Association of polymorphisms in low-density lipoprotein receptor-related protein 5 gene with bone mineral density in postmenopausal Chinese women1
Introduction
Osteoporosis is characterized by a decrease in bone mass as well as a deterioration of the bone architecture, resulting in an increased risk of fracture. The disease is multifactorial, and it depends on environmental and genetic factors. Twin studies have shown that genetic factors account for 60%–80% of the variance in bone mineral density (BMD)[1–3], which is the best predictor of the risk of osteoporosis. Several candidate genes that may contribute to BMD have been identified. Vitamin D receptor (VDR), estrogen receptor-alpha (ER-alpha), and collagen type I alpha 1 (COL1A1) genes are three important candidate genes that could potentially regulate BMD. Association and linkage studies have been performed in order to identify these candidate genes in the pathogenesis of osteoporosis[4–8]. However, their effect on the variation of BMD in the general population is controversial[9–11].
Recently, osteoporosis-pseudoglioma (OPPG), an autosomal recessive disease characterized by low bone mass, childhood fractures and abnormal eye development, has been shown to be due to an inherited loss of function of the gene for low-density lipoprotein receptor related protein 5 (LRP5)[12]. Moreover, two independent studies have suggested that a mutation (G171V) in the LRP5 gene is associated with high bone mass (HBM)[13,14]. In addition to the above mentioned G171V mutation in the LRP5 gene, Gong et al[12] identified three potential disease-associated missense mutations in regions encoding the LRP5 extracellular domain: R494Q, R570W and V667M. More recently, mutation analysis have identified seven novel sequence variants in the human LRP5 gene[15]. Two of them are missense mutations (c.314A>G: Q89R and c.4037C>T: A1330V). Although Koller et al[16] found the evidence that a quantitative trait locus (QTL) contributed to normal variation in BMD on chromosome 11q12–13 (the chromosome region where LRP5 is located), only a few studies have investigated the association between LRP5 gene polymorphisms and variation in BMD in the general population[17–20]. However, these studies yielded inconsistent reports of the association between candidate loci and BMD, and only small groups of subjects were studied in the Japanese and Korean studies. In the present study, we investigated the association between BMD and Q89R, A1330V, and N740N polymorphisms in the LRP5 gene in 647 postmenopausal Chinese women.
Materials and methods
Study population The study population comprised of 647 unrelated, postmenopausal, healthy volunteers aged 43–76 years (mean±SD, 60.1±6.3 years) living in Shanghai, China. All participants were of the Han ethnic group. The clinical data taken included questions on medical history, including medication, and a survey of the incidence of disease. A physical checkup was carried out on all subjects, and all were found to be in good health. No participant had medical complications or was undergoing treatment for conditions known to affect bone metabolism, such as hyperthy-roidism, diabetes mellitus, primary hyperparathyroidism, renal failure, pituitary and adrenal disease, or rheumatic disease. Postmenopausal women who had experienced early menopause (before 40 years of age) and those who had undergone ovariectomy or who were receiving estrogen replacement therapy were excluded. The study protocol was approved by the Committee on the Ethics of Human Research of Shanghai Jiaotong University Affiliated Sixth People’s Hospital.
BMD measurements A total of 647 subjects were measured for BMD. The BMD of the lumbar spine 1–4 (L1–4) and the left proximal femur including femoral neck, trochanter, and Ward’s triangle was measured by dual-energy X-ray aborptionmetry (DXA) on a Hologic QDR 2000 (Hologic, Bedford, MA, USA). The machine was calibrated daily, and the coefficient of variation (CV) values of the DXA measurements (which were obtained from 7 individuals repeatedly measured 5 times) at L1–4, the femoral neck, trochanter, and Ward’s triangle were 0.9%, 1.93%, 1.48% and 2.85%, respectively[6,21]. The long-term reproducibility of our DXA data during the trial based on weekly repeated phantom measurements was 0.45%.
