Effects of CpG oligodeoxynucleotide on transcription factors GATA-3 and T-bet mRNA expression in asthmatic mice
Introduction
Asthma is a chronic airway inflammatory disease involving multiple inflammatory cells and cytokines. T-helper type 2 cells (Th2) are considered to play central role in the pathogenesis of asthma by producing a variety of cytokines such as interleukin (IL)-4, IL-5, IL-9, and IL-13[1,2]. These cytokines promote IgE synthesis and mediate airway inflammatory cells (such as eosinophil, mast cell, T lymphocyte and neutrophil) infiltration. On the other hand, T-helper type 1 cells (Th1) inhibit Th2 function by producing interferon (IFN)-γ. Recent studies show that bacterial DNA containing unmethylated CpG motifs, or immunostimulatory sequences (ISS), can induce strong Th1-polarized immune responses both in vivo[3] and in vitro[4,5]. The induction of Th1 responses is thought to result from the ability of ISS (containing CpG) to induce activation and secretion of IL-12 and IL-18 by macrophages and dendritic cells[6,7], which promote IFN-γ production and polarize T-helper type 0 cells (Th0) to Th1. Studies also show that T-box expressed in T cells (T-bet) and GATA binding protein 3 (GATA-3) are 2 T cell-specific transcription factors. T-bet promotes Th1 polarization while GATA-3 enhances Th2 polarization. In the present study, we investigated the effects of the CpG oligodeoxynucleotide (ODN) on the mRNA expression of transcription factors GATA-3 and T-bet in splenocytes and lung tissue in a mouse model of asthma.
Materials and methods
Mice and reagents Thirty-six male BALB/c mice (approxi-mately 6–10 weeks old, purchased from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences, Shanghai, China) were maintained in specific pathogen free (SPF) animal facility at Renji Hospital, Shanghai Second Medical University (Shanghai, China). The CpG ODN consisted of 20 bases containing 2 CpG motifs: TCCATGACGTT-CCTGACGTT was synthesized and purified by Sangon Biological Engineering and Technology (Shanghai, China), and ovalbumin (OVA) was purchased from Sigma (St Louis, MO, USA). IL-4 and IFN-γ enzyme-linked immunosorbent assay (ELISA) kits were purchased from JingMei Biotech (Shen-zhen, China). Trizol reagent was purchased from Invitrogen (Carlsbad, CA, USA). Oligo(dT)18 primer and 2×Master Mix were purchased from MBI Fermentas (Hanover, MD, USA).
OVA sensitization and challenge Mice were divided into 3 groups: (i) group A, control group; (ii) group B, OVA-sensitized and -challenged; and (iii) group C, OVA-sensitized and challenged, CpG ODN-treated. Mice were sensitized and challenged with chicken OVA as described previously[8]. Briefly, on d 0, d 7, d 14, and d 21, all animals were injected with 0.2 mL phosphate-buffered saline (PBS) containing 100 µg OVA and 2 mg aluminum hydroxide (alum). On d 26, d 30, d 36, and d 37, animals were challenged with 2% OVA aerosol for 30 mins. Control mice (group A) received a saline-only aerosol.
Administration of CpG oligodeoxynucleotide On d 24, d 28, and d 32, mice in group C were injected with 0.1 mL PBS containing 50 µg CpG ODN. Mice were assessed for pulmonary cellular infiltration histopathology on d 38.
Cellular count in branchoalveolar lavage fluid and lung histology Bronchoalveolar lavage (BAL) was carried out as in Kline et al[9]. After euthanasia, the trachea was cannulated and the lungs underwent lavage 3 times with 0.5 mL PBS. The lavage samples were processed immediately for total and differential cell counts. BAL fluid (BALF; 10 µL) was used for counting total inflammatory cells. The residual BALF was centrifuged (10 min, 2000×g) and the supernatant was stored at -30 °C for cytokine detection. The precipitated cells were used to prepare cell slides, which were stained with hematoxylin and eosin (HE), and 200 cells were counted for differential cell counting. The right lung was preserved in liquid nitrogen for mRNA extraction, while the left lung was fixed with 10% formalin and embedded in paraffin. Sections 4-µm thick were used to prepare slides, which were stained with HE.
Measurement of cytokines Approximately 5×106 single suspended splenocytes were stimulated with 20 µg/mL OVA for 72 h, centrifuged (10 min, 2000×g) and the supernatant was preserved for cytokine detection. The concentrations of IL-4 and IFN-γ in BALF and splenocyte culture supernatants were determined by using ELISA kits according to the manufacturer’s recommendations.
