Different Na+/K+-ATPase signal pathways was involved in the increase of [Ca2+]i induced by strophanthidin in normal and failing isolated guinea pig ventricular myocytes
Introduction
Cardiotonic steroids (CS) increase the force of contraction and have been widely used for congestive heart failure (CHF) for more than 200 years. However, the mechanisms underlying its positive inotropic effects are still unknown. It is primarily believed that CS produce positive inotropic action in the failing heart via the inhibition of Na+/K+-ATPase activities, which leads to an increase in the intracellular Na+ concentration ([Na+]i). This in turn raises the intracellular Ca2+ concentration ([Ca2+]i) via reversing the mode of the Na+–Ca2+ exchanger, thus increasing the uptake and subsequent release of Ca2+ by the sarcoplasmic reticulum (SR)[1].
We observed an elevation of Na+/K+-ATPase activities by nanomolar concentrations of strophanthidin (Str; a CS) in both normal and failing isolated guinea pig hearts. [Ca2+]i increase and cardiac contractility was enhanced, thus the Na+/K+-ATPase inhibition theory may not properly explain the inotropic action of glycosides[2]. Although we observed that dihydroouabain (a CS) could increase [Ca2+]i in non-beating myocytes via stimulating the ICa-L and ICa, or directly triggering a release of [Ca2+]i using whole-cell patch-clamp and the confocal microscopy techniques[3], these experiments did not detect [Ca2+]i and contractility simultaneously, which still can not effectively explain the mechanisms of the inotropic actions of CS.
Recent studies have revealed several previously unknown effects of ouabain on cardiac myocytes. In addition to being the key regulator of intracellular Na+ and K+ concentrations, Na+/K+-ATPase may act as a signal transducer. Na+/K+-ATPase interacts with neighboring membrane proteins, organizes cytosolic cascades of signaling proteins from the plasma to the nucleus, and regulates cell growth and gene expression[4].
The binding of CS to Na+/K+-ATPase in cardiac myocytes results in the activation of 2 main signal transduction pathways. One is CS/Na+/K+-ATPase/Src/epidermal growth factor receptor (EGFR)/Ras/reactive oxygen species (ROS) cascade (ROS pathway). The other is CS/Na+/K+-ATPase/Src/EGFR/Ras/mitogen-activated protein kinase (MAPK) cascade (MAPK pathway; Figure 1A)[5]. Na+/K+-ATPase regulates cell growth and gene expression, and increases [Ca2+]i through these 2 signal transduction pathways. ROS and [Ca2+]i are essential second messengers. In addition, p42/44 MAPK can open the L-type Ca2+ channel to make the connection between the long-term regulation of cell growth and the short-term increase in [Ca2+]i[6,7]. So is the signal-transducing function of Na+/K+-ATPase that increases [Ca2+]i involved in mediating positive inotropic actions?
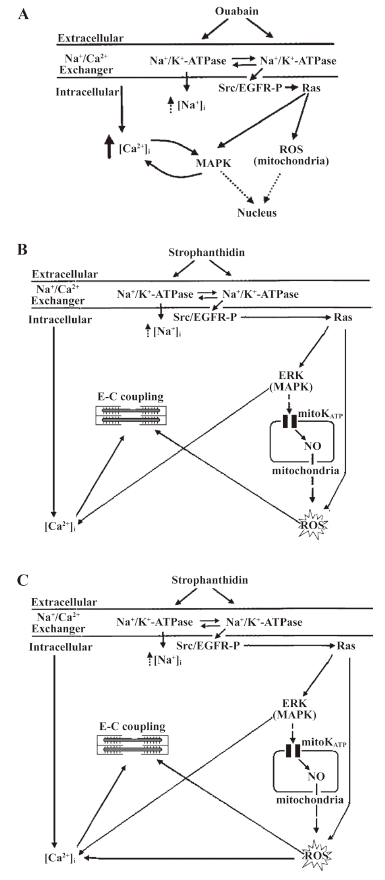
Recently, Xie et al reported that in neonatal and adult rat cardiac myocytes, ouabain at non-toxic concentrations caused a partial inhibition of Na+/K+-ATPase and increased [Ca2+]i through the MAPK pathway. They found that both [Ca2+]i and ROS work together to raise contractility in cardiac myocytes[8], but they did not do it in cardiac myocytes from failing hearts, and they did not detect the changes of [Ca2+]i and contractility simultaneously.