Genotyping DNA was isolated from peripheral blood leukocytes using conventional methods. Using the methods described by Okubo et al[15], we genotyped subjects with the LRP5 Q89R, N780N and A1330V polymorphisms. Genomic DNA (0.1 µg) was carried out in 30 µL buffer solution (10 mmol/L Tris-HCL, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 200 µmol/L of each of the four deoxyribonucleotides [dNTPs], 2.5 U of Tag polymerase, and 0.25 µmol/L of each primer). The polymerase chain reaction (PCR) was performed by using the following steps: 94 oC for 5 min and then 94 oC for 1 min, 56 oC for 1 min, 72 oC for 1 min, for 30 cycles, and 72 oC for 7 min. The PCR primers and restriction endonucleases used for genotyping are summarized in Table 1. The PCR products were digested with AvaI I, Ase I, and DraIII restriction endonucleases, respectively. The Q89R genotypes were separated by electrophoresis in 1.5% agarose gel. The QQ genotype produces a 436 bp fragment, the RR genotype produces 274 bp and 162 bp fragments, and the heterozygous QR genotype produces 436 bp, 274 bp and 162 bp fragments. The N740N and A1330V genotypes were separated by electrophoresis on 8.0% polyacrylamide gel electrophoresis gels. The N740N CC genotype produces a 237 bp fragment, the TT genotype produces 216 bp and 21 bp fragments, and heterozygous TC genotypes produce 237 bp, 216 bp and 21 bp fragments. The A1330V AA genotype produces a 143 bp fragment, the VV genotype produces 119 bp and 24 bp fragments, and the heterozygous AV genotype produces 143 bp, 119 bp and 24 bp fragments.
