Effects of Ganoderma lucidum polysaccharides on proliferation and cytotoxicity of cytokine-induced killer cells
Introduction
The fungus Ganoderma lucidum (Leyss, ex Fr) Karst (known in China as “lingzhi” and in Japan as “reishi”) has a long history of use in traditional Chinese medicine. Polysaccharides from Ganoderma lucidum have been reported to have promising immune modulating effects; for example, they have been shown to promote the function of the mononuclear phagocyte system, antigen-presenting cells and cytotoxic T lymphocytes (CTL) induced by dendritic cells[1–3], to enhance lymphocyte proliferation and antibody production[4–6], to potentiate cytokine production by splenocytes and macrophages[7], and to inhibit spontaneous and Fas-mediated apoptosis in neutrophils[8]. The antitumor effect of Gl-PS is reportedly due to its effects on the immune system, and there is reportedly no associated toxicity in humans[9].
Over the past 20 years or so, investigators have attempted to develop adoptive cellular immunotherapy for cancer treatment. Cytokine-induced killer (CIK) cells have been shown to generate effector cells with higher proliferative capacity, increased cytotoxicity and fewer side effects than lymphokine activated killer (LAK) cells[10,11]. Some reports have demonstrated that Gl-PS can enhance the cytotoxicity of CTL and natural killer (NK) cells[1], but little is known about the effects of Gl-PS on the development and cytotoxic activity of CIK cells. The present study was undertaken to elucidate the effects (and mechanisms thereof) of Gl-PS on LAK cells and CIK cells induction and anti-tumor activity.
Materials and methods
Animals and drugs Male C57BL/6j mice 6–8-week-old were purchased from the Department of Experimental Animals, Health Science Center, Peking University, Beijing, China. Gl-PS were isolated from Ganoderma lucidum (Leyss, ex Fr) Karst by placing the fungus in boiling water, followed by ethanol precipitation, dialysis, and protein depletion using the Sevag method. The isolated molecule was a polysaccharide peptide with a molecular weight of 584 900, and a ratio of polysaccharides to peptides of 93.61%:6.49%. The polysaccharides included D-rhamnose, D-xylose, D-fructose, D-galactose, D-mannose and D-glucose with a molar ratio of 0.793:0.964:2.944:0.167:0.389:7.94, linked together with β-glycosidic linkages. The peptides contained 16 kinds of amino acids[2]. Gl-PS was isolated as a water-soluble powder that was dissolved in serum-free RPMI-1640 medium (Gibco BRL, NY, USA), then filtered through a 0.22 μm filter and stored at 4 °C. This solution was further diluted to the required concentrations before assay.
Isolation and culture of LAK cells LAK cells were generated as previously described[9]. Briefly, mice were killed by cervical dislocation. Suspensions of spleen single cells were pooled in serum-free RPMI-1640 medium by filtering the suspension through mesh with the aid of a glass homogenizer to exert gentle pressure on the spleen fragments. Erythrocytes were lysed with an ammonium chloride solution [0.15 mol/L NH4Cl, 10 mmol/L KHCO3, 0.1 mmol/L edetic acid (EDTA), pH 7.2]. Obtained cells were washed twice with phosphate-buffered saline (PBS) and resuspended in the complete RPMI-1640 medium [RPMI-1640 medium supplemented with 10% inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, 25 mmol/L NaHCO3, 1 mmol/L sodium pyruvate, 25 mmol/L N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES), 100 kU/L penicillin G, and 100 mg/L strepto-mycin]. Adherent cells were removed via adherence to glass surfaces. Nonadherent mononuclear cells were obtained and the percentage of viable cells was >95%. Murine recombinant interleukin-2 (rIL-2) (300 kU/L) was added on d 0. Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 at a concentration of 1×109 cells/L. Cell density was determined every 4 d, and cells were subcultured in fresh complete medium containing 150 kU/L IL-2. LAK cells were cultured in complete medium with 300 kU/L IL-2 for the positive control, with 75 kU/L IL-2 plus 400 mg/L or 100 mg/L Gl-PS for experimental groups 1 and 2, respectively, and with 75 kU/L IL-2 plus 400 or 100 mg/L soluble starch (SS) or methylcellulose (MC) as negative controls 1–4, respectively.
