special Administrative Region. The hearts were immediately rinsed several times and transported back to the laboratory in ice cold, oxygenated Krebs–Ringer bicarbonate solution (composition in mmol/L: NaCl 118.3, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.5, NaHCO3 25.0, and glucose 11.1; control solution). The right coronary arteries were dissected. The surrounding fat and connective tissue were removed, and the artery was cut into rings (with the endothelium; 4–5 mm in length). The rings were placed in jacketed organ chambers filled with 5 mL control solution. The solution was maintained at 37 oC and continuously aerated with 95% O2/5% CO2.
The rings were suspended vertically between 2 stainless-steel stirrups. One stirrup was anchored in the organ chamber, and the other was connected to a strain gauge for isometric tension recording. The rings were subjected to 5 g tension, which in preliminary experiments, was shown to be the optimal tension for rings of pig right coronaries obtained from the same source. Isometric tension was measured by means of force transducers (FT03; Grass Instruments, Quincy, MA, USA) coupled to an amplifier and a personal computer for data collection (PICO data logger; Pico Technology, Cambridge, UK).
The viability of each ring was determined by first contracting it 2–3 times with 30 mmol/L KCl followed by a single maximal contraction to 60 mmol/L KCl during which relaxation to bradykinin (1 µmol/L) was obtained. Rings that failed to produce a contraction greater than 4 g with 30 mmol/L KCl or relaxation greater than 40% with bradykinin were discarded. The rings were then washed repeatedly with the control solution.
Effect of pertussis toxin on EDHF-mediated relaxation After the re-establishment of a baseline tension of 5 g, the rings were incubated in control solution for 2 h in the presence or absence of pertussis toxin (400 ng/mL)[6]. The rings were then contracted with prostaglandin F2α (1−3 µmol/L) to a tension level approximating 30% of the maximal contraction produced by 60 mmol/L KCl. Once the contraction had stabilized, various agonists were added to produce relaxation. Indomethacin (10 µmol/L) was added to all rings 30 min prior to the initiation of contraction with prostaglandin F2α. When other inhibitors, including Nω-nitro-L-arginine methyl ester (L-NAME; 100 µmol/L), charybdotoxin (0.1 µmol/L), apamin (0.1 µmol/L), or ketanserin (a selective 5-HT2 antagonist that inhibits the direct activating effect of serotonin on vascular smooth muscle; 10 µmol/L) were required, they were added at the same time as indomethacin. Pertussis toxin was allowed to remain in contact with the rings throughout the experiment.
Statistical analysis Data are presented as mean±SEM, with n representing the number of pig hearts used in the experiment. Curve fitting was performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA) by fitting the data to the sigmoidal logistic equation:
(1)
where R is the reduction in tone, A is the concentration of the agonist, Rmax is the maximal relaxation, and H the slope function.
ANOVA was used for the comparison of curves among multiple treatment groups. A post-hoc comparison was performed by Newman-Keuls multiple comparison test where appropriate. Student’s t-test for paired observations was used in the case of 2 group comparisons. P-values less than 0.05 were indicated statistically significant differences.
Drugs Indomethacin, ketanserin, L-NAME, charybdotoxin, apamin, prostaglandin F2α, bradykinin, substance P, serotonin, UK14304, thrombin, and SFLLRN were purchased from Sigma (St Louis, MO, USA), and pertussis toxin was purchased from List Biological (Campbell, CA, USA). A stock solution of indomethacin was prepared in a 5 mmol/L sodium bicarbonate solution. A stock solution of pertussis toxin was prepared in 0.1 mol/L sodium phosphate (pH 7.0) and 0.5 mol/L NaCl. All other compounds were dissolved in deionized water. Concentrations are expressed as final molar concentrations in the bathing solution.
Results
Effect of pertussis toxin on EDHF-mediated relaxations by receptors agonists that may involve non-Gi/o coupling Bradykinin caused concentration-dependent relaxations which were reduced significantly by L-NAME (100 µmol/L). The presence of pertussis toxin (400 ng/mL) and L-NAME did not further inhibit relaxation. Charybdotoxin (0.1 µmol/L) and apamin (0.1 µmol/L) added to L-NAME nearly abolished the relaxations evoked by bradykinin (Figure 1A; Table 1). Similar results were obtained with substance P (Figure 1B; Table 1).