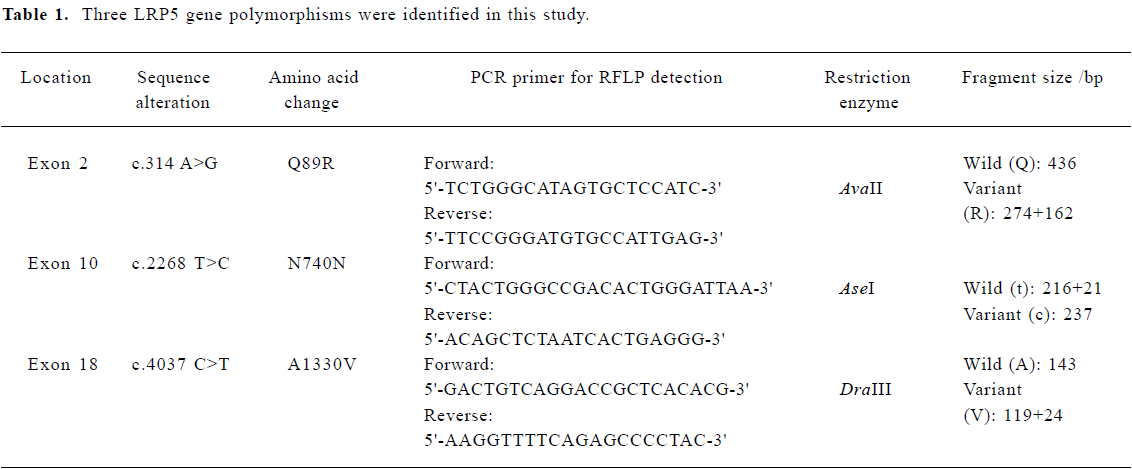
Full table
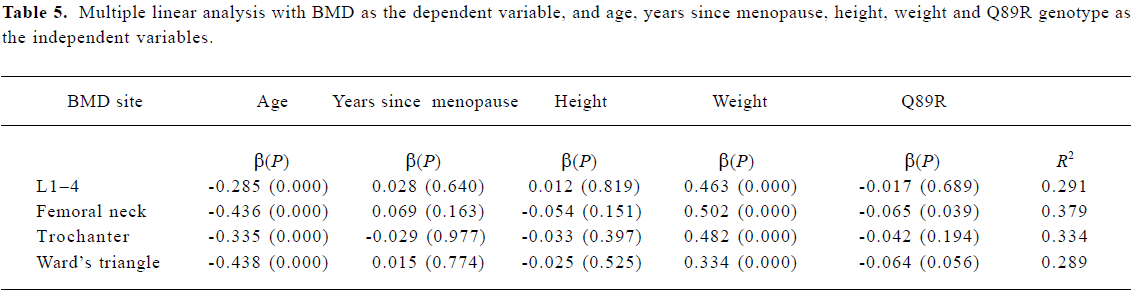
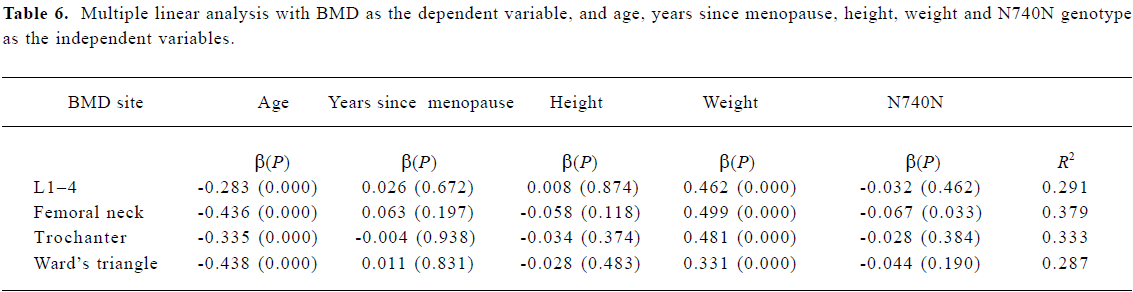
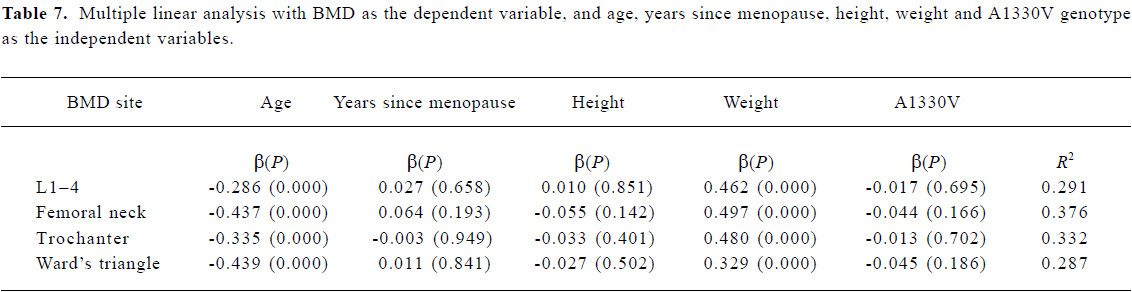
Statistical analysis Allele frequencies were estimated by using the gene counting method, and the chi-squared test was used to identify significant departures from the Hardy-Weinberg equilibrium and linkage disequilibrium between genotypes. The relationship between various LRP5 genotypes and BMD was analyzed by using the unpaired Student’s t-test. All associations were further evaluated using multiple linear regression analysis to adjust for risk factor. BMD was a dependent variable and the independent variables included age, height, weight, years since menopause, and LRP5 Q89R genotype (0=QQ, 1=QR+RR), N740N genotype (0=TT, 1=TC+CC), and A1330V genotype (0=AA, 1=AV+VV). P<0.05 was considered to be statistically significant. All statistical calculations were performed using the SPSS 9.0 program (SPSS, Chicago, IL, USA).
Results
Frequency distribution of LRP5 gene polymorphisms The genotype distribution and allele frequencies of polymorphisms of the LRP5 gene are shown in Table 2. Frequencies of the Q89R, N740N, and A1330V genotypes and alleles did not deviate from Hardy-Weinberg equilibrium. Strong linkage disequilibrium was found between Q89R and A1330V polymorphisms in our population (χ2=13.50, P<0.01; Table 3). However, no linkage disequilibrium was found between Q89R and N740N or between A1330V and N740N polymorphisms in these subjects.