In the present study, the cell shortening and calcium transient induced by Str were simultaneously assessed in guinea pig normal ventricular myocytes (NC), or its CHF model (failing ventricular myocytes [FC]) with or without a special signaling inhibitor to explore if and how the MAPK and ROS signal transduction pathways of Na+/K+-ATPase are involved in the [Ca2+]i increase and the positive inotropic effects of Str, which will aid us to further understand the therapeutic mechanism of CS.
Materials and methods
Drugs and chemicals Str, Fura-2-AM, genistein, PD98059, and N-acetylcysteine (NAC) were purchased from Sigma (St Louis, MO, USA). Str, Fura-2-AM, genistein, and PD98059 were all dissolved in DMSO, and NAC was dissolved in water as stock solution. Collagenase type II was purchased from Worthing Biochemical Co (Lakewood, NJ, USA), and PP2 was purchased from Calbiochem (San Diego, California, USA). All other reagents were of analyticl reagent (AR) grade.
Development of a heart failure model in guinea pigs CHF was induced in adult male Charles River guinea pigs (250–300 g) by chronic pressure overload using constricting descending thoracic aorta as described[9]. The sham-operated animals underwent the same operation, except that the aorta was not constricted.
Preparation of left ventricular myocytes in guinea pigs The guinea pigs were killed by sodium pentobarbitone solution (1 mL of 360 mg/mL, intraperitoneally). Single left ventricular myocytes were prepared essentially as described by Wang et al[10] and resuspended in the Krebs’ solution supplemented with 2% bovine serum albumin. Ca2+ was slowly added to the cell suspension until [Ca2+]i reached a final concentration of 1.8 mmol/L. The cells were used within 16 h after isolation.
Measurement of cell shortening and [Ca2+]i transient The contraction and [Ca2+]i transient of ventricular myocytes were assessed by a video-based, motion-edge detection system (IonOptix, Milton, MA, USA)[11] simultaneously. Briefly, the myocytes were loaded with Fura-2-AM (0.5 mol/L) for 30 min in darkness at room temperature. Then the cells were placed in a chamber mounted onto the stage of an inverted microscope (Olympus, Tokyo, Japan) and superfused (1 mL/min, 25 ºC) with Tyrode’s solution. The cells were field stimulated at a frequency of 0.5 Hz at a duration of 5 ms using a pair of platinum electrodes placed on the opposite sides of the chamber. The myocyte being studied was displayed on the computer monitor imaged through a 40×objective using an IonOptix Myocam camera. At the same time, the fluorescence measurement was recorded by a dual-excitation fluorescence photomultiplier system (IonOptix, USA). The 340 nm excitation scan was repeated at the end of the protocol, and qualitative changes in the [Ca2+]i transient were inferred from the ratio of the fluorescence intensity at 340 and 380 nm wavelengths[12]. Under each experimental condition, ~30 single myocytes from 6 independent hearts were imaged.
Statistical analysis The baseline cell length and the fluorescence ratio before the drug administration were assigned a value of 100% to calculate the percentages of changes in cell shortening and the fluorescence ratio after drug administration. Values are presented as mean±SEM. Data analysis were performed with Origin software (OriginLab Corporation, Northampton, MA, USA). Data were compared by using paired t-test or unpaired Student’s t-test. Statistical significance was considered to be P<0.05.
Results
Effect of Str on contractile function in NC or FC The extent of cell shortening induced by Str was normalized using the value before Str perfusion as 100%. In NC, Str at 1, 10, and 25 µmol/L increased the extent of cell shortening by 135.72%±7.44%, 167.02%±15.84%, and 265.80%±32.65% in a concentration-dependent manner (r=0.989, P<0.05), respectively. Str at 0.1 µmol/L had no effect on cell shortening, and Str at 50 µmol/L caused the irregular cell contraction. The cells even became “round-up”. In FC, Str at 0.1, 1, 10, and 25 µmol/L also increased the extent of cell shortening by 137.83%±9.78%, 174.73%±18.98%, 226.18%±29.74%, and 423.18%±59.62% in a concentration-dependent manner (r=0.988, P<0.01), respectively. At the same concentration, increased degrees of cell shortening by Str in FC were much greater than those in NC (Figure 2A).