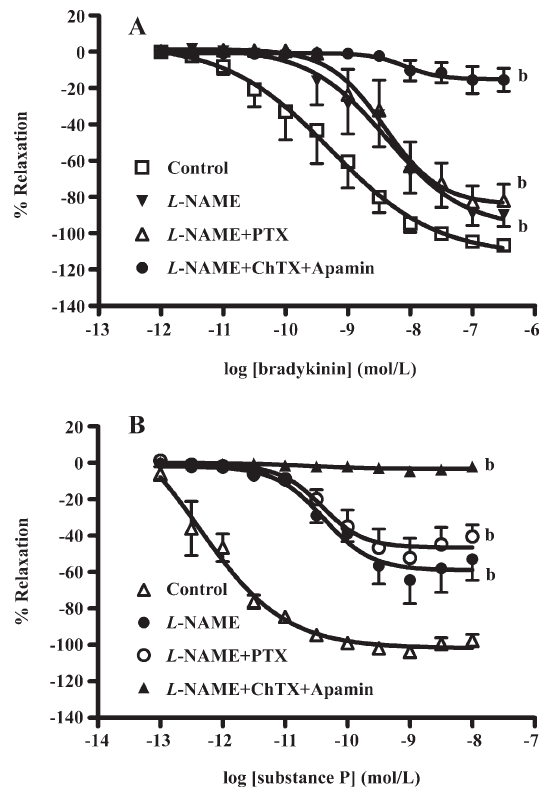

Full table
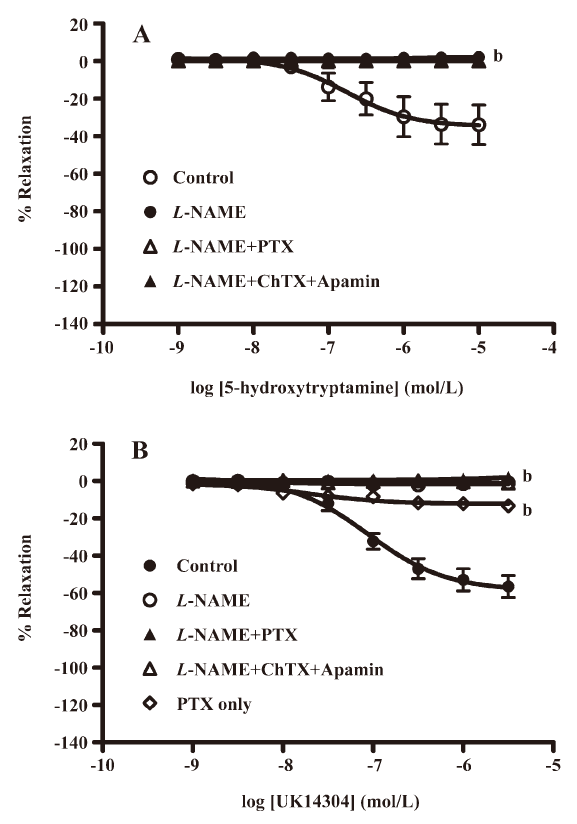
Effect of pertussis toxin on EDHF-mediated relaxation by receptor agonists that may involve Gi/o coupling Relaxations to thrombin (Figure 2A; Table 1) were attenuated by L-NAME (100 µmol/L) and abolished by charybdotoxin (0.1 µmol/L) and apamin (0.1 µmol/L) on top of L-NAME. Incubation with pertussis toxin (400 ng/mL) did not significantly affect the L-NAME-resistant component of relaxation. A similar pattern of inhibition was observed for SFLLRN (Figure 2B; Table 1) with L-NAME, charybdotoxin, and apamin. Pertussis toxin did not significantly affect L-NAME-resistant relaxation to SFLLRN. No relaxation was produced by serotonin or UK14304 in the presence of L-NAME (100 µmol/L; Figure 3A, 3B). NO-dependent relaxation to UK14304 was significantly inhibited by pertussis toxin (Figure 3B).