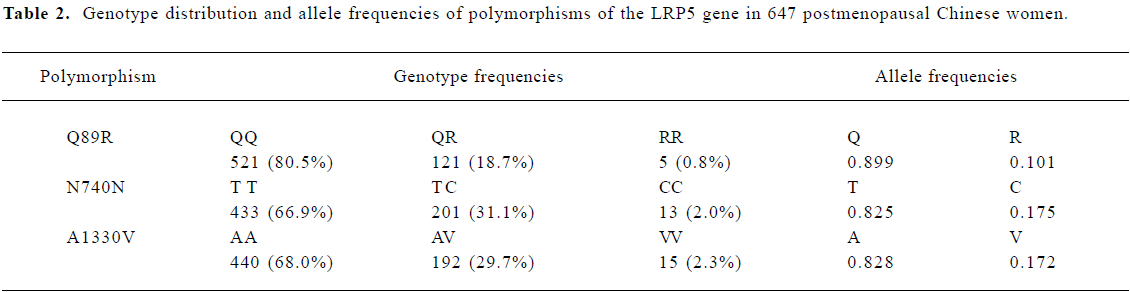
Full table
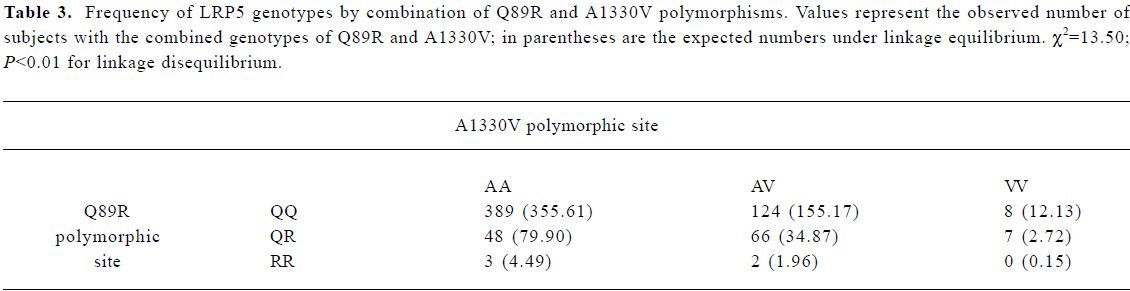
Full table
Association between BMD and LRP5 genotype Because the frequencies of the Q89R RR, N780N CC and A1330V VV genotypes were all very low, we compared the background parameters and BMD in the QQ and QR/RR, TT and TC/CC, and AA and AV/VV groups for further analysis. The association between BMD and LRP5 genotype was analyzed using the unpaired Student’s t-test (Table 4). BMD at the femoral neck was significantly higher in subjects with the Q89R QQ genotype than in the combined group with QR/RR genotypes (P<0.05). A similar finding was observed for the N740N genotype; that is, that subjects with the N740N TT genotype had significantly higher BMD at the femoral neck compared with those with TC/CC genotypes (P<0.05). Moreover, we further used multiple linear analysis to adjust for age, years since menopause, height, and weight. We found that BMD at the femoral neck was significantly associated with the Q89R polymorphism and the N740N polymorphism (P<0.05; Tables 5, 6). However, no significant association was found between the A1330V genotype and BMD at any site according to the unpaired Student’s t-test or multiple linear analysis (Tables 4, 7).
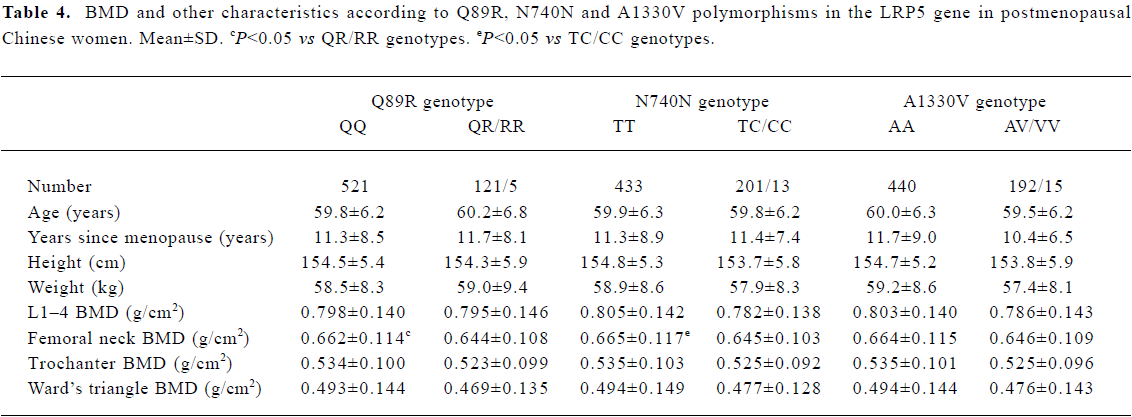
Full table
Full table
Full table
Full table
Discussion
In this cross-sectional study of postmenopausal Chinese women, we found that differences existed in the frequencies of the Q89R, N740N and A1330V LRP5 polymorphisms compared with frequencies in Caucasian people; in particular, the Q89R polymorphism is very rare in Caucasian people[22,23]. However, the frequencies of genotypes and alleles of Q89R, N740N and A1330V LRP5 polymorphisms in Chinese women are similar to those found in Japanese and Korean subjects[15,17]. We found that the prevalent frequencies of the Q, T and A alleles in Chinese women were 89.9%, 82.5%, and 82.8%, respectively, as compared with 92%, 81%, and 82%, respec-tively, in Japanese subjects; furthermore, the frequencies of the Q89R Q and A1330V A alleles were 92% and 85% in Korean subjects. Therefore, LRP5, similar to other candidate genes (eg, VDR and COL1A1) had significantly different frequencies of genotypes and alleles in various ethnic groups[9,24].
