Resveratrol protects bone marrow mesenchymal stem cell derived chondrocytes cultured on chitosan-gelatin scaffolds from the inhibitory effect of interleukin-1β
Introduction
Despite osteoarthritis (OA) being one of the most common afflictions in the elderly, adult articular cartilage is reluctant to spontaneously repair even minor injuries to its structure. This apparent inability provides the rationale for current efforts to develop cartilage tissue engineering techniques and because of a shortage of adult articular cartilage it is imperative to use stem cells in tissue engineering to both repair and regenerate cartilage. Further, pro-inflammatory cytokines, such as IL-1β, mediate the catabolic degradation of extracellular matrix (ECM) in articular cartilage and play a key role in osteoarthritis (OA) pathogenesis. These cytokines induce chondrocytes and synoviocytes to synthesize proteolytic enzymes, such as matrix metalloproteinases (MMP) and inflammatory mediators. Overexpression of MMPs and prostaglandins are implicated in cartilage loss[1]. Additionally, these pro-inflammatory cytokines induce chondrocyte apoptosis, a process that is thought to play a pivotal role in joint diseases[2]. Thus, an altered imbalance between biosynthesis and degradation of matrix components leads to progressive tissue destruction, resulting in extensive articular cartilage damage and further synovial inflammation. These deleterious effects are mediated by the activation of various transcription factors. Among them, NF-κB, a sequence-specific transcription factor that regulates the expression of numerous genes, exerts either protective or detrimental effects depending on the cellular context[3].
Resveratrol, a natural phytoalexin, was reported to exert immunomodulatory, antioxidative, and anti-inflammatory functions[4]. A recent article indicated that resveratrol had been shown to inhibit tetradecanoylphorbol acetate-induced phosphorylation, degradation of IκBα and the subsequent nuclear translocation of the p65 protein[5]. However, the effects of resveratrol on chondrocytes in an inflammatory environment have not been fully investigated.
Integrins, the trans-membrane adhesion and signalling receptors, which physically link ECM to the cytoskeleton, are key players in transducing mechanical signals via mitogen-activated protein kinases (MAPK) and NF-κB[6]. Given the internal relations between resveratrol, NF-κB and the β1-integrin signalling pathway, the study was to test the hypothesis that when MSC-derived chondrocytes were cultured on the CGS, resveratrol acts as both an anti-inflammatory and antioxidant agent against the catabolic effects of IL-1β through the inhibition of NF-κB nuclear translocation. In addition, we also tested whether the β1-integrin signalling pathway was involved in this process. Such a unique procedure allows researchers to mimic the in vivo inflammatory environment.
Materials and methods
Preparation of chitosan-gelatin scaffolds Chitosan (C-3646, Sigma, St Louis, USA) solution (20 mg/mL) was prepared by dissolving chitosan in a 0.5 mol/L acetic acid solution. Chitosan-gelatin solution was then prepared by mixing chitosan solution with gelatin (G-2500, Sigma) solution (20 mg/mL) at a 1:1 ratio. The mix solution was poured into 48-well plates (Cellstar) and then frozen at –80 °C. The frozen gel in the 48-well plates was lyophilized in a freeze-dryer (Christ Beta 1-16). The CGS were cut into 3-mm thick discs and sterilized with ethylene oxide.
MSC isolation, expansion, chondrogenetic differentiation, and culture Mesenchymal stem cells were obtained from adult Wistar rat femurs and tibias using a previously reported method[7]. MSCs were suspended in a complete medium (low glucose, DMEM, Gibco) containing 20% fetal bovine serum (FBS, Hyclone) in T75 flasks (Cellstar). The medium was exchanged after the first 24 h, and then subsequently 3 times a week. For expansion, the cells were subcultured to passage 6. Expanded MSCs were suspended in a 0.15 mol/L NaCl solution containing 1.25% alginate at a density of 2×106/mL. The cell suspension was slowly passed through a 21-gauge needle in a drop-wise fashion into a 102 mmol/L CaCl2 solution. The beads were then allowed to polymerize for 10 min. Alginate beads were then cultured in the chondrogenetic-inducing high glucose, FBS free DMEM medium containing the following: 10 ng/mL TGF-β3 (Peprotech), 100 ng/mL IGF-I (Peprotech), 100 nmol/L dexamethasone, 50 µg/mL ascorbate-2-phosphate, 40 µg/mL proline, 100 µg/mL pyruvate, and ITS+1 (Sigma, 10 µg/mL insulin, 5.5 µg/mL human transferrin, 5 ng/mL sodium selenite, 0.5 mg/mL bovine serum albumin and 4.7 µg/mL linoleic acid). The medium was changed every other day. After 3 weeks, the alginate beads were dissolved in a 0.9% sodium chloride solution containing 55 mmol/L sodium citrate. Cells were pelleted and reseeded once in a monolayer manner. Confluent cells were cultured on the CGS at a density of 1×106/construct for another 3 weeks.