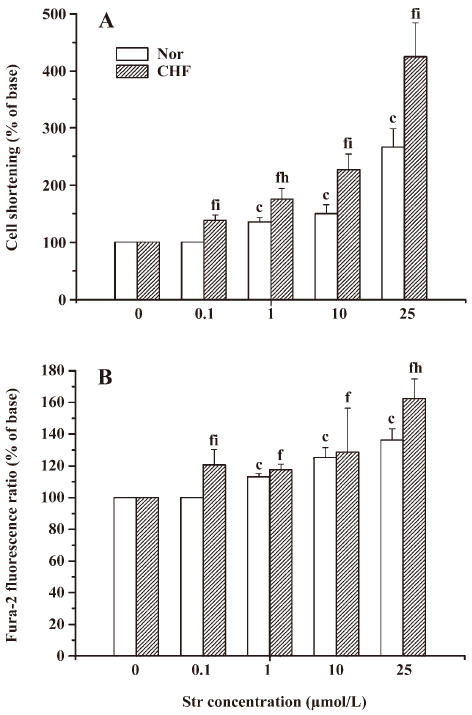
Effect of Str on the calcium transient in NC or in FC In order to study the relationship between the positive inotropic effects of Str and [Ca2+]i, the calcium transient was detected simultaneously along with cell shortening. In NC, Str at 1, 10, and 25 µmol/L concentration-dependently elevated the calcium transient by 113.04%±2.17%, 125.28%±6.22%, and 137.13%±7.31% (r=0.988, P<0.05), respectively. In FC, Str at 0.1, 1, 10, and 25 µmol/L increased the calcium transient by 120.60%±6.33%, 117.60%±3.64%, 128.59%±7.54%, and 162.30%±12.60%, also in a concentration-dependent manner (r=0.980, P<0.01). Str at 0.1 and 25 µmol/L resulted in a greater amplitude of the calcium transient in FC than in NC (P<0.01 and 0.05; Figure 2B). These findings suggest that the alternations of Str on cell shortening and [Ca2+]i are similar. We also found that the change of [Ca2+]i occurred before the change of cell shortening (data not shown), indicating that Ca2+ plays a key role in the cell shortening of NC and FC.
ROS pathway is involved in an increase in the positive inotropic effects induced by Str in NC The myocytes were pretreated with 100 µmol/L genistein (a Src kinase inhibitor), 10 µmol/L PD98059 (a MAPK kinase inhibitor), or 2.5 mmol/L NAC (a ROS scavenger) for 30 min, and 1 µmol/L PP2 (a specific Src kinase inhibitor) for 20 min, respectively, and exposed to 25 µmol/L Str for 10 min. The cell shortening and calcium transient were recorded. In NC, 25 µmol/L Str increased contractility by 198.47%±30.57%; genistein, PP2, and NAC decreased the effects of 25 µmol/L Str to 161.83%±17.69%, 163.12%±12.73%, and 158.97%±17.41% (P<0.05), respectively, but PD98059 did not (168.50%±14.76%, P>0.05; Figure 3A). These findings indicate that the ROS signal transduction pathway of Na+/K+-ATPase was involved in an increase in the positive inotropic effects induced by Str in NC (Figure 1B).
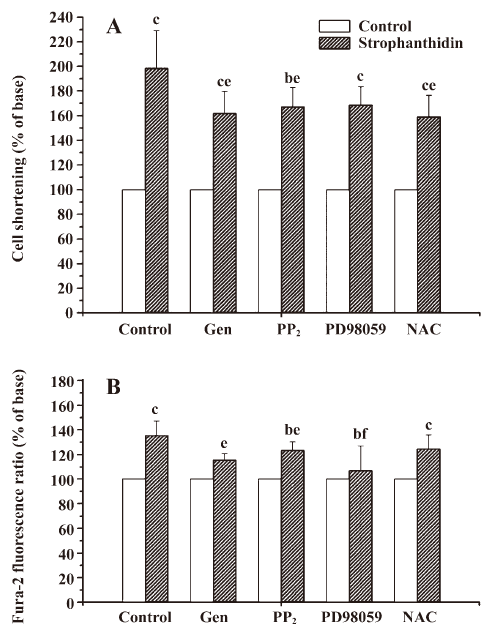
MAPK signal transduction pathway of Na+/K+-ATPase is involved in an increase in the calcium transient induced by Str in NC The myocytes were pretreated with 100 µmol/L genistein, 10 µmol/L PD98059, 2.5 mmol/L NAC (for 30 min), and 1 µmol/L PP2 (for 20 min), and exposed to 25 µmol/L Str for 10 min. The cell shortening and calcium transient were recorded. In NC, genistein, PP2, and PD98059 decreased the amplitudes of the calcium transient increased by Str 25 µmol/L (115.60%±5.12%, 123.28%±7.15%, and 106.96%±2.28% vs 135.44%±11.65%, P<0.05 or 0.01), but NAC did not (124.43%±11.46% vs 135.44%±11.65%, P>0.05; Figure 3B). These findings indicate the MAPK signal transduction pathway of Na+/K+-ATPase was involved in an increase of the calcium transient induced by Str in NC (Figure 1B).
