Intra-herb pharmacokinetics interaction between quercetin and isorhamentin1
Introduction
Many herbs containing flavonoids have been widely used in traditional medicines. Their extracts are increasingly introduced into modern medicines as complementary and alternative medicines, such as Gingko flavones (GF), total flavones of Hippophae rhamnoides L (TFH), Soy isoflavones, Pycnogenol (procyanidins extracted from Pinus maritima), grape seeds extracts and Saint John’s Wort extracts, etc. With the gradually widening use of these herb extracts as food supplements, more and more clinical affairs derived from herb-drug interactions have emerged and are being investigated[1–3]. Most interactions are associated with pharmacokinetics[4].
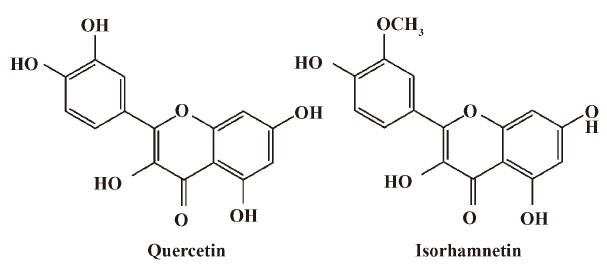
Quercetin and related bioflavonoids, such as isorhamnetin, were common constituents of some of these extracts, such as GF[5] and TFH[6]. Isorhamnetin, which is also called 3′-O-methylquercetin, was a metabolite of quercetin not only in plants, but also in some mammalian species, such as rats[7], pigs[8] and human[9] after oral administration. The O-methylation of quercetin in mammals is catalyzed by catechol-O-methyltranferase (COMT)[10]. Both quercetin and isorhamnetin exhibited considerable anti-oxidant activities, such as modification of eicosanoid biosynthesis, protection of low-density lipoprotein from oxidation, prevention of platelet aggregation and promotion of relaxation of cardiovascular smooth muscle[11]. On the other hand, quercetin and related bioflavonoids had been proven to modulate the function and expression of P-glycoprotein[12,13] and CYP3A4[14,15]. Consequently, quercetin and related bioflavonoids affected the pharmacokinetics and pharmacodynamics of some chemical drugs.
Interestingly, similar to the drug-herb interaction, co-occurrence of quercetin and related bioflavonoids in one herb extract would possibly cause potential intra-herb interaction. There was some evidence that not only quercetin but also isorhamnetin interacted with P-glycoprotein[16]. Therefore, we doubted that if there was any pharmacokinetics interaction between quercetin and isorhamnetin, and investigated the interaction by cell transport assays and in vivo pharmacokinetics assay.
Materials and methods
Quercetin (QU, 99%), isorhamnetin (IS, 99%) (Figure 1) and baicalein (internal standard, 99.9%) were purchased from Tauto Biotech (Shanghai, China). β-Glucuronidase (type H-3, from Helix pomatia) and sulfatase (type H-1, from Helix pormatia) were obtained from Sigma-Aldrich (St Louis, MO, USA). The human colon carcinoma cell line (Caco-2) was obtained from American Type Culture Collection (Rockville, MD, USA). MDCK cell clones strain II[17] (MDCKII) and its human MDR1 cDNA transfected strain[18] (MDR1-MDCKII) were gifts from Professor Borst P of the Netherlands Cancer Institute (NKI). Dulbecco’s Modified Eagle’s medium (DMEM, high glucose, Cat: 61975), Hanks’ Balance Salt Solution (HBSS, Cat: 14175), penicillin-streptomycin (Cat: 15070) and Trypsin-EDTA (Cat: 25300) were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Fetal calf serum (FCS, Cat: SH30084) was purchased from Hyclone (Logan, UT, USA). Dimethyl sulfoxide (DMSO) was purchased from Millipore Inc (USA). Methanol, glacial acetic acid and formic acid (HPLC grade) were purchased from TEDIA Inc (Fairfield, IA, USA).