The experiment was specifically designed to mimic the inflammatory environment in vitro. Serum-starved cells on CGS were either treated with 100 µmol/L resveratrol (Sigma) or stimulated with 10 ng/mL IL-1β (R&D Systems) for 24 h. Afterwards, the IL-1β-stimulated CGS with cells were co-treated with IL-1β and resveratrol for another 24 h in the absence and presence of the specific β1-integrin blocking antibody (BD Pharmingen, dilution 1:100).
Morphological assessment Alginate beads with MSCs, induced for 3 weeks were stained overnight in a 70% ethanol solution containing 0.2% (w/v) toluidine blue, and then rinsed with a 95% ethanol solution to visualize the pericellular matrix accumulation.
Immunostaining was used to assess collagen type II and aggrecan expression in MSC-derived chondrocytes. Briefly, the differentiated cells released from alginate beads were reseeded on cover glasses. After being fixed with 4% paraformaldehyde, the cells were blocked in 5% FBS. After being incubated overnight at 4 °C with the primary antibodies (Santa Cruz), the cells were then incubated for 1 h with either a rhodamine or a fluorescein isothiocyanate (FITC) conjugated secondary antibody. The cells were observed under a fluorescent microscope (Leica).
A scanning electron microscope (SEM, S-570, Hitachi) and a laser confocal microscope (LCM, Leica, TCS-SP) were used to evaluate MSC-derived chondrocyte morphology. For the SEM, the scaffold cultures were fixed with 2.5% (v/v) glutaraldehyde in a 0.1 mol/L sodium cacodylate buffer at pH 7.2 for 2 h, and then were postfixed in 1% (w/v) OsO4 for 1 h. Dehydration in an ethanol series followed. Finally, the samples were dried in a critical point dryer and coated with gold. The coated samples were then observed under the SEM. For the LCM, the CGS were stained with acridine orange (AO). Briefly, the scaffold cultures were first fixed with 4% paraformaldehyde and then were washed with PBS twice, each for 5 min. The scaffolds were then submerged in a 1% acetic acid solution for 30 s and stained with a 0.01% AO solution for 5 min, followed by 2 more PBS washes. Finally, the scaffolds were submerged with 1% CaCl2 for 30 s, followed by a thorough PBS wash and then observation with the LCM.
RT-PCR assay Total RNA was isolated using a Trizol reagent (Invitrogen). Using a ReverTra Ace Kit (Toyobo), 1 µg of total RNA was reverse transcribed into cDNA. Then 1 µL of cDNA was amplified using a PCR Master Mix kit (Promega). The mRNA levels were quantified with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which served as an internal standard. Primers used for PCR were the following: 5'-GAA GCA CAT CTG GTT TGG AG-3' (sense) and 5'-TTG GGG TTG AGG GTT TTA CA-3' (antisense) for type II collagen (448 bp); 5'-TAG AGA AGA AGA GGG GTT AGG-3' (sense) and 5'-AGC AGT AGG AGC CAG GGT TAT-3' (antisense) for aggrecan (322 bp); 5'-ACC ACA GTC CAT GCC ATC AC-3' (sense) and 5'-TCC ACC ACC CTG TTG CTG TA-3' (antisense) for GAPDH (452 bp). DNA amplification included an initial denaturation at 94 °C for 3 min, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 56 °C (type II collagen and aggrecan), or 65 °C (GAPDH) for 30 s, and then extension at 72 °C for 45 s. The PCR products were analyzed by electrophoresis in 1.2% agarose gels stained with ethidium bromide.
Preparation of nuclear and cytosolic extracts After the incubation, MSC-derived chondrocytes were trypsinized from the CGS and pelleted at 500×g for 3 min. Nuclear and cytosolic extracts were obtained with NE-PER™ nuclear and cytoplasmic extraction reagents (Pierce) and protease inhibitor cocktail set III reagent (Calbiochem) according to the manufacturer’s instructions.