In the present study, a significant association was observed between the Q89R genotype or the N740N genotype and BMD at the femoral neck both before and after adjusting for confounding factors, and subjects with the QQ genotype or the TT genotype had significantly higher BMD. Our findings are consistant with those of Koh et al[17], who recently reported that the Q89R polymorphism was significantly associated with BMD at the femoral neck and Ward’s triangle in 219 young Korean men. After adjusting for age, weight, and height, a marginal association was observed at the femoral neck (P=0.098). However, the N740N polymorphism was not investigated in Korean men. The Q89R and A1330V polymorphisms are located in exon 2 and exon 18 in the LRP5 gene, respectively. The Q89R and A1330V polymorphisms were in linkage disequilibrium in our study population. This finding is similar to that for a Korean population[17], but differs from that for a European population[22]. Although the A1330V polymorphism is a functional mutation, no significant association was observed between the A1330V polymorphism and BMD either in postmenopausal Chinese women or in young Korean men. Similarly, Ferrari et al[18] failed to find a significant association between c.4037C>T (A1330V) polymorphism and lumbar bone mineral content and bone area in 889 healthy Caucasian people of both sexes.
LRP5 is a single pass membrane receptor whose extracellular domain contains four modules consisting of six YWTD repeats followed by an epidermal growth factor (EGF)-like motif and an LDLR-like ligand-binding domain[25,26]. Recent studies have shown that LRP5 and its closely related LRP family member, LRP6, are Wnt co-receptors that are capable of interacting with several key components of the Wnt pathway, and research regarding the signaling mechanisms involved in bone regulation by LRP5 has focused on this pathway[27–29]. The LRP5 gene has 23 exons. In addition to the G171V substitution in HBM, nine disease-causing mutations in exons encoding the LRP5 extracellular domain have been identified in patients with OPPG[12,13]. We found that the Q89R and N740N polymorphisms were only associated with BMD at the femoral neck, and were not associated with BMD at the lumbar spine in this large group of postmenopausal Chinese women, which suggests that the Q89R and N740N polymorphisms can influence the attainment of peak bone mass. Although the molecular mechanisms that underlie the association of the Q89R and N740N polymorphisms of the LRP5 gene with BMD remain unclear, we consider that the Q89R and N740N or related linked polymorphisms in the region might alter LRP5 protein function and might be associated with BMD.
In conclusion, we found a significant association between the Q89R and N740N polymorphisms in the LRP5 gene and BMD at the femoral neck in postmenopausal Chinese women, but we failed to observe a significant association between the A1330V polymorphism and BMD at any site. Our findings suggest that the LRP5 gene is a candidate for the genetic determination of BMD in postmenopausal Chinese women. Further studies will be needed to determine an association between the LRP5 gene polymorphisms and the risk of osteoporosis in the general population.
References
- Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Bone mass, lean mass and fat mass: same genes or same environments. Am J Epidemiol 1998;147:3-16.
- Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S. Genetic determinants of bone mass in adults: a twin study. J Clin Invest 1987;80:706-10.
- Hopper JL, Green RM, Nowson CA, Young D, Sherwin AJ, Kaymakci B, et al. Genetic, common environment, and individual specific components of variance for bone mineral density in 10- to 26-year-old females: a twin study. Am J Epidemiol 1998;147:17-29.
- Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, et al. Prediction of bone density by vitamin D receptor alleles. Nature 1997;367:284-7.
- Grant SF, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH. Reduced bone density and osteoporotic fracture associated with a polymorphism Sp1 binding site in the collagen type I 1 gene. Nat Genet 1996;14:203-5.
- Zhang ZL, Qin YJ, Huang QR, He JW, Li M, Hu YQ, et al. Association of estrogen receptor-alpha and vitamin D receptor genotypes with therapeutic response to calcium in postmenopausal Chinese women. Acta Pharmacol Sin 2004;25:1690-7.