Cell transport assay Caco-2 cells and MDCKII/MDR1-MDCKII cells were seeded on Transwell polycarbonate membrane filters (0.4 µm pore diameter, 12 mm diameter, Coring Costar Inc. Cambridge, MA, USA) at a density of 5×105 cells/well and 2×106 cells/well, respectively. Cells were maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin G and 100 µg/L streptomycin sulfates. For Caco-2 cells, culture medium was replaced 3 times a week for 14 d and daily thereafter until 3 weeks. For MDCKII/MDR1-MDCKII cells, culture medium was replaced every day for 3–4 d. The integrity and confluence of the cells monolayers was determined by measuring the transepithelial electrical resistance (TEER) by and Millicell-ERS voltohmmeter. Inserts were used for transport studies with TEER values >350 Ω·cm2 for Caco-2 cells, TEER values >400 Ω·cm2 for MDCKII cells, and TEER values >4000 Ω·cm2 for MDR1-MDCKIIcells[19].
The inserts were washed twice with warm transport buffer (HBSS containing 1% DMSO [v/v] and 1 mmol/L ascorbic acid [to prevent oxidation of quercetin]). The trans-epithelial permeability of QU and IS was measured at a concentration of 50 µmol/L. Transport buffer containing QU or/and IS was added to donor compartment (either the apical [2.0 mL] or basolateral [2.5 mL] side of the inserts), while the receiver compartment contained the corresponding volume of fresh transport buffer. During the incubation at 37 °C for 80 min, samples (100 µL) were collected from both sides at 20, 40, and 80 min and stored at –70 °C until analysis by HPLC. After sampling, 100 µL of fresh transport buffer were added to both transport compartments.
In control experiments, paracellular flux of [3H]inulin (0.15 µCi/mL) through the monolayer was always less than 1.5%, indicating intactness of the monolayer.
Pharmacokinetics study This experiment was approved by the Ethics Committee of the West China Center of Medicine, Sichuan University. A latin square study design was introduced into the pharmacokinetic studies. 12 male Wistar rats (200±20 g, Experimental Animals Center, Sichuan University, China) were randomly divided into 6 groups (Group A–F, 2 rats/group). The rats were housed and fed as described in our former published paper[20]. The dose orders of each group of rats were according to Table 1. The wash-out period was 10 d.
Full table
In each experimental period, blood samples (0.3–0.4 mL of venous blood) were collected before administration and at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 24, 48, and 72 h postadministration from the tail vein of rats. Blood samples in tubes containing lithium-heparin were centrifuged (1500×g for 10 min) within 30 min following collection (EBA21 table centrifuge, Hettich, Germany). Then, due to instability of flavonols at high pH[7], 100 µL of harvested plasma was acidified with 10 µL of 0.5 mol/L acetic acid, which contained 2 mg of vitamin C per mL.
Samples analysis An adjusted HPLC method based on the report of Wang et al[16] was used to determine the concentrations of samples of transport studies. After adding 100 µL of methanol, the mixture was vortexed for 1 min at 2000 r/min (Vortex Genius 3, IKA, Germany) and centrifuged for 3 min at 5000×g (EBA21 table centrifuge, Hettich, Germany). A mobile phase of methanol-acetonitril-0.5% acetic acid in water (5:23:72, v/v/v) was delivered at a rate of 1.0 mL/min using a Shimadzu LC-2010C HPLC system. The Shimadzu VP-ODS column (150×4.6 mm, 5 µm) was maintained at 40 °C, and the wavelength of the UV detector was set at 370 nm.
The ESI-LC-MS/MS method to determine plasma samples was published previously[20]. After being treated with β-glucuronidase and sulfatase, the analytes were extracted with the internal standard (baicalein). The chromatographic separation was carried out on a Diamonsil C18 column with a mobile phase of 2% formic acid-methanol (10:90, v/v) at a flow rate of 1.00 mL/min, with a split of 200 µL to mass spectrometer. The mass spectrometer was operated in the positive ion mode with the TurboIonspray heater set at 450 °C (API3000 LC/MS/MS system, Applied Biosystems, Foster City, CA, USA). The samples were analyzed using the transition of m/z 303→153 amu for QU, m/z 317→153 amu for IS and m/z 271→123 amu for baicalein.