Western blotting assay Cell lysis was carried out using M-PER mammalian protein extraction reagent (Pierce) and protease inhibitor cocktail set III (Calbiochem) plus 5 mmol/L EDTA. A total volume of 20 µg of protein was separated by 10% SDS-PAGE. The separated proteins were transferred to a polyvinylidene difluoride membrane. After being blocked with 5% non-fat milk in Tris-buffer saline containing 0.05% Tween 20 (TBST), the membrane was incubated with the corresponding antibodies (Santa Cruz) in TBST overnight at 4 °C. The membrane was then washed 3 times with TBST and incubated with the horseradish peroxidase-conjugated secondary antibody in TBST at room temperature. After being washed with TBST 5 times, the bands on the membrane were visualized with a 3,3'-diaminobenzidine (DAB) kit and analyzed with GeneSnap image acquisition software.
Statistical analysis All data are presented as mean± SD. Statistical analysis was carried out using a one-way ANOVA test and P values less than 0.05 were deemed significant.
Results
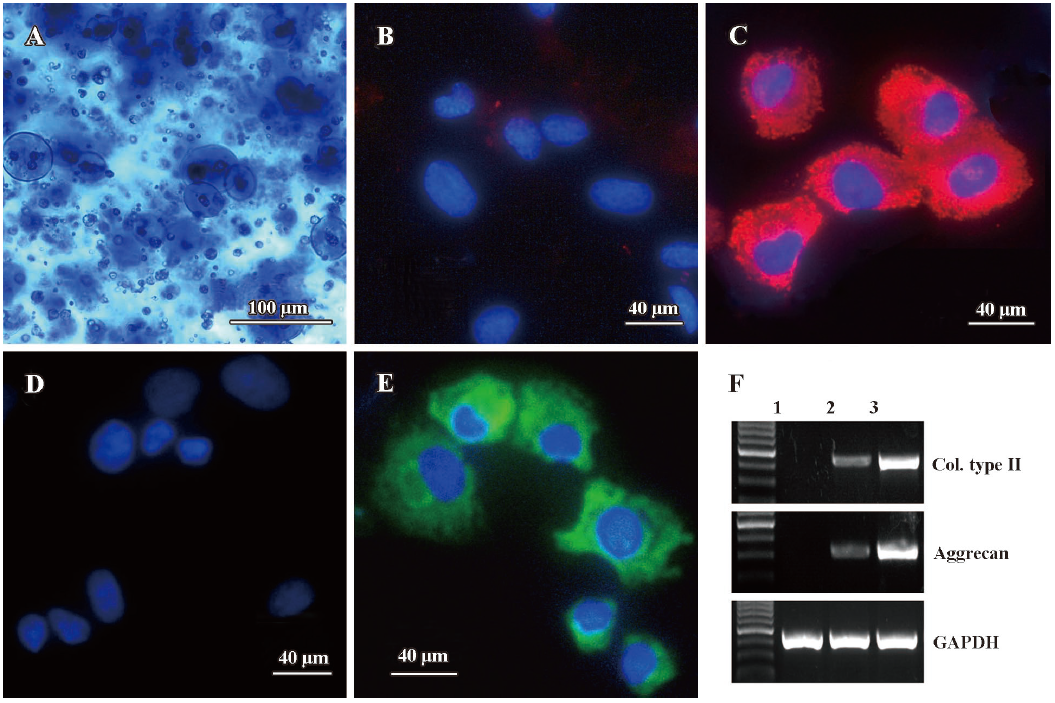
Chondrogenetic differentiation of MSC encapsulated in alginate beads To determine whether TGF-β3 combined with IGF-I had an effect on the chondrogenetic differentiation of MSC, we first measured the pericellular matrix formation using toluidine blue staining. Collagen type II and aggrecan expressions were evaluated using immunostaining and RT-PCR assays, respectively. As shown in Figure 1, at the end of the 3 week culture, MSCs encapsulated in alginate beads under a chondrogenetic medium produced abundant pericellular matrix which was circumscribed by the alginate hydrogel (Figure 1A). When compared with the control groups (Figure 1B, 1D, MSCs were encapsulated in alginate beads without the chondrogenic medium), MSC chondrogenesis was confirmed by intense collagen type II (Figure 1C) and aggrecan expression (Figure 1E). In addition, RT-PCR results showed similar mRNA level changes of collagen type II and aggrecan (Figure 1F).