- Qin YJ, Zhang ZL, Huang QR, He JW, Hu YQ, Zhou Q, et al. Association of vitamin D receptor and estrogen receptor- gene polymorphism and peak bone mass and bone size in Chinese women. Acta Pharmacol Sin 2004;25:462-8.
- Kobayashi S, Inoue S, Hosoi T, Ouchi Y, Shiraki M, Orimo H. Association of bone mineral density with polymorphism of the estrogen gene. J Bone Mineral Res 1996;11:306-11.
- Gong G, Haynatzki G. Association between bone mineral density and candidate genes in different ethnic populations and its implications. Calcif Tissue Int 2003;72:113-23.
- Han KO, Moon IG, Kang YS, Chung HY, Min HK, Han IK. Nonassociation of estrogen receptor genotypes with bone mineral density and estrogen responsiveness to hormone replacement therapy in Korean postmenopausal women. J Clin Endocrinol Metab 1997;82:991-5.
- Ioannidis JP, Stavrou I, Trikalinos TA, Zois C, Brandi ML, Gennari L, et al. Association of polymorphism of the estrogen receptor gene with bone mineral density and fracture risk in women: A meta-analysis. J Bone Miner Res 2002;17:2048-60.
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001;107:513-23.
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, et al. A mutation in the LDL receptor related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 2002;70:11-9.
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 2002;346:1513-21.
- Okubo M, Horinishi A, Kim DH, Yamamoto TT, Murase T. Seven novel sequence variants in the human low density lipoprotein receptor related protein 5 (LRP5) gene. Hum Mutat 2002;19:186.
- Koller DL, Rodriguez LA, Christian JC, Slemenda CW, Econs MJ, Hui SL, et al. Linkage of a QTL contribution to normal variation in bone mineral density to chromosome 11q12–13. J Bone Mineral Res 1998;13:1903-8.
- Koh JM, Jung MH, Hong JS, Park HJ, Chang JS, Shin HD, et al. Association between bone mineral density and LDL receptor-related protein 5 gene polymorphism in young Korean men. J Korean Med Sci 2004;19:407-12.
- Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, et al. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in Whites. Am J Hum Genet 2004;74:866-75.
- Urano T, Shiraki M, Ezura Y, Fujita M, Sekine E, Hoshino S, et al. Association of a single-nucleotide polymorphism in low-density lipoprotein receptor protein 5 gene with bone mineral density. J Bone Miner Metab 2004;22:341-5.
- Koay MA, Woon PY, Zhang Y, Miles LJ, Duncan EL, Ralston SH, et al. Influence of LRP5 polymorphisms on normal variation in BMD. J Bone Miner Res 2004;19:1619-27.
- Qin YJ, Shen H, Huang QR, Zhao LJ, Zhou Q, Li MX, et al. Estrogen receptor α gene polymorphism and peak bone density in Chinese nuclear families. J Bone Miner Res 2003;18:1028-35.
- Twells RC, Mein CA, Phillips MS, Hess JF, Veijola R, Gilbey M, et al. Haplotype structure, LD blocks, and uneven recombination within the LRP5 gene. Genome Res 2003;13:845-55.
- Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Benichou O, Scopelliti D, et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 2003;72:763-71.
- Lau EM, Young RP, Ho SC, Woo J, Kwok JL, Birjandi Z, et al. Vitamin D receptor gene polymorphisms and bone mineral density in elderly Chinese men and women in Hong Kong. Osteoporos Int 1999;10:226-30.
- Brown MS, Herz J, Goldstein JL. LDL-receptor structure: Calcium cages, acid baths and recycling receptors. Nature 1997;388:629-30.
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, et al. Arrow encodes and LDL-receptor-related protein essential for Wingless signalling. Nature 2000;407:527-30.
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, et al. LDL-receptor-related proteins in Win signal transduction. Nature 2000;407:530-5.
- Mao J, Wang J, Liu B, Pan W, Farr GH 3rd, Flynn C, et al. Low-density lipoprotein receptor-related protein-5 binds to axin and regulates the canonical Win signaling pathway. Mol Cell 2001;7:801-9.
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA 2nd, et al. Cbfa1-inderpendent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in LRP5, a Wnt coreceptor. J Cell Biol 2002;157:303-14.