Reverse transcription-polymerase chain reaction Total RNA was extracted from lung tissue and splenocytes using Trizol reagent, according to the manufacturer’s instructions. Reverse transcription (RT) was carried out with 2 μg of total RNA using an oligo (dT)18 primer, and polymerase chain reaction (PCR) was carried out using 2×Master Mix. The T-bet primer was designed according to Chakir et al[10], while the GATA-3 primer was designed according to Ise et al[11]. The primer sequences were as follows: β-actin, sense 5'-GTGGGC-CGCTCTAGGCACCA-3', antisense 5'-CGGTTGGCCTTAG GGTTCAGGGGG-3'; GATA-3, sense 5'-GAAGGCATCCA-GACCCGAAAC-3', antisense 5'-ACCCATGGCGGTGA-CCATGC-3'; T-bet, sense 5'-AACCAGTATC CTGGTCCCA-GC-3', antisense 5'-TGTC GCCACTGGAA GGATAG-3'. PCR were run for 35 cycles and the annealing temperatures were as follows: GATA-3 and β-actin, 55 °C; T-bet, 57 °C. Semiquantitative RT-PCR was carried out using β-actin as an internal control to normalize gene expression for the PCR templates. The PCR product sizes were as follows: T-bet, 436 bp; GATA-3, 255 bp; β-actin, 245 bp. PCR products were electrophoresed in 2% agrose gel and the net intensity of the corresponding bands was analysed using Tian-Neng gel image software (Shanghai Tian-neng Technology Corpora-tion, Shanghai, China).
Statistical analysis All data were expressed as mean± SD. Using Prism software, one-way ANOVA analysis was used to assess statistical significance between the different groups. P<0.05 was considered to be significant.
Results
CpG oligodeoxynucleotide reduced the total number of inflammatory cells and the percentage of eosinophils in branchoalveolar lavage fluid The total number of inflammatory cells and the percentage of eosinophils were (100.4±7.1)×105/mL and 43.3%±2.5%, respectively, in BALF from the asthma group of mice (group B). CpG ODN treatment reduced the total number of inflammatory cells and the percentage of eosinophils to (40.5±2.9)×105/mL and 10.2%±0.5%, respectively (Table 1).
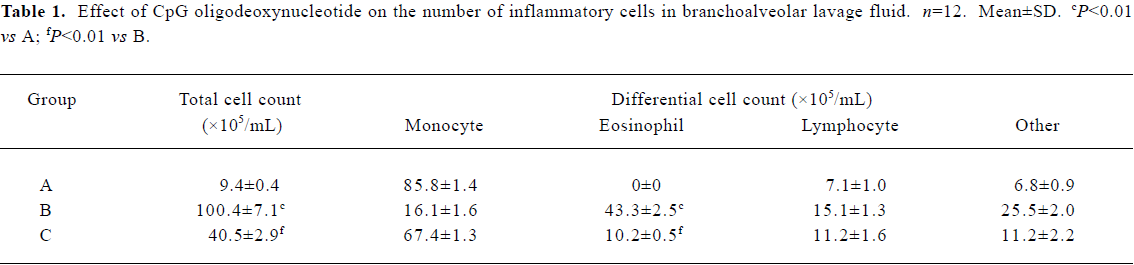