Isolation and culture of CIK cells CIK cells were generated as previously described[10]. Nonadherent splenocytes were suspended in complete RPMI-1640 medium at a concentration of 1×109 cells/L. Murine γ-interferon (IFN-γ; 1000 kU/L) was added on d 0. After 24 h of incubation, 50 mg/L monoclonal antibodies (mAb) against CD3 (dilution of supernatant from hybridoma 145-2C11), 300 kU/L IL-2, and 100 kU/L murine rIL-1 were added. Fresh IL-2 (150 kU/L) and fresh medium were added every 3 d to maintain a cell density of 1.5×109–2.0×109 cells/L for 14–21 d. All the cytokines were purchased from PeproTech EC, London, UK. CIK cells were prepared by using the standard protocol described earlier for the positive control. Experimental groups 1 and 2 were prepared according to the standard protocol except that Gl-PS (400 or 100 mg/L) was added, and the dose of anti-CD3 and IL-2 was decreased by 50% and 75%, respectively. For negative control groups 1–4, Gl-PS in the experimental protocol was replaced with SS or MC (400 or 100 mg/L).
LAK and CIK cell proliferation assay Cell density was assessed every 3 d, and cells were subcultured in fresh complete medium containing IL-2 as described previously[10]. Viable cell numbers were determined by the Trypan blue dye exclusion method at each time point. LAK and CIK cell proliferation was analyzed by cell growth curves.
Preparation of target cells P815 (NK-resistant) and YAC-1 (NK-sensitive) cells proliferating in a logarithmic manner were used as target cells for the cytotoxicity assay. Cells (2×108 cells/L) were maintained in complete RPMI-1640 medium as described earlier. On the day of testing, cells were washed once with PBS and re-suspended in a complete medium at a concentration of 2×108 cells/L.
Cytotoxicity assay The MTT colorimetric assay was used for testing cytotoxic activity in vitro[12]. LAK cells or CIK cells were used as effectors, and were coincubated with P815 or YAC-1 cells as targets for 4 h at effector:target ratios of 20:1. Cells were plated in triplicate in 96-well microculture plates (Corning Costar, MA, USA). MTT (Sigma, St Louis, MO, USA) was dissolved at 5 g/L in PBS and filtered through a 0.22 μm filter. MTT solution (20 μL) was added to each well, and the microplates were further incubated at 37 °C for 4 h. The 96-well microplates were centrifuged at 200×g for 15 min, then supernatants were discarded, and 150 μL of Me2SO was added into each well. The tray was gently shaken thoroughly for 10 min to dissolve the dark blue crystals of formazan that could be measured spectrophotometrically. Data are expressed as mean absorbance value (optical density, OD) of samples±SD. Percentage-specific cytotoxicity (% C) was calculated as follows: % C={1–[(OD of effectors+targets)–OD of effectors]/OD of targets}×100%.
Flow cytometric assays Effector cells were harvested and washed twice with ice cold FACScan buffer (PBS containing 2% FCS and 0.1% sodium azide). To block nonspecific antibody binding, 20% mixed mouse and rat serum was used and then cells were stained with mAb against CD3 coupled to fluorescein isothiocyanate (FITC) and/or mAb against NK1.1 coupled to phycoerythrin (PE) (antibodies from Santa Cruz, CA, USA or Pharmingen, CA, USA) for 45 min at 4 °C in the dark. The stained cells were washed twice and fixed with 1% paraformaldehyde in FACScan buffer and then analyzed by using a FACScan flow cytometer (Becton Dickinson, NJ, USA). Dead cells and debris were gated out.