Effect of pertussis toxin on EDHF-mediated relaxation independent of endothelial surface receptors The relaxation to calcimycin (Figure 4; Table 1) was attenuated by L-NAME (100 µmol/L) and was nearly abolished with charybdotoxin (0.1 µmol/L) and apamin (0.1 µmol/L) on top of L-NAME. Incubation with pertussis toxin (400 ng/mL) again did not significantly affect the L-NAME-resistant component of the relaxation.

Discussion
This study investigates the dependence, if any, of EDHF-mediated relaxations of pig coronary arteries on pertussis toxin-sensitive Gi/o proteins based on previous findings that certain NO-mediated responses are Gi/o protein dependent[2,3]. EDHF-mediated responses were obtained after the inhibition of the NO pathway with L-NAME and the prostacyclin pathway with indomethacin in arteries with endothelia. These responses were abolished in the presence of charybdotoxin and apamin, confirming that these are EDHF-mediated responses[1].
Hyperpolarization of vascular smooth muscles in EDHF-mediated responses may follow that of the endothelial cells via gap junctions, changes in interstitial K+ concentrations, release of EET, lipoxygenase derivatives, CNP, endocannabinoids, or hydrogen peroxide[1,4,7–9]. As the final effector mechanisms are so varied, there are likely to be multiple pathways linking the initial activation of endothelial receptors to these numerous mechanisms. As Gi/o proteins may be involved in both transduction and effector mechanisms, it is intuitively quite possible that they are involved in at least part of the entire EDHF-mediated response. Pertussis toxin inhibits EDHF-mediated responses to carbachol, the endocannabinoid anandamide, and ∆9-tetrahydrocannabinol, as well as calcimycin in small mesenteric arteries of rats[8,10]. It also inhibits relaxation by CNP, a putative EDHF, again in the rat mesenteric artery[7]. However, this was found not to be the case in pig coronary arteries in the present study. This may be explained by the different agonists used to induce EDHF responses in the present experiment or by species differences. Thus, pertussis toxin does not cause inhibition of the EDHF-mediated response in the rabbit carotid artery[11].
Previous studies on regenerated endothelia after denudation by balloon angioplasty also support the notion that EDHF-mediated responses are independent of Gi/o proteins in pig coronary arteries. The dysfunction of the Gi/o protein pathway[12,13] is of major importance in the impairment of endothelium-dependent relaxations in coronaries with regenerated endothelium[3,14], however, these arteries frequently exhibit a compensatory increase in the EDHF-mediated response[15]. Similar observations have also been made in hyperlipidemia and atherosclerosis, as Gi/o protein pathway dysfunction has been observed under these conditions[16–18]. Again, a compensatory increase in EDHF-mediated relaxation is observed[19], despite Gi/o protein pathway dysfunction. Lastly, Gi/o protein expression is low in small coronary arteries[20] and preparations known to exhibit prominent EDHF activity[21–23].
The findings of the present study are in line with these observations. In pig coronary arteries, all of the EDHF-mediated responses studied appear to be independent of Gi/o protein pathways as they were unaffected by pertussis toxin. This was the case for agonists, such as bradykinin and substance P that elicit a prominent EDHF-component during endothelium-dependent relaxations in this preparation. Bradykinin induces relaxation by activating B2 kinin receptors, and substance P induces relaxation via the NK1 receptor[24]. Both receptors are coupled to the Gq protein and involve the phospholipase C, inositol 1,4,5-triphosphate, and diacylglycerol system as effector systems[25]. As pertussis toxin selectively ADP-ribosylates the α-subunits of Gi/o proteins, it is plausible that the effects of bradykinin and substance P are not affected by pertussis toxin. The absence of the effect of pertussis toxin on bradykinin-induced relaxation has been reported previously[2].