Full table
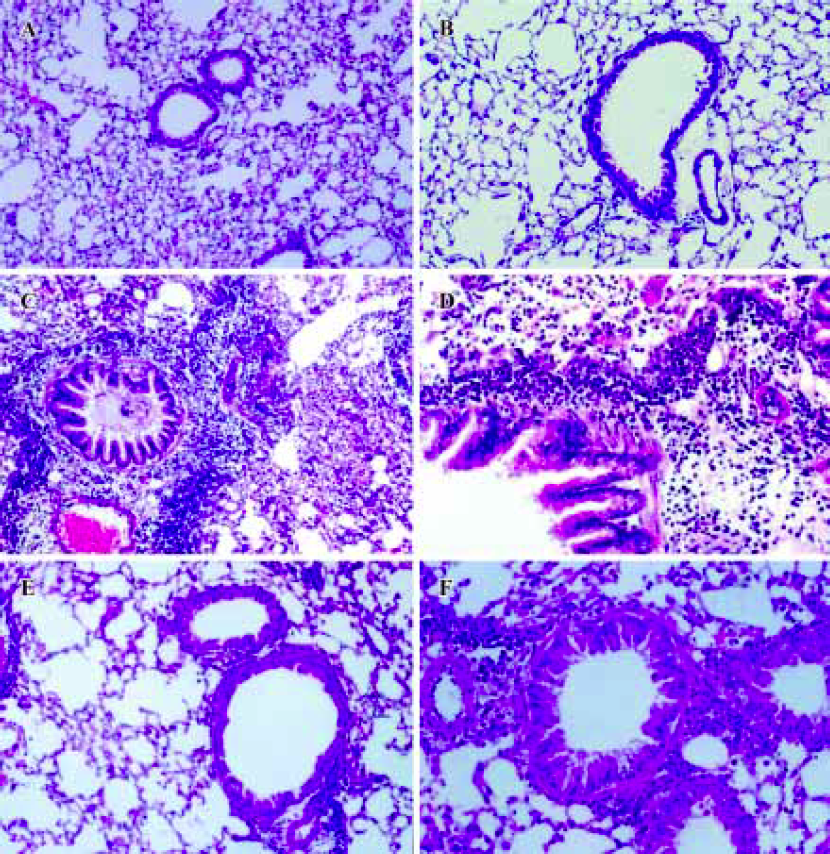
CpG oligodeoxynucleotide inhibited infiltration of airway inflammatory cells In asthma-group mice, lung pathological slides showed significant bronchial epithelial proliferation and shedding, bronchoconstriction and mucus production, as well as abundant inflammatory cell infiltration in the airway wall. In CpG ODN-treated mice, lung pathological slides showed that bronchoconstriction, airway inflammatory cell infiltration and mucus production were alleviated significantly, compared with asthma-group mice (Figure 1).
Effects of CpG oligodeoxynucleotide on the interferon-γ concentration and the interleukin-4 concentration Compared with the asthma group, IFN-γ was increased in the CpG ODN-treated group in both BALF and splenocyte culture supernatants, but IL-4 was reduced in both BALF and splenocyte culture supernatants (Table 2).
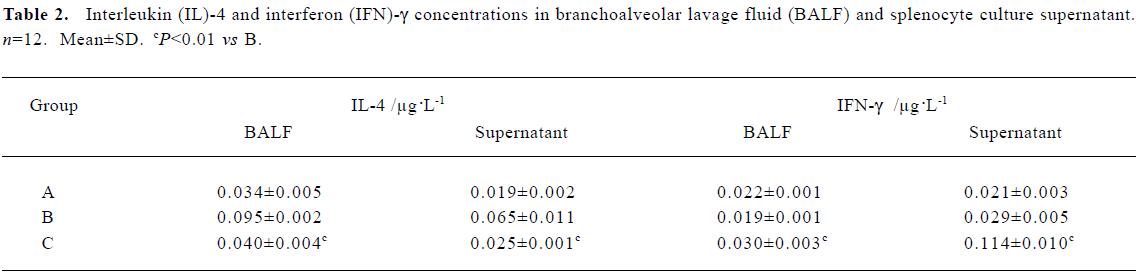
Full table
Effects of CpG oligodeoxynucleotide on GATA-3 and T-bet mRNA expression Compared with the asthma group, GATA-3 mRNA expression was reduced in splenocytes in the CpG ODN-treated group, although no difference was found in T-bet mRNA expression between the 2 groups. However, the mRNA ratio of T-bet to GATA-3 was increased in the CpG ODN-treated group compared with the asthma group (Figure 2). In lung tissue, CpG ODN treatment also decreased mRNA ratio of GATA-3 to β-actin compared with the asthma group (0.19±0.04 vs 0.35±0.04, P<0.02). T-bet mRNA was not detected under the same conditions.
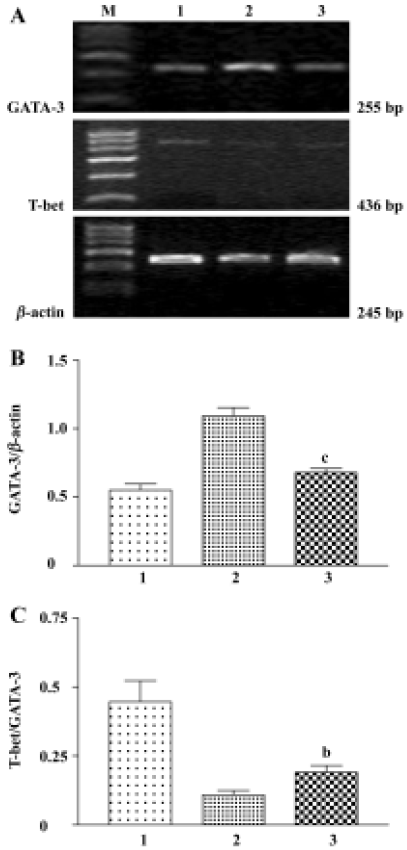
Discussion
Airway eosinophil infiltration is one of the most prominent characteristics of asthma[12–14]. In the present study, a typical asthmatic mouse model was established, as evidenced by increased total inflammatory cells, increased percentage of eosinophils in BALF, and obvious bronchoconstriction, mucus secretion, epithelial proliferation and shedding, and inflammatory cell infiltration in the lungs in the asthma group compared with the control group.