Antibody block experiment CIK cells were induced by using the standard protocol described earlier for the positive control, except that Gl-PS (400 or 100 mg/L) was added and the dose of anti-CD3 and IL-2 was decreased by 50% and 75% (experimental groups 1 and 2, respectively) or Gl-PS in the experimental group protocol was replaced by SS (400 mg/L or 100 mg/L) or MC (400 mg/L or 100 mg/L) as negative controls 1–4, respectively. Anti-CR3 (eBioscience, San Diego, CA, USA) or its isotype immunoglobulin G (IgG) was added 30 min prior to Gl-PS in the experimental group protocol, in anti-CR3 groups 1 or 2 and isotype antibody groups 1 or 2. Cells were harvested on d 7. The proliferation and cytotoxicity of different groups were determined by MTT colorimetric assay.
Statistical analysis Data were analyzed using general linear models (2-way ANOVA) in SPSS statistical software (version 10.0) with a significance level of α=0.05. Comparisons between means were made using the least significant difference multiple comparisons t- test of Dunnett’s t-test. Results are presented as the mean±SD. Values of P<0.05 were considered to be statistically significant.
Results
Effects of Gl-PS on LAK cell proliferation Nonadherent splenocytes were incubated at 1×109 cells/L in complete medium with IL-2 alone or IL-2 at different concentrations plus Gl-PS at different concentrations. Cells were harvested after 4 d to generate LAK cells. Statistical tests of the between-subject effects showed that there were significant differences according to univariate analyses of variance (two-way ANOVA). The interaction between IL-2 and Gl-PS was significant, which suggests a synergy between IL-2 and Gl-PS. The effect of IL-2 was bigger than that of Gl-PS. For the factor “IL-2”, there was no statistically significant difference between neither the 150 kU/L nor the 75 kU/L level when compared with the 300 kU/L level (P>0.05). For the factor “Gl-PS ”, the 400 mg/L and 100 mg/L levels were significantly different from the 0 mg/L level (P<0.01). The combination of 75 kU/L IL-2 and 400 mg/L or 100 mg/L Gl-PS was optimal for the least usage of IL-2 (75 kU/L), and LAK cell proliferation induced by the combination was not less than that of the positive control, in which only IL-2 (300 kU/L) was added (Table 1). Compared with only 75 kU/L IL-2 in the LAK cell culture, the effects of SS or MC in different concentrations plus 75 kU/L IL-2 were not significant, therefore SS and MC were chosen as negative controls (data not shown).
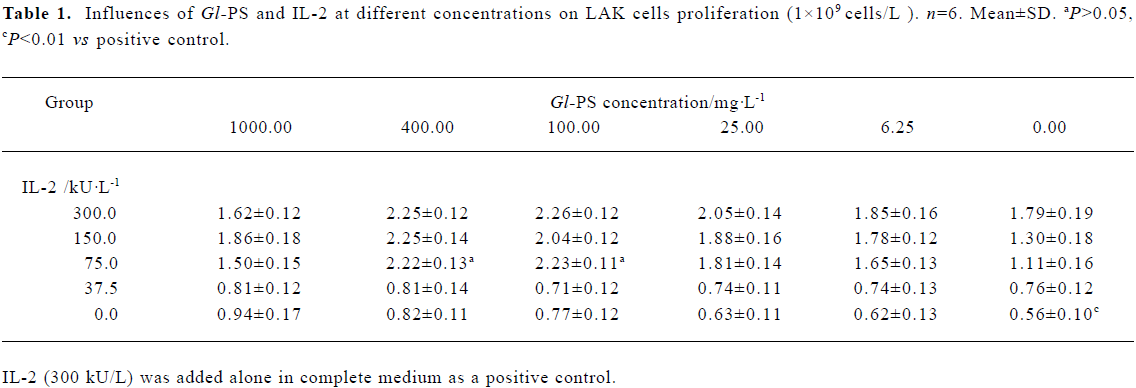
Full table
Cells in different groups were harvested and viable cell numbers were determined on d 1, 4, 8, and 12. The difference in cell numbers across groups was not significant on d 1. Following 4 d of culture, the number of viable cells of the positive control and experimental groups increased 2-fold in comparison to the negative controls, but the difference between experimental groups and the positive control was not significant at that time. The number of viable cells in the positive control and experimental groups increased markedly compared with negative controls on d 8. However, at that time, there was no significant difference between the positive control and experimental groups. After 8 d of culture, cells in all groups no longer proliferated, died gradually, and decreased on d 12 relative to the number present on d 1 (Figure 1).