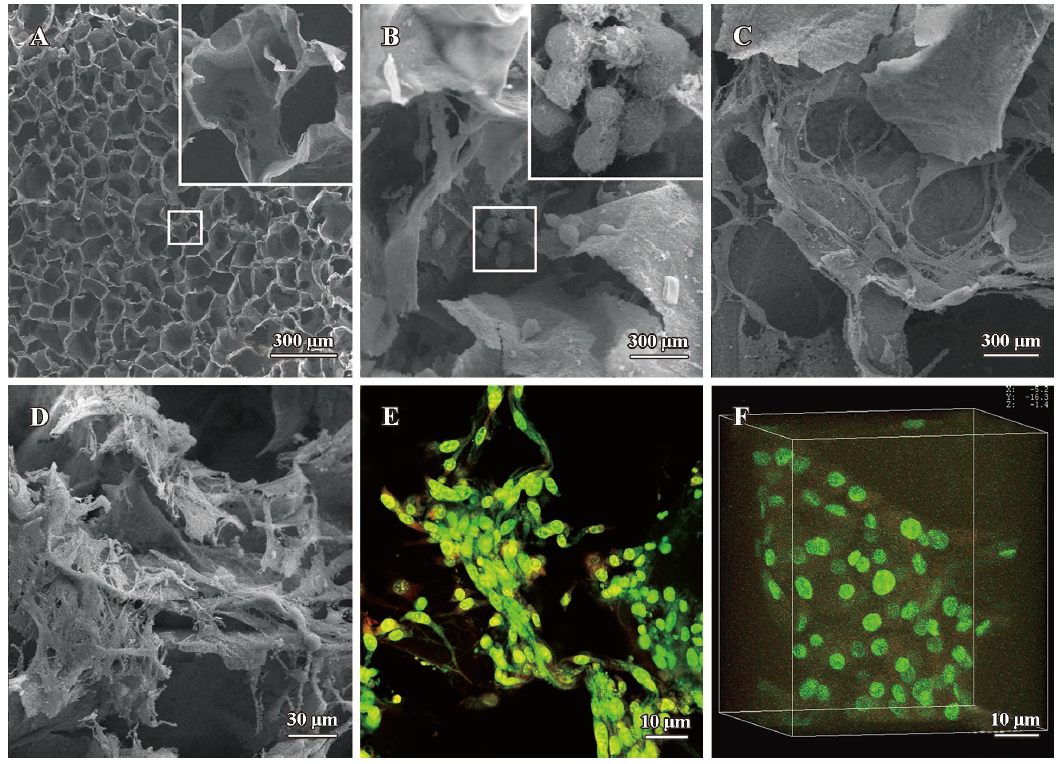
Evaluation of MSC derived chondrocytes cultured on the CGS Morphology of the MSC-derived chondrocytes cultured on the CGS for 3 weeks was evaluated by SEM and LCM (Figure 2). The blank scaffold exhibited a honeycombed structure and had a smooth surface. Pore diameter ranged from 50 µm to 200 µm. Average pore size was about 100 µm (Figure 2A). On d 7, the round-shaped MSC-derived chondrocytes congregated and attached to the scaffold surfaces. In addition, there was some extracellular matrix-like material adherent to the cell surface (Figure 2B). On d 14 the MSC derived chondrocytes transformed from a round shape into a polygon. Cells proliferated, spread out and migrated around (Figure 2C). On d 21, the number of MSC-derived chondrocytes noticeably increased, and then entwined together to form a mesh (Figure 2D). At the end of the third week LCM images showed similar 2D (Figure 2E) and 3D (Figure 2F) chondrocyte morphology on the scaffolds.
Effects of resveratrol and β1 integrin blocking antibody on IL-1β induced collagen type II, MMP-13 expression and NF-κB translocation To examine the effects of resveratrol on IL-1β induced MSC-derived chondrocytes when cultured on CGS, we first evaluated collagen type II, aggrecan and MMP-13 expression, respectively. As shown in Figure 3, compared with the control groups (lane 1), collagen type II expression decreased (Figure 3A) while expression of aggrecan (Figure 3B) and MMP-13 (Figure 3C) were not significantly affected by resveratrol alone (lane 2). Stimulation with IL-1β (lane 3) resulted in a significant downregulation of collagen type II and aggrecan, whereas MMP-13 expression increased. However, resveratrol reversed the effects of IL-1β-induced collagen type II, aggrecan and MMP-13 (lane 4) expression.