Data analysis Apparent permeability coefficients (Papp) across Caco-2 monolayer were calculated using the following equation:
In this equation, Q is the amount of analytes in the receiving compartment, in which the sampling amount was added; A is the membrane surface area (4.71 cm2); C0 is the initial concentration in the donor compartment; dQ/dt is the change of analytes in the receiver solution over time. The permeability ratio (Pratio) was calculated according to the following equation:
The pharmacokinetics parameters were calculated using DAS 2.0 pharmacokinetics software (Drug and Statistics, Version 2.0) with non-compartment models. All of the data statistics were carried out with SPSS (version 10.0). AUC0–72 h, AUC0–∞ and Cmax were converted to logarithms before entering the statistics.
Results
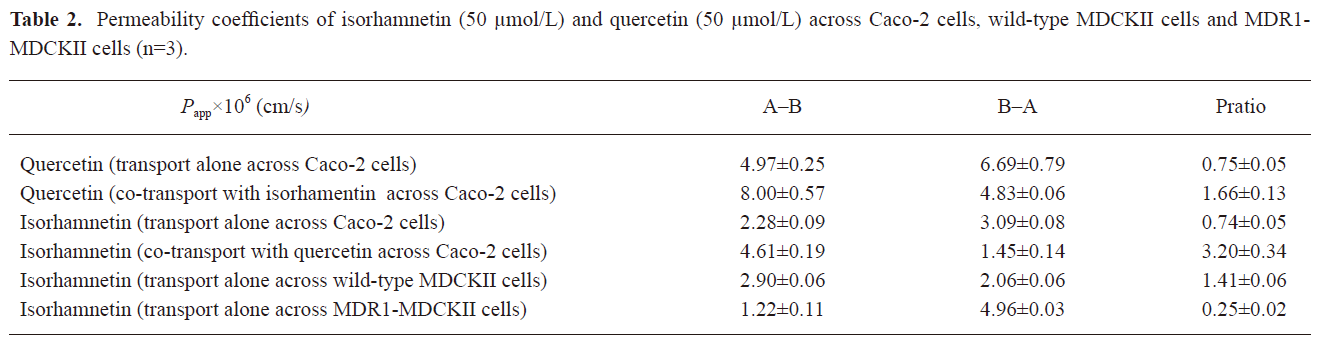
Transport of IS and QU No biotransformation between QU and IS was found in Caco-2 cells and MDCKII cells transport systems. Table 2 shows the permeability coefficients (Papp) of QU (50 µmol/L)/IS (50 µmol/L) transport alone or together in the Caco-2 cells, wild-type MDCKII cells and MDR1-MDCKII cells. The MDR1-MDCKII cells model was used to validate if IS was the substrate of P-gp. The Pratio of IS in the MDR1-MDCKII cells model was significantly less than the one in the wild-type MDCKII cells model (P<0.001, Student’s t-test). This result proved the role of P-gp in the cell efflux of IS. In Caco-2 cells transport assay, both Papp (A–B) and Papp (B–A) of QU were significantly larger than the ones in IS. The secretion (basolateral to apical) rates of transport were higher than absorptive (apical to basolateral) rates of transport (P<0.05 for IS, P<0.05 for QU, Student’s t-test). The Pratio of IS and QU was 0.74 and 0.75, respectively, indicating that an efflux mechanism was involved in the transport of either IS or QU.
Full table
Although co-transport occurred across Caco-2 cells, the Papp (A–B) of both IS and QU were increased and the Papp (B–A) were decreased (P<0.001 for IS, P<0.05 for QU, Student’s t-test). Subsequently, the Pratio of co-transport studies increased from 0.74 and 0.75 to 3.20 and 1.66 for IS and QU, respectively. These results indicated that IS and QU could facilitate the absorptive permeation and inhibit the secretion permeation of each other across the Caco-2 cells monolayer. Drug efflux pumps mediated drug-drug interaction might be one of the main reasons[12,15,21].