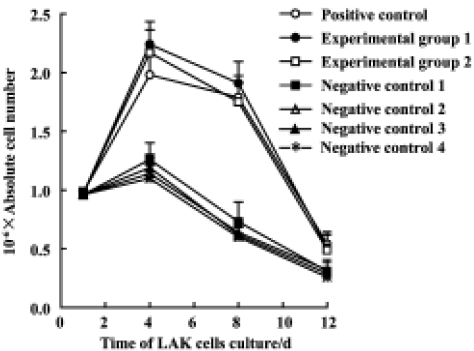
Effects of Gl-PS on CIK cell proliferation With respect to the synergistic interaction of Gl-PS (400 mg/L or 100 mg/L) and IL-2 (75 kU/L), experimental groups 1 and 2 were the same as the positive control except for the addition of 400 or 100 mg/L Gl-PS and a reduction in the doses of anti-CD3 and IL-2 by 50% and 75%, respectively. For negative controls 1–4, Gl-PS was replaced with SS or MC (400 mg/L or 100 mg/L) in the protocol used in the experimental groups. We observed that the proliferation of CIK cells in the experimental groups was also similar to that of the positive control. The reduction of cell numbers in the negative controls was significant compared with the positive control on d 6 to d 21. The number of CIK cells in the positive control and experimental groups peaked at d 21 and was expanded about 80-fold relative to the beginning culture. Their increase in activity was much larger than that observed in LAK cells (LAK proliferation peaked at d 4–8 and the cell number increased 2-fold relative to the initial culture). This suggests that 400 mg/L or 100 mg/L Gl-PS decreased the usage of IL-2 and anti-CD3 by 75% and 50%, respectively, and that the influence on CIK cell proliferation was almost equal to that of cytokines alone at higher doses (Figure 2).
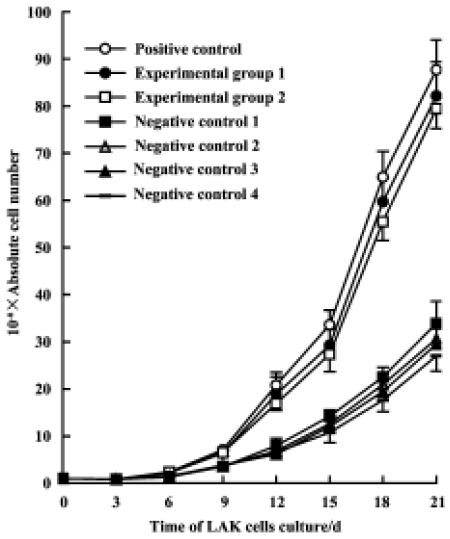
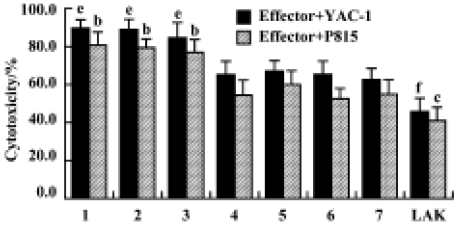
Effects of Gl-PS on cytotoxicity of LAK Compared with induction by 300 kU/L IL-2 alone, no significant difference in the cytotoxicity of LAK cells induced by 75 kU/L IL-2 plus 400 mg/L or 100 mg/L Gl-PS was observed, but cytotoxicity induced by 75 kU/L IL-2 plus SS or MC instead of Gl-PS in the negative controls was reduced. This suggests that a certain concentration of Gl-PS increases the cytotoxicity of LAK cells (Figure 3).
Effects of Gl-PS on cytotoxicity of CIK cells Compared with the standard protocol for preparing CIK cells, the cytotoxicity of CIK cells in the experimental groups was not significantly different, but was higher than that in the negative control groups. We found that CIK cells in every group achieved higher cytotoxicity than LAK cells. This suggests that 400 mg/L or 100 mg/L Gl-PS decreases the usage of IL-2 and anti-CD3 by 75% and 50%, respectively, but has no influence on CIK cell cytotoxicity induced by cytokines alone at higher doses (Figure 4).