Thrombin induces endothelium-dependent relaxations by activating protease-activated receptor (PAR)1, which is coupled to Gi/o proteins[2,26]. In the present experiment, however, EDHF-mediated responses to PAR activation remained intact after incubation with pertussis toxin. This was the case with both the natural agonist of PAR, thrombin, as well as with the PAR1-selective tethered ligand thrombin receptor activating peptide, SFLLRN. Both produce endothelium-dependent relaxations, but differences exist between the 2 PAR agonists. Thus, in pig coronary arteries, the non-NO component of endothelium-dependent relaxations to thrombin, but not that to SFLLRN, are inhibited by L-type voltage-operated calcium channel blockers, such as nifedipine[27]. In the present study, a similar EDHF-mediated relaxation was obtained with both thrombin and SFLLRN. This relaxation was not affected by pertussis toxin in both cases. This then prompts the conclusion that the Gi/o protein pathway is not involved, at least in the EDHF-mediated relaxation following PAR1 activation. This finding is of particular importance because of the role of thrombin as a key enzyme in blood coagulation, platelet activation, and inflammation[28]. Thrombin is likely to be present in the vicinity of the endothelium during critical events, such as thrombus formation and plaque rupture. The present study confirms that thrombin maintains its ability to induce EDHF-mediated relaxations independent of Gi/o proteins. This may allow thrombin to produce a more favorable vascular tone during such critical events.
This study also investigates 2 agonists with known dependence on the Gi/o protein pathway to produce endothelium-dependent relaxations, namely serotonin[14] and the α2-adrenergic agonist UK14304. However, in the present study both serotonin and UK14304 failed to produce EDHF-mediated relaxations. Therefore, the contribution of the Gi/o protein pathway to EDHF responses induced by these 2 agonists cannot be assessed in pig coronary arteries.
In addition to being coupled to endothelial surface receptors, Gi/o proteins, and thus pertussis toxin-sensitive pathways, may also be involved in the mechanisms leading to hyperpolarization of vascular smooth muscle cells. For instance, the relaxations induced by CNP, which is a possible candidate for EDHF, may involve the action of CNP on a Gi/o-coupled smooth muscle K+ channel[29]. This and other similar mechanisms are investigated in this study using calcimycin to directly increase endothelial intracellular Ca2+ concentrations, bypassing the effects of G-protein coupling to endothelial surface receptors. Again, no pertussis toxin-dependent effect was observed.
The major conclusion derived from the present experiments is that EDHF-mediated relaxations of pig coronary arteries are not dependent on pertussis toxin-sensitive Gi/o protein pathways. This independence of EDHF-mediated relaxations on Gi/o proteins is universal across a variety of agonists and probably a full range of different mechanisms of EDHF generation. This shows that in large coronary arteries, which are the major sites affected by atherosclerosis and plaque formation, and also the principal site of coronary interventions, NO- and EDHF-mediated endothelium-dependent relaxations are distinct mechanisms. These mechanisms not only differ in terms of the mediator(s) involved, but also have distinct signal transduction systems. These 2 pathways can therefore serve complementary roles, particularly under diseased conditions, such as the dysfunction caused by regeneration or atherosclerosis.
Acknowledgements
We would like to thank Prof David D KU of the Department of Pharmacology at the University of Alabama (Birmingham, USA) and all the staff at the Vascular Biology Laboratory of the University of Hong Kong, in particular Mr GSK MAN, Ms YH CHUNG, and Mr D YEUNG for their advice and assistance.
References
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 2006;26:1215-25.
- Flavahan NA, Shimokawa H, Vanhoutte PM. Pertussis toxin inhibits endothelium-dependent relaxations to certain agonists in porcine coronary arteries. J Physiol 1989;408:549-60.
- Shimokawa H, Flavahan NA, Vanhoutte PM. Natural course of the impairment of endothelium-dependent relaxations after balloon endothelium removal in porcine coronary arteries. Possible dysfunction of a pertussis toxin-sensitive G protein. Circ Res 1989;65:740-53.
- Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Ann Med 2007;39:495-516.
- Zygmunt PM, Edwards G, Weston AH, Larsson B, Hogestatt ED. Involvement of voltage-dependent potassium channels in the EDHF-mediated relaxation of rat hepatic artery. Br J Pharmacol 1997;121:141-9.