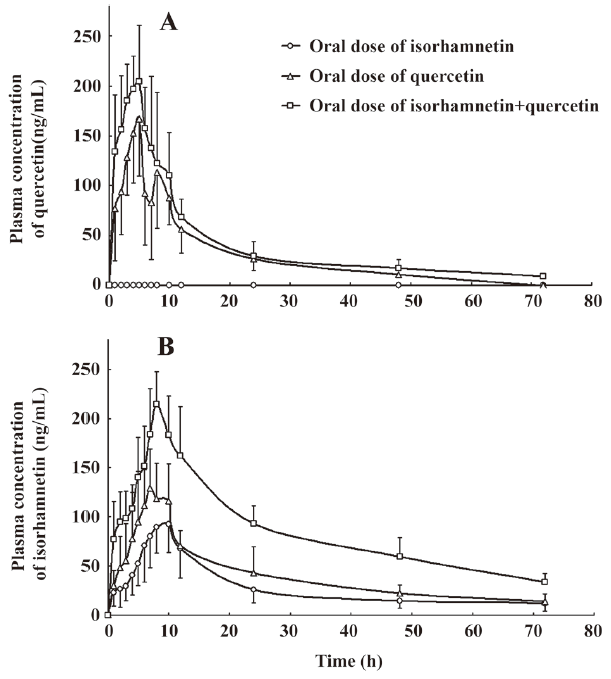
Pharmacokinetic interaction of isorhamnetin and quercetin The plasma concentration of QU and IS versus time curve is illustrated in Figure 2. Corresponding to the previous pharmacokinetics studies in human[22,23], QU and IS showed two or more peaks of maximum plasma concentration in individual rats. The multiple peaks phenomenon was weakened with coadministration. QU was not detected after oral administration of IS alone, whereas IS was detected soon after the oral dose of QU alone. The IS in plasma after the oral dose of IS or QU came from the oral absorption of IS and the biotransformation of QU, respectively. IS in plasma after coadministration of IS and QU was the sum IS absorbed and IS biotransformed from QU. These results confirmed that QU is metabolized to IS, but not vice versa[20]. The main pharmacokinetics parameters of QU and IS were listed in Table 3 and Table 4.
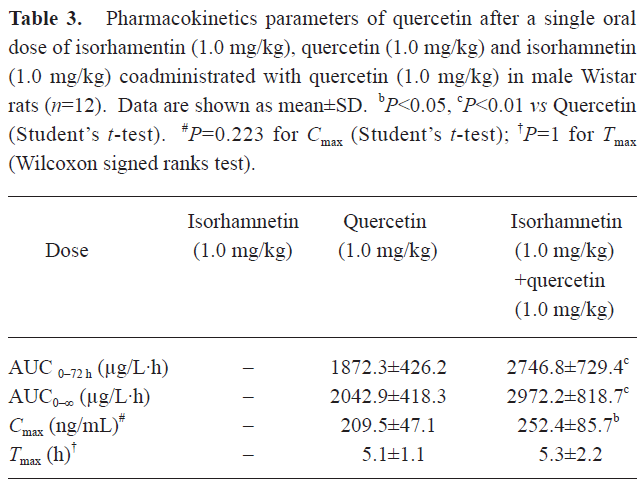
Full table
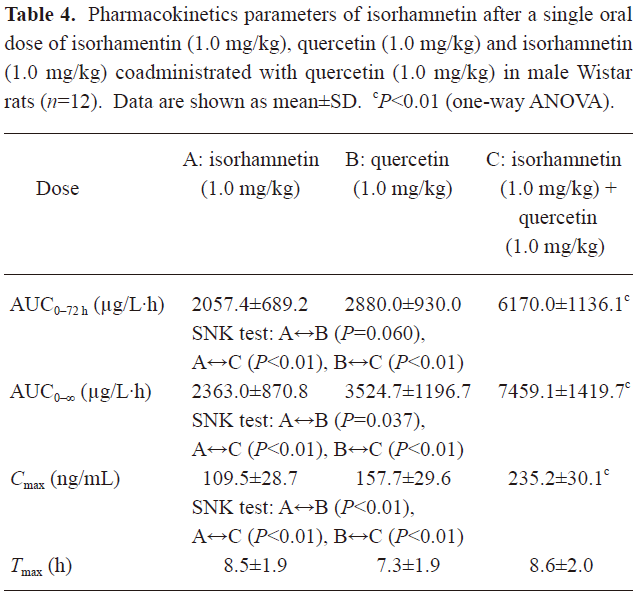
Full table
In Table 3, it can be seen that the AUC of QU after the oral dose of QU was significantly different from the ones after coadministration of IS and QU (P<0.01 for both AUC0–72 h and AUC0–∞, Student’s t-test). After coadministration with IS, the oral bioavailability of QU was significantly enhanced. In Table 4, it can be seen that the AUC of IS prominently increased after coadministration of QU with IS to rats [P<0.01 for both AUC0–72 h and AUC0–∞, Student-Newman-Keuls (SNK) test]. In Table 5, the AUC of IS after coadministration of IS and QU is compared with the sum of AUC of IS after the oral doses of IS alone and QU alone. Significant differences were found in the AUC 0–72 h and AUC0–∞ (P<0.05, Student’s t-test). Assuming the metabolism of QU was not affected by the oral dose of IS, it could be concluded that QU could enhance the oral bioavailability of IS in rats. Therefore, it was concluded that IS and QU could enhance the oral bioavailability of each other. The results of the pharmacokinetics studies were consistent with the results of Caco-2 cells monolayer transport studies. The drug efflux pumps mediated interaction of intestinal epithelia efflux might contribute to the improvements of oral bioavailability.