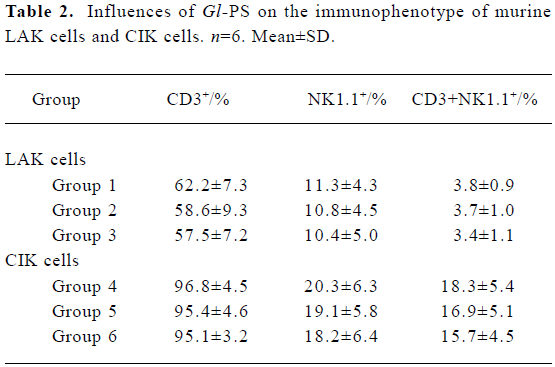
Immunophenotype of effector cells in the presence of Gl-PS Flow cytometric assays showed that LAK cells represented a heterogeneous population, in which the major effector cells generally had an NK1.1+ cell surface phenotype. Compared with LAK cells in the positive control groups, the phenotype of LAK cells in the experimental groups was not significantly different. Most CIK cells expressed CD3, a surface marker of T cells. Treatment with 400 mg/L or 100 mg/L Gl-PS in the experimental groups did not alter the phenotype of CIK cells (Table 2).
Full table
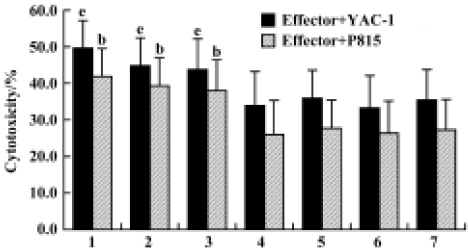
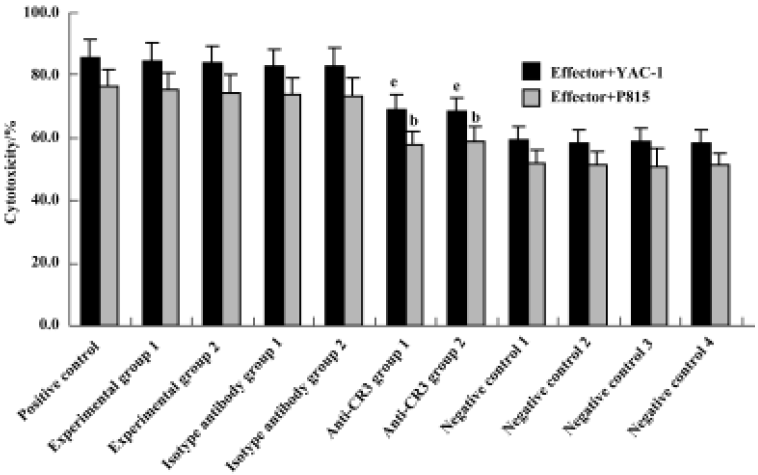
Effect of anti-CR3 on proliferation and cytotoxicity of CIK cells induced by stimuli containing Gl-PS CIK cell proliferation and cytotoxicity were reduced to <40% of that in the experimental groups by the addition of anti-CR3 30 min prior to the addition of Gl-PS in CIK cell culture, but not by nonspecific isotype IgG (Figures 5, 6).

Discussion
Polysaccharides isolated from Ganoderma are high-molecular-weight polysaccharides linked together by β-glycosidic linkages. These polysaccharides exhibit immunological activity, for example promoting cellular immunity and humoral immunity[1–8,13–15]. Biological and immunopharma-cological activities depend on the conformation of polysac-charides, molecular mass, solubility in water and triple-helical structure. Soluble starch is an α-(1,4)-bonding glucose polymer, and methylcellulose links by uniform β-(1,4) glycosidic linkages[16]. Because they lack a helical structure, neither of these compounds have the immunological activity, so they served as negative controls. Flow cytometry indicated that LAK and CIK cells populations were hetero-geneous. Effector cells in LAK cell culture generally have the CD3-NK1.1+ cell surface phenotype[17]; however, most CIK cells express T-cell markers. Earlier studies have shown that cytotoxicity correlates with the expansion of CD3+NK1.1+ lymphocytes, which are the main effector cells in CIK cell cultures[18]. The present study demonstrated that the optimal combination of Gl-PS (400 mg/L or 100 mg/L) and certain cytokines did not alter the phenotype of LAK cells or CIK cells, and had no effect on the percentage of effector cells. For this reason, the cytotoxicity of LAK cells and CIK cells in the experimental groups was similar to that of the positive control, which contained cytokines alone at higher doses.