Prior studies have demonstrated that CpG ODN treatment inhibits asthmatic airway inflammation and increases Th1 cytokine IFN-γ while decreasing Th2 cytokine, IL-4 and IL-5 levels in asthmatic mouse models[15–17]. In the present study, our data showed that CpG ODN treatment reduced total inflammatory cells and the percentage of eosinophils in BALF when CpG ODN was administered (ip) after allergen sensitization. Lung tissue pathological slides showed that inflammatory cell infiltration were significantly alleviated in CpG ODN treated group compared with asthma group. The inhibitory effect of CpG ODN on lung inflammation was accompanied by increased IFN-γ levels but decreased IL-4 levels in both BALF and splenocyte culture supernatants. IL-4-producing Th2 cells are considered to play an important role in the immune reaction of local tissue inflammation[18]. Xie et al demonstrated that in a rat asthma model, the decrease in IFN-γ level was accompanied by an increase in IL-4 level, which resulted in a decreased IFN-γ/IL-4 ratio in the BALF after the sensitized rats were challenged with aerosol antigen[19]. These results suggest that the inhibitory effect of CpG ODN on airway inflammatory cell infiltration maybe the result of a downregulation of Th2 cytokine synthesis with an upregulation of Th1 cytokine synthesis.
Recent studies have shown that T-bet and GATA-3 are 2 major T helper-specific transcription factors that regulate the expression of Th1 and Th2 cytokine genes, and play a crucial role in T-helper cell differentiation. T-bet is thought to initiate Th1 development while inhibiting Th2 cell differentiation[20]. GATA-3 plays a pivotal role in the development of the Th2 phenotype while inhibiting Th1 cell differentiation[21]. Because asthma is a Th2-mediated airway inflammatory disease, both T-bet and GATA-3 may play an important role in the pathogenesis of asthma by directing Th0 differentiation. Finotto et al demonstrated that expression of T-bet in T cells from airways of patients with asthma was reduced compared with that in T cells from airways of non-asthmatic patients, suggesting that loss of T-bet might be associated with asthma[22]. Nakamura et al demonstrated that GATA-3 mRNA expression was increased significantly in the airways of asthmatic subjects and the number of cells expressing GATA-3 mRNA correlated significantly with reduced airway caliber and airway hyperresponsiveness[23]. Furthermore, GATA-3 mRNA-positive cells correlate significantly with the number of cells expressing IL-5 mRNA, and double in situ hybridization demonstrates that approximately 76% of GATA-3 mRNA-positive cells coexpress IL-5 mRNA and 91% of IL-5 mRNA-positive cells coexpress GATA-3 mRNA[23]. Blockade of GATA-3 mRNA expression by GATA-3 antisense oligonucleotides in lung tissue significantly inhibits Th2 cytokine production and airway inflammatory cells infiltration in an asthmatic mouse model[24].
Most studies have focused on the effect of CpG ODN on the Th1/Th2 cytokine balance in a murine model, but data regarding the effect of CpG ODN on the mRNA expression of transcription factors GATA-3 and T-bet have not been reported. In the present study, we first investigated the effects of CpG ODN on GATA-3 and T-bet mRNA expression in a mouse model of asthma. Our data showed that, compared with the asthma group, CpG ODN treatment reduced GATA-3 mRNA expression and increased the mRNA ratio of T-bet to GATA-3 in splenocytes, although no difference was found in T-bet mRNA expression between the 2 groups. In lung tissue, GATA-3 mRNA expression was also decreased in the CpG ODN-treated group compared with the asthma group. Chakir et al demonstrated that T-bet and GATA-3 gene expression reflected changes in Th1-specific cytokine IFN-γ and Th2-specific cytokine IL-4 in DO11.10 CD4+ T cells as well as in a mixed population of BBc rat splenocytes, and the T-bet/GATA-3 ratio reflects the Th1/Th2 differentiation status[10].