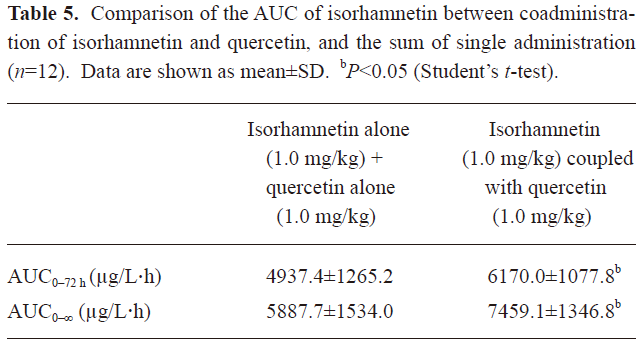
Full table
Discussion
Drug efflux pumps, such as P-gp, MRP1, MRP2 (multi-drug resistance associate protein 2) and BCRP (breast cancer resistance protein), were expressed not only in Caco-2 cells and intestinal epithelial cells[24–26], but also at the canalicular surface of hepatocytes[27,28]. All might play potential roles in the transport interactions of IS and QU in Caco-2 cells and intestinal epithelial cells. The role of these drug efflux pumps in the ADME of IS and QU might also be taken into consideration while considering the mechanism of the pharmacokinetics interactions.
It had been documented that some naturally-occurring glucosides of QU can be absorbed via sodium dependent glucose transporters (SLGT)[29]. However, the uptake of QU from the intestinal lumen into plasma is limited because of incomplete intestinal absorption, rapid biliary efflux[30,31] or a certain extent of intestinal efflux[20], in which P-gp, MRP2 and BCRP[32,33] were considered to be involved. For most QU glycosides, the transcellular permeation was hindered by their large molecular weight. The intestinal micro flora exhibited an important role in glycosides absorption[34,35]. In the enterocytes and hepatocytes, quercetin and its conjugates were partly transformed to isorhamentin by catechin-O-transferase[10]. Both IS and QU undergo extensive conjugations with glucuronide and sulfate after entering intestinal epithelium[20,36]. No CYP metabolism of QU or IS was found. The bile excretion was one of the main routes of elimination, in which MRP2 and BCRP were supposed to be involved[33,36]. Then, considerable reabsorption occurred after bile excretion. Consequently, multiple peaks of maximum plasma drug concentration usually occurred in pharmacokinetics studies. In the present pharmacokinetics study, the attenuation of the multiple peaks phenomenon also hinted that QU and IS might influence the bile excretion of each other.