CIK cells with enhanced cytotoxicity and a higher proliferative capacity relative to LAK cells were described first by Schmidt-Wolf and colleagues in 1991. They reported that treatment of tumor cells from bone marrow with CIK cells effectively reduced the tumor cell burden, allowing animals to survive[10]. As demonstrated here, LAK and CIK cells possess cytotoxic effects against both NK-sensitive and NK-resistant tumor cells, and CIK cells are more cytotoxic than LAK cells. Previous studies have shown that CIK cells express both the Fas receptor and FasL. Fas-sensitive and previously activated T cells are eliminated in the early stages of CIK cell culture through either IFN-γ, activation-induced cell death or both. To generate CIK cells, splenocytes are stimulated with IFN-γ 24 h prior to anti-CD3 and IL-2 treatment. This combination of cytokines might select for a population of Fas-resistant cells[19]. In the present study, the CIK cell growth curve decreased in the initial stages, then increased. The initial decrease might be related to the downregulation of cell proliferation caused by IFN-γ. In the present study, a synergistic interaction between IL-2 (75–300 kU/L) and Gl-PS (400 mg/L or 100 mg/L) enhanced LAK cell development and cytotoxicity. In combination with 1000 kU/L IFN-γ, 75 kU/L IL-2, 25 mg/L anti-CD3 and 100 kU/L IL-1, Gl-PS (400 mg/L or 100 mg/L) decreased the dose of IL-2 and anti-CD3 by 75% and 50% respectively, but did not influence CIK cell proliferation and cytotoxicity. These results show that only intermediate concentrations (not too high or too low) of Gl-PS enhance immunocyte development; and the results also suggest that a certain concentration of Gl-PS could be an immune potentiator to reduce the dose of cytokine inducing LAK and CIK cells. Previous studies have shown that Gl-PS can stimulate splenocytes and macrophages to produce cytokines, including IL-1, IL-2, IFN-γ and tumor necrosis factor[5,20–23]. Whether Gl-PS exerts its activity by promoting the release of cytokines by the precursors of CIK cells is currently under investigation.
β-Glucans are known to bind to receptors, for example CR3. The binding site of CR3 has a broad specificity for certain polysaccharides containing glucose and mannose, as well as N-acetyl-D-glucosamine[24]. Gl-PS is a hetero-glycan that contains primarily glucose[2], and CR3 is expressed mostly by activated lymphocytes, NK cells, macrophages and granulocytes[25]. Our results demonstrated that the activity of CIK cells mediated by Gl-PS was drastically inhibited (60%–70%) by prior treatment of murine splenocytes with anti-CR3. These results correspond with those of Xia et al, who found that β-Glucans bound to CR3 on macrophages, neutrophils, and NK cells in humans and mice, and cytotoxicity mediated by β-glucans can be blocked by anti-CR3 added before or after β-glucan treatment[25]. It has been confirmed that anti-CR3 had no effect on the cytotoxic activity of CIK cells[26]. These results suggest that the immune-modulating effect of Gl-PS on CIK cells is mediated primarily through CR3. Our study provides useful data for the use of Gl-PS in combination with other immuno-activators.
Acknowledgements
This research was supported by the Research Fund of Shanghai Green Valley Holdings Co. The Gl-PS was kindly provided by Prof Shu-qian LIN and Associate Prof Sai-zhen WANG from the Fuzhou Institute of Green Valley Bio-Pharm Technology.
References
- Lin ZB, Zhang HN. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol Sin 2004;25:1387-95.