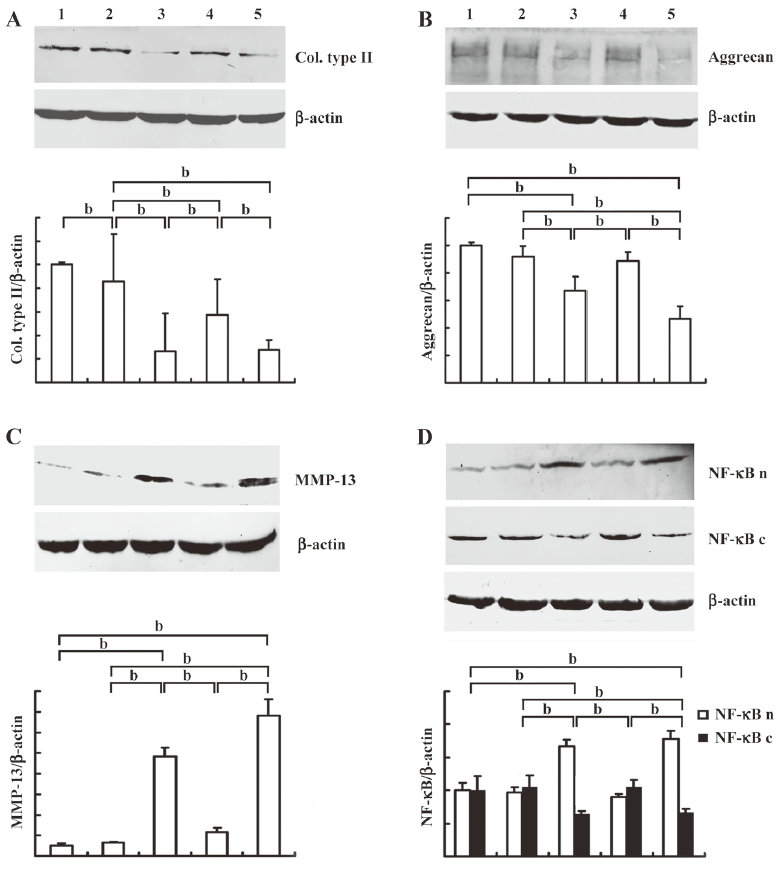
Next, to investigate the mechanism of resveratrol, we evaluated the cytosolic and nuclear fraction of NF-κB, respectively. As shown in Figure 3D, compared with the control groups (lane 1), the nuclear translocation of NF-κB was not significantly affected by resveratrol (lane 2). When stimulated with IL-1β (lane 3), there was a significant shift of NF-κB from the cytoplasm to the nucleus. However, co-treatment with resveratrol and IL-1β resulted in an increase in cytosolic NF-κB and a decrease in nuclear NF-κB.
We subsequently investigated the role of β1-integrin in the effects of resveratrol on IL-1β-stimulated chondrocytes. We observed that the specific β1-integrin blocking antibody (lane 5) abrogated the effects of resveratrol on IL-1β-induced collagen type II, aggrecan and MMP-13 expression. Furthermore, the nuclear fraction of NF-κB increased, while the cytosolic fraction decreased (P<0.05).
Discussion
Despite there being reports on the protective efficacy of resveratrol either in an experimental animal OA model[8] or in the pro-inflammatory cytokine stimulated chondrocytes[9,10], the mechanism of resveratrol in protecting chondrocytes is not fully understood. Furthermore, applying tissue engineered cartilage under an inflammatory environment has been seldom reported. The study results did confirm that IL-1β both suppressed anabolism and induced catabolism in the differentiated chondrocytes derived from MSCs. As further documented, resveratrol reversed the IL-1β effects on these chondrocytes by inhibiting the NF-κB nuclear translocation. However, blocking the β1-integrin abrogated the effects of resveratrol. In this complicated process, integrin signalling played a pivotal role in chondrocyte response to IL-1β.