The present results proved that QU and IS could significantly facilitate the absorptive permeation, inhibit the secretion permeation of each other across the Caco-2 cells monolayer, and enhance the oral bioavailability of each other. Considering the pharmacokinetics of QU and IS, the first possible mechanism of the pharmacokinetics interaction might exist in the methylation of QU. It had been proven that demethylation of IS to QU could not occur in our previous study[20]. Theoretically according to the enzyme kinetics, the increase of metabolic product (IS) could inhibit the methylation of IS, which will result in increase of the plasma level of QU and decrease of IS. In fact, significantly increased systemic exposures of both quercetin and isorhmanetin were found in the present study. Furthermore, COMT is abundant in human tissues and organs and might not be saturated in the tested dose. The metabolic interaction might play a minor role in the present pharmacokinetics interaction between quercetin and isorhamnetin. Therefore, the most possible potential mechanism existed in the intestinal excretion and bile excretion, in which P-pg, MRP2[36] and BCRP[32] might be involved. Because both QU and IS had been identified to interact with P-gp[16,21,37,38]. It could be concluded that P-gp might contribute to the present pharmacokinetics interaction between QU and IS. But whether MRP2 and BCRP were involved in this intra-herb interaction need further validation.
In the present study, it was proven that IS and QU could enhance the oral absorption of each other in rats. P-gp might play an important role in the absorptive pharmacokinetics interaction between IS and QU, whereas other ABC transporters, such as MRP2 and BCRP, might be involved. Therefore, it could be deduced that the oral dose of mixed flavonols in herb extracts could gain a significant biopharmaceutical advantage compared with the oral dose of single flavonol. Accordingly, besides the drug-herb interactions, inter-herb interaction and intra-herb interaction might be brought into view with the wide use of herbal-based remedies.
Acknowledgements
The MDCKII cells and MDR1-MDCKII cells were kindly given by Prof Borst P of the Netherlands Cancer Institute (NKI).
References
- Aruna D, Naidu MU. Pharmacodynamic interaction studies of Ginkgo biloba with cilostazol and clopidogrel in healthy human subjects. Br J Clin Pharmacol 2007;63:333-8.
- Meijerman I, Beijnen JH, Schellens JH. Herb-drug interactions in oncology: focus on mechanisms of induction. Oncologist 2006;11:742-52.
- Flanagan D. Understanding the grapefruit-drug interaction. Gen Dent 2005;53:282-5.
- Brazier NC, Levine MA. Drug-herb interaction among commonly used conventional medicines: a compendium for health care professionals. Am J Ther 2003;10:163-9.
- Oh SM, Chung KH. Estrogenic activities of Ginkgo biloba extracts. Life Sci 2004;74:1325-35.
- Hibasami H, Mitani A, Katsuzaki H, Imai K, Yoshioka K, Komiya T. Isolation of five types of flavonol from seabuckthorn (Hippophae rhamnoides) and induction of apoptosis by some of the flavonols in human promyelotic leukemia HL-60 cells. Int J Mol Med 2005;15:805-9.
- Manach C, Morand C, Demigne C, Texier O, Regerat F, Remesy C. Bioavailability of rutin and quercetin in rats. FEBS Lett 1997;409:12-16.
- Ader P, Wessmann A, Wolffram S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radic Biol Med 2000;28:1056-67.
- Gugler R, Leschik M, Dengler HJ. Disposition of quercetin in man after single oral and intravenous doses. Eur J Clin Pharmacol 1975;9:229-34.
- Cornish KM, Williamson G, Sanderson J. Quercetin metabolism in the lens: role in inhibition of hydrogen peroxide induced cataract. Free Radic Biol Med 2002;33:63-70.
- Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol 1995;33:1061-80.
- Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J Chemother 2005;17:86-95.
- Zhou S, Lim LY, Chowbay B. Herbal modulation of P-glycoprotein. Drug Metab Rev 2004;36:57-104.
- Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci 2006;78:2131-45.
- Patel J, Buddha B, Dey S, Pal D, Mitra AK. In vitro interaction of the HIV protease inhibitor ritonavir with herbal constituents: changes in P-gp and CYP3A4 activity. Am J Ther 2004;11:262-77.
- Wang Y, Cao J, Zeng S. Involvement of P-glycoprotein in regulating cellular levels of Ginkgo flavonols: quercetin, kaempferol, and isorhamnetin. J Pharm Pharmacol 2005;57:751-8.
- Fuller S, von Bonsdorff CH, Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell 1984;38:65-77.