- Hoi PM, Hiley CR. Vasorelaxant effects of oleamide in rat small mesenteric artery indicate action at a novel cannabinoid receptor. Br J Pharmacol 2006;147:560-8.
- Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA 2003;100:1426-31.
- White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol 1997;122:1573-84.
- Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci 2007;28:61-7.
- O’Sullivan SE, Kendall DA, Randall MD. The effects of delta9-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br J Pharmacol 2005;145:514-26.
- Ayajiki K, Ozaki M, Shiomi M, Okamura T, Toda N. Comparison of endothelium-dependent relaxation in carotid arteries from Japanese white and Watanabe heritable hyperlipidemic rabbits. J Cardiovasc Pharmacol 2000;36:622-30.
- Borg-Capra C, Fournet-Bourguignon MP, Janiak P, Villeneuve N, Bidouard JP, Vilaine JP, et al. Morphological heterogeneity with normal expression but altered function of G proteins in porcine cultured regenerated coronary endothelial cells. Br J Pharmacol 1997;122:999-1008.
- Shibano T, Codina J, Birnbaumer L, Vanhoutte PM. Pertussis toxin-sensitive G proteins in regenerated endothelial cells of porcine coronary artery. Am J Physiol 1994;267:H979-H981.
- Flavahan NA, Vanhoutte PM. G-proteins and endothelial responses. Blood Vessels 1990;27:218-29.
- Thollon C, Fournet-Bourguignon MP, Saboureau D, Lesage L, Reure H, Vanhoutte PM, et al. Consequences of reduced production of NO on vascular reactivity of porcine coronary arteries after angioplasty: importance of EDHF. Br J Pharmacol 2002;136:1153-61.
- Flavahan NA. Lysophosphatidylcholine modifies G protein-dependent signaling in porcine endothelial cells. Am J Physiol 1993;264:H722-7.
- Liao JK, Clark SL. Regulation of G-protein alpha i2 subunit expression by oxidized low-density lipoprotein. J Clin Invest 1995;95:1457-63.
- Tsutsui M, Shimokawa H, Tanaka S, Kuwaoka I, Hase K, Nogami N, et al. Endothelial Gi protein in human coronary arteries. Eur Heart J 1994;15:1261-6.
- Brandes RP, Behra A, Lebherz C, Boger RH, Bode-Boger SM, Phivthong-Ngam L, et al. NG-nitro-L-arginine- and indomethacin-resistant endothelium-dependent relaxation in the rabbit renal artery: effect of hypercholesterolemia. Atherosclerosis 1997;135:49-55.
- Shimokawa H, Tsutsui M, Mizuki T, Hase K, Kuwaoka I, Nogami N, et al. Endothelial Gi protein expression is markedly low in human coronary microvessels. J Cardiovasc Pharmacol 1996;27:297-302.
- Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang PL, Vanhoutte PM, et al. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci USA 2000;97:9747-52.
- Urakami-Harasawa L, Shimokawa H, Nakashima M, Egashira K, Takeshita A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J Clin Invest 1997;100:2793-9.
- Woolfson RG, Poston L. Effect of NG-monomethyl-L-arginine on endothelium-dependent relaxation of human subcutaneous resistance arteries. Clin Sci (Lond) 1990;79:273-8.
- Cathieni MM, Taban CH, Sors P, Mastrangelo D. Binding of gold–protein ligand complexes to neurokinin receptors. Visualization with the electron microscope on pig coronary artery and rat portal vein. J Vasc Res 1999;36:59-67.
- Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol 2006;147 Suppl 1:S46-55.
- Barnes JA, Singh S, Gomes AV. Protease activated receptors in cardiovascular function and disease. Mol Cell Biochem 2004;263:227-39.
- Hamilton JR, Cocks TM. Heterogeneous mechanisms of endothelium-dependent relaxation for thrombin and peptide activators of protease-activated receptor-1 in porcine isolated coronary artery. Br J Pharmacol 2000;130:181-8.
- Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med 2005;15:254-9.
- Leuranguer V, Vanhoutte PM, Verbeuren T, Feletou M. C-type natriuretic peptide and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br J Pharmacol 2008;153:57-65.