Because of cartilage’s slow turnover and inability to self-repair, stem cell-based chondrogenesis that provides a sufficient quantity of cells is vital in cartilage tissue engineering. In the first part of our experiment we used a three dimensional culture with a chondrogenic medium to induce MSC differentiation into chondrocytes. An abundant pericellular matrix and intense collagen type II and aggrecan expressions suggested that MSC encapsulation in alginate beads induced by the chondrogenic medium was an ideal method for chondrogenesis. Additionally, chitosan and its derivatives, the biocompatible and biodegradable polymers, have been reported to increase articular chondrocyte density after joint injection[11], as well as maintain the chondrogenic phenotype in vitro[12]. Our data indicated that CGS contributed to the attachment, proliferation, migration and ECM formation of MSC-derived chondrocytes and these cell-CGS were suitable biomaterial for cartilage tissue engineering. Or, the constructs could be stimulated with pro-inflammatory factors to investigate arthritic cartilage function in an inflammatory environment, which was important for tissue engineered cartilage in clinical application.
A primary impetus for research on resveratrol was initiated from the so-called French paradox: a low incidence of cardiovascular diseases co-exists with a high-fat diet intake and moderate red wine consumption. A more recent review provided interesting insights into its potential as an anti-aging agent in treating age-related human diseases[4]. Previous studies have also shown that NF-κB was essential for the expression of numerous genes such as iNOS, MMP-1, MMP-13 and collagen type II in response to inflammatory IL-1[13,14]. We demonstrated that when MSC-derived chondrocytes were stimulated by IL-1β alone or co-incubated with resveratrol, collagen type II, aggrecan and MMP-13 expression changes were correlated with nuclear translocation of NF-κB. This result suggests that NF-κB activation, initiated by IL-1β stimulation, could play a pivotal role in chondrocyte function. Moreover, IL-1β induced catabolic effects, including collagen type II expression inhibition, MMP-13 biosynthesis and NF-κB nuclear translocation, were reversed by resveratrol. Consistent with our results, Shakibaei and Csaki observed that resveratrol inhibited IL-1β-induced caspase-3 stimulation, poly ADP ribose polymerase (PARP) cleavage, reactive oxygen species (ROS) production, and p53-dependent apoptosis of human chondrocytes in a time- or dose-dependent manner[9,10]. In another study, NF-κB was required in resveratrol’s protective effects[15].
Similarly, the loss of chondrocyte survival signals contributes to OA articular cartilage degradation. Further, integrin signalling has been implicated in the effects of ECM anchorage signals on cell survival, both as cell adhesion receptors and as intracellular signalling receptors. This ubiquitous system, through phosphorylation and consequent activation of a cascade of proteins that modulate gene transcription, regulates essential cellular functions such as growth and differentiation[16]. Blocking β1 integrin inhibited both DNA and proteoglycan synthesis of chondrocyte response to TGF-β1[17]. In addition to mediating cell matrix interactions, integrins were also involved in regulating OA inflammatory mediators. Increased β1-integrin expression was observed in OA cartilage[18]. Other studies suggested that in OA cartilage, integrins were involved in the upregulation or downregulation of several inflammatory mediators including IL-1β via receptor interactions[19]. The point of salience is that although these important links have been found, the regulatory mechanism of β1-integrin-mediated chondrocyte-ECM interaction in OA cartilage has yet to be explored.
When the cell-CGS constructs were co-treated with IL-1β and resveratrol, the protective effects of resveratrol were abrogated by the specific β1-integrin blocking antibody. In addition, the elevated collagen type II and aggrecan expression was reversed and MMP-13 expression increased. Meanwhile, NF-κB nuclear translocation was reversed. Since inhibition of cell-matrix interactions leads to chondrocyte apoptosis[22], interactions between the extracellular cartilage matrix and chondrocytes are important for the proliferation, differentiation, and cell survival[20-22]. Cell-matrix interactions are primarily mediated via multifunctional β1 integrin[22], which organize cell surface mechanoreceptor complexes[23] and functioned as signal transduction molecules[24] that stimulate MAPK pathways[20]. The decreased expression of signalling proteins might be a primary process of reduced cell-matrix interactions and β1-integrin seems to be a marker for synthetic activity as the chondrocytes try to generate ECM suitable for their survival. Therefore, our study explanation could be the following: when IL-1β, via activation of NF-κB, induced the catabolic effects of collagen type II and aggrecan degradation, along with MMP-13 synthesis, the β1 integrin cellular survival signalling pathway was also compromised. After resveratrol reversed NF-κB nuclear translocation and upregulated β1-integrin expression, the cell-matrix interactions between MSC-derived chondrocytes, ECM and CGS functioned via the β1 integrin receptor as a signal transduction molecule. Next, essential cellular survival signalling was modulated through phosphorylation and consequent activation of a MAPK cascade. When this survival signalling was blocked, the effects of resveratrol were cancelled and the imbalance between matrix component biosynthesis and degradation persisted.