- Cao LZ, Lin ZB. Regulation on maturation and function of dendritic cells by Ganoderma lucidum polysaccharides. Immunol Lett 2002;83:163-9.
- Cao LZ, Lin ZB. Regulatory effect of Ganoderma lucidum polysaccharides on cytotoxic T-lymphocytes induced by dendritic cells in vitro. Acta Pharmacol Sin 2003;24:321-6.
- Zhang J, Tang Q, Zimmerman-Kordmann M, Reutter W, Fan H. Activation of B lymphocytes by GLIS, a bioactive proteoglycan from Ganoderma lucidum. Life Sci 2002;71:623-38.
- Bao XF, Zhen Y, Ruan L, Fang JN. Purification, characterization, and modification of T lymphocyte-stimulating polysaccharide from spores of Ganoderma lucidum. Chem Pharm Bull (Tokyo) 2002;50:623-9.
- Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Crit Rev Immunol 1999;19:65-96.
- Lin ZB, Zhang QH. Effect of Ganoderma lucidum polysaccharide B on TNF-α and IFN-γ production and their mRNA expression. J Beijing Med Univ 1999;31:179-83.
- Hsu MJ, Lee SS, Lin WW. Polysaccharide purified from Ganoderma lucidum inhibits spontaneous and Fas-mediated apoptosis in human neutrophils through activation of the phosphatidylinositol 3 kinase/Akt signaling pathway. J Leukoc Biol 2002;72:207-16.
- Lin ZB. Progress of studies on the anti-tumor activity and immunomodulating effect of Ganoderma. J Beijing Med Univ 2002;34:493-8.
- Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent anti-tumor cell activity. J Exp Med 1991;174:139-49.
- Lu PH, Negrin RS. A novel population of expended human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol 1994;153:1687-96.
- Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods 1990;131:165-72.
- Zákány J, Chihara G, Fachet J. Effect of Lentinan on tumor growth in murine allogeneic and syngeneic host. Int J Cancer 1980;25:371-6.
- Zákány J, Chihara G, Fachet J. Effect of Lentinan on the production of migration inhibitory factor induced by syngeneic tumor in mice. Int J Cancer 1980;26:783-8.
- Bao XF, Wang XS, Dong Q, Fang JN, Li XY. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry 2002;59:175-81.
- Chen HL. Studies of the conformation and activity of polysaccharides. Shanghai: Shanghai Medical University Press; 1997.
- Schmidt-Wolf IG, Lefterova P, Johnston V, Scheffold C, Csipai M, Mehta BA, et al. Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cell Immunol 1996;169:85-90.
- Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, et al. Phenotypic characterization and identification of effector cells involved in tumor recognition of cytokine-induced killer cells. Exp Hematol 1993;21:1673-9.
- Verneris MR, Kornacker M, Mailänder V, Negrin RS. Resistance of ex vivo expanded CD3+CD56+ T cells to Fas-mediated apoptosis. Cancer Immunol Immunother 2000;49:335-45.
- Wang YY, Khoo KH, Chen ST, Lin CC, Wong CH, Lin CH. Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorg Med Chem 2002;10:1057-62.
- Ma L, Lin ZB, Li RZ, He YQ. Effects of Ganoderma polysaccharides on IL-2 production by mouse splenocytes in vitro. J Beijing Med Univ 1991;23:412-6.
- Lei LS, Lin ZB. Effects of Ganoderma polysaccharides on T cell subpopulations and production of interleukin 2 in mixed lymphocyte response. Acta Pharmacol Sin 1992;27:331-5.
- Zhang QH, Lin ZB. The antitumor activity of Ganoderma lucidum (Curt Fr) P Karst (Ling Zhi) (Aphyllophoromycetideae) polysaccharides is related to tumor necrosis factor-α and interferon-γ. Int J Med Mushroom 1999;1:207-15.
- Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol 1996;156:1235-46.
- Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol 1999;162:2281-90.
- Schmidt-Wolf IG, Lefterova P, Johnston V, Huhn D, Blume KG, Negrin RS. Propagation of large numbers of T cells with natural killer cell markers. Br J Haematol 1994;87:453-8.