Our data demonstrated that CpG ODN downregulated GATA-3 mRNA expression both in lung tissue and in splenocytes, which may be an important mechanism for reducing Th2 cytokine synthesis in asthmatic mouse model. Moreover, as the mRNA ratio of T-bet to GATA-3 in spleno-cytes was increased in the CpG ODN-treated group compared with the asthma group, CpG ODN may also regulate T cell differential status by promoting Th0 differentiation to Th1. In summary, our data have shown that reducing GATA-3 mRNA expression both in lung tissue and in splenocytes may be an important mechanism for CpG ODN to regulate Th1/Th2 cytokine synthesis, thereby alleviating airway eosinophilia in an asthmatic mouse model.
Acknowledgement
We thank Dr Yi-hong HU’s help in experimental work from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China.
References
- Tournoy KG, Kips JC, Pauwels RA. The allergen-induced airway hyperresponsiveness in a human-mouse chimera model of asthma is T cell and IL-4 and IL-5 dependent. J Immunol 2001;166:6982-91.
- Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperrespon-siveness. J Exp Med 1998;188:1307-20.
- Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of T-helper type 2 cell and airway eosinophilia by transmucosal coadministration of antigen and oligodeoxynucleotides containing CpG motifs. Am J Respir Cell Mol Biol 2000;22:176-82.
- Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med 1998;188:2335-42.
- Sun S, Kishimoto H, Sprent J. DNA as an adjuvant: capacity of insect DNA and synthetic oligodeoxynucleotides to augment T cell responses to specific antigen. J Exp Med 1998;187:1145-50.
- Hartmann G, Weiner GJ, Krieg AM. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci USA 1999;96:9305-10.
- Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol 1999;73:329-68.
- Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, et al. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol 1998;161:7054-62.
- Kline JN, Krieg AM, Waldschmidt TJ, Ballas ZK, Jain V, Businga TR. CpG oligodeoxynucleotides do not require TH1 cytokines to prevent eosinophilic airway inflammation in a murine model of asthma. J Allergy Clin Immunol 1999;104:1258-64.
- Chakir H, Wang H, Lefebvre DE, Webb J, Scott FW. T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine profile in mixed cell populations: predominant role of GATA-3. J Immunol Methods 2003;278:157-69.
- Ise W, Totsuka M, Sogawa Y, Ametani A, Hachimura S, Sato T, et al. Naive CD4+ T cells exhibit distinct expression patterns of cytokines and cell surface molecules on their primary responses to varying doses of antigen. J Immunol 2002;168:3242-50.
- Kang H, Wei EQ, Yang XH, Zhang WP, Shen JZ. VCAM-1 expression, eosinophil infiltration, and pharmacological modulation in rat allergic airway inflammation. Acta Pharmacol Sin 2002;23:157-61.
- Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004;305:1773-6.
- Zhang YJ, Zhang L, Wang SB, Shen HH, Wei EQ. Montelukast modulates lung CysLT(1) receptor expression and eosinophilic inflammation in asthmatic mice. Acta Pharmacol Sin 2004;25:1341-6.
- Broide DH, Stachnick G, Castaneda D, Nayar J, Miller M, Cho JY, et al. Systemic administration of immunostimulatory DNA sequences mediates reversible inhibition of Th2 responses in a mouse model of asthma. J Clin Immunol 2001;21:175-82.
- Serebrisky D, Teper AA, Huang CK, Lee SY, Zhang TF, Schofield BH, et al. CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. J Immunol 2000;165:5906-12.
- Santeliz JV, Van Nest G, Traquina P, Larsen E, Wills-Karp M. Amb a 1-linked CpG oligodeoxynucleotides reverse established airway hyperresponsiveness in a murine model of asthma. J Allergy Clin Immunol 2002;109:455-62.
- Muller KM, Jaunin F, Masouye I, Saurat JH, Hauser C. Th2 cells mediate IL-4-dependent local tissue inflammation. J Immunol 1993;150:5576-84.
- Xie QM, Chen JQ, Shen WH, Bian RL. Correlative changes of interferon-gamma and interleukin-4 between cortical layer and pulmonary airway of sensitized rats. Acta Pharmacol Sin 2002;23:248-52.
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000;100:655-69.
- Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem 1997;272:21597-603.
- Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science 2002;295:336-8.
- Nakamura Y, Ghaffar O, Olivenstein R, Taha RA, Soussi-Gounni A, Zhang DH, et al. Gene expression of the GATA-3 transcription factor is increased in atopic asthma. J Allergy Clin Immunol 1999;103:215-22.
- Finotto S, De Sanctis GT, Lehr HA, Herz U, Buerke M, Schipp M, et al. Treatment of allergic airway inflammation and hyperresponsiveness by antisense-induced local blockade of GATA-3 expression. J Exp Med 2001;193:1247-60.