In conclusion, our results indicate that resveratrol, a polyphenolic compound, could be used as a natural NF-κB inhibitor to protect chondrocytes from the deleterious effects of pro-inflammatory cytokines. In addition, this mechanism might be involved in the β1 integrin receptor signalling pathway.
References
- Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res 2004.S37-46.
- Kühn K, D'Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage 2004;12:1-16.
- Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 2006;14:839-48.
- Saiko P, Szakmary A, Jaeger W, Szekeres T. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res 2008;658:68-94.
- Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis 2006;27:1465-74.
- Shaw A, Xu Q. Biomechanical stress-induced signaling in smooth muscle cells: an update. Curr Vasc Pharmacol 2003;1:41-58.
- Ahmed N, Dreier R, Göpferich A, Grifka J, Grässel S. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem 2007;20:665-78.
- Elmali N, Esenkaya I, Harma A, Ertem K, Turkoz Y, Mizrak B. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm Res 2005;54:158-62.
- Shakibaei M, John T, Seifarth C, Mobasheri A. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann NY Acad Sci 2007;1095:554-63.
- Csaki C, Keshishzadeh N, Fischer K, Shakibaei M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol 2008;75:677-87.
- Lu JX, Prudhommeaux F, Meunier A, Sedel L, Guillemin G. Effects of chitosan on rat knee cartilages. Biomaterials 1999;20:1937-44.
- Donati I, Stredanska S, Silvestrini G, Vetere A, Marcon P, Marsich E, et al. The aggregation of pig articular chondrocyte and synthesis of extracellular matrix by a lactose-modified chitosan. Biomaterials 2005;26:987-98.
- Martin G, Bogdanowicz P, Domagala F, Ficheux H, Pujol JP. Rhein inhibits interleukin-1 beta-induced activation of MEK/ERK pathway and DNA binding of NF-kappa B and AP-1 in chondrocytes cultured in hypoxia: a potential mechanism for its disease-modifying effect in osteoarthritis. Inflammation 2003;27:233-46.
- Fahmi H, Di Battista JA, Pelletier JP, Mineau F, Ranger P, Martel-Pelletier J. Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-1beta-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum 2001;44:595-607.
- Kundu JK, Shin YK, Surh YJ. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as prime targets. Biochem Pharmacol 2006;72:1506-15.
- Goggs R, Carter SD, Schulze-Tanzil G, Shakibaei M, Mobasheri A. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. Vet J 2003;166:140-58.
- Lee JW, Qi WN, Scully SP. The involvement of beta1 integrin in the modulation by collagen of chondrocyte-response to transforming growth factor-beta1. J Orthop Res 2002;20:66-75.
- Loeser RF, Carlson CS, McGee MP. Expression of beta 1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp Cell Res 1995;217:248-57.
- Attur MG, Dave MN, Clancy RM, Patel IR, Abramson SB, Amin AR. Functional genomic analysis in arthritis-affected cartilage: yin-yang regulation of inflammatory mediators by alpha 5 beta 1 and alpha V beta 3 integrins. J Immunol 2000;164:2684-91.
- Shakibaei M, John T, De Souza P, Rahmanzadeh R, Merker HJ. Signal transduction by beta1 integrin receptors in human chondrocytes in vitro: collaboration with the insulin-like growth factor-I receptor. Biochem J 1999;342:615-23.
- Shakibaei M, Schulze-Tanzil G, de Souza P, John T, Rahmanzadeh M, Rahmanzadeh R, et al. Inhibition of mitogen-activated protein kinase kinase induces apoptosis of human chondrocytes. J Biol Chem 2001;276:13289-94.
- Cao L, Lee V, Adams ME, Kiani C, Zhang Y, Hu W, et al. beta-Integrin-collagen interaction reduces chondrocyte apoptosis. Matrix Biol 1999;18:343-55.
- Mobasheri A, Carter SD, Martín-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int 2002;26:1-18.
- Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J 1990;4:2868-80.