- Evers R, Kool M, Smith AJ, van Deemter L, de Haas M, Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br J Cancer 2000;83:366-74.
- Hammerle SP, Rothen-Rutishauser B, Kramer SD, Gunthert M, Wunderli-Allenspach H. P-Glycoprotein in cell cultures: a combined approach to study expression, localisation, and functionality in the confocal microscope. Eur J Pharm Sci 2000;12:69-77.
- Lan K, Jiang X, He J. Quantitative determination of isorhamnetin, quercetin and kaempferol in rat plasma by liquid chromatography with electrospray ionization tandem mass spectrometry and its application to the pharmacokinetic study of isorhamnetin. Rapid Commun Mass Spectrom 2007;21:112-20.
- Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med 2004;36:592-604.
- Schulz HU, Schurer M, Bassler D, Weiser D. Investigation of pharmacokinetic data of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin revealed from single and multiple oral dose studies with a hypericum extract containing tablet in healthy male volunteers. Arzneimittelforschung 2005;55:561-8.
- Schulz HU, Schurer M, Bassler D, Weiser D. Investigation of the bioavailability of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin following single and multiple oral dosing of a hypericum extract containing tablet. Arzneimittelforschung 2005;55:15-22.
- Braun A, Hammerle S, Suda K, Rothen-Rutishauser B, Gunthert M, Kramer SD, et al. Cell cultures as tools in biopharmacy. Eur J Pharm Sci 2000;11 Suppl 2:S51-60.
- Walgren RA, Karnaky KJ Jr, Lindenmayer GE, Walle T. Efflux of dietary flavonoid quercetin 4′′-beta-glucoside across human intestinal Caco-2 cell monolayers by apical multidrug resistance-associated protein-2. J Pharmacol Exp Ther 2000;294:830-6.
- Xia CQ, Liu N, Yang D, Miwa G, Gan LS. Expression, localization, and functional characteristics of breast cancer resistance protein in Caco-2 cells. Drug Metab Dispos 2005;33:637-43.
- Gant TW, Silverman JA, Thorgeirsson SS. Regulation of P-glycoprotein gene expression in hepatocyte cultures and liver cell lines by a trans-acting transcriptional repressor. Nucleic Acids Res 1992;20:2841-6.
- Lee CH, Bradley G, Zhang JT, Ling V. Differential expression of P-glycoprotein genes in primary rat hepatocyte culture. J Cell Physiol 1993;157:392-402.
- Ader P, Block M, Pietzsch S, Wolffram S. Interaction of quercetin glucosides with the intestinal sodium/glucose co-transporter (SGLT-1). Cancer Lett 2001;162:175-80.
- Crespy V, Morand C, Besson C, Cotelle N, Vezin H, Demigne C, et al. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am J Physiol Gastrointest Liver Physiol 2003;284:G980-8.
- Arts IC, Sesink AL, Faassen-Peters M, Hollman PC. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br J Nutr 2004;91:841-7.
- Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun 2004;317:269-75.
- Sesink AL, Arts IC, de Boer VC, Breedveld P, Schellens JH, Hollman PC, et al. Breast cancer resistance protein (Bcrp1/Abcg2) limits net intestinal uptake of quercetin in rats by facilitating apical efflux of glucuronides. Mol Pharmacol 2005;67:1999-2006.
- Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J Nutr 2005;135:48-52.
- Schneider H, Simmering R, Hartmann L, Pforte H, Blaut M. Degradation of quercetin-3-glucoside in gnotobiotic rats associated with human intestinal bacteria. J Appl Microbiol 2000;89:1027-37.
- O’Leary KA, Day AJ, Needs PW, Mellon FA, O’Brien NM, Williamson G. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol 2003;65:479-91.
- Wang Y, Cao J, Zeng S. Establishment of a P-glycoprotein substrate screening model and its preliminary application. World J Gastroenterol 2004;10:1365-8.
- Mitsunaga Y, Takanaga H, Matsuo H, Naito M, Tsuruo T, Ohtani H, et al. Effect of bioflavonoids on vincristine transport across blood-brain barrier. Eur J Pharmacol 2000;395:193-201.