Only the ROS pathway is involved in increasing the positive inotropic effects by Str in FC In FC, genistein, 2 µmol/L PP2, and NAC also decreased the extent of contractility increased by 25 µmol/L Str from 254.13%±40.47% to 203.80%±33.70%, 153.48%±17.35%, and 196.19%±28.19% (P<0.05 or 0.01). Genistein, PP2, and NAC all lowered the contractility increase induced by Str, but PD98059 had no such effect, indicating that only the ROS pathway is involved in the increase of the positive inotropic effects of Str (Figure 1C, 4C).
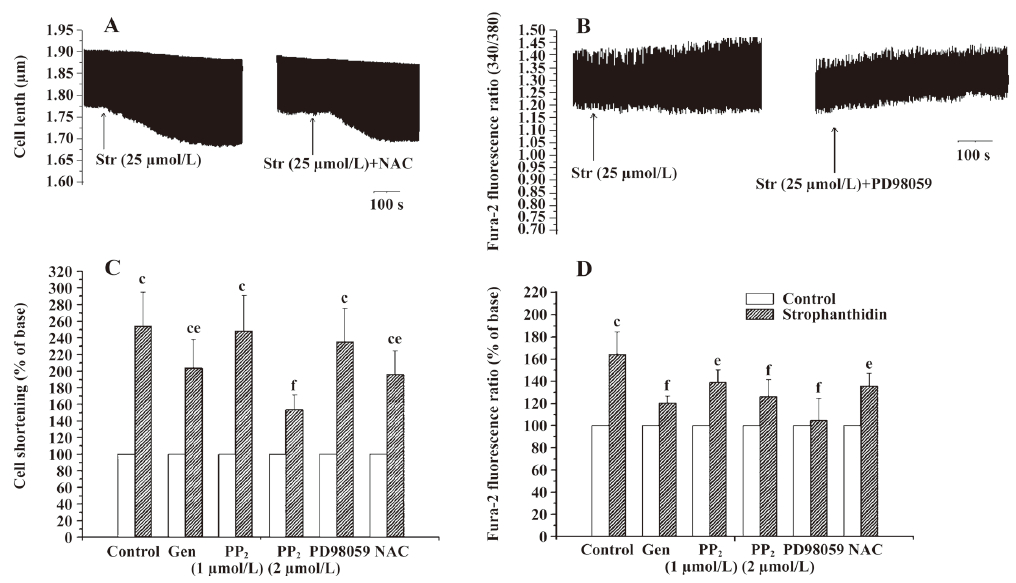
Both the MAPK and ROS signal transduction pathways of Na+/K+-ATPase are involved in an increase in the calcium transient induced by Str in FC In FC, genistein, 1 µmol/L PP2, 2 µmol/L PP2, PD98059, and NAC decreased the extent of the calcium transient increased by 25 µmol/L Str from 164.44%±20.19% to 120.34%±6.30% (P<0.01), 139.12%±11.08% (P<0.05), 126.33%±15.12% (P<0.01), 104.84%±19.49% (P<0.01), and 135.92%±11.07% (P<0.05), suggesting that both the Na+/K+-ATPase MAPK and ROS signal transduction pathways are involved in increasing the calcium transient induced by Str in FC (Figure 1C, 4D).
Discussion
Generally, E–C coupling mediated by Ca2+ is the key to the contractility of cardiac myocytes, which is the mechanism of the inotropic activity induced by CS. In the present experiment, we simultaneously assessed the effects of Str on contractility and the calcium transient in NC or FC from guinea pigs and demonstrated that Str could increase the amplitudes of the calcium transient and positive inotropic effects in a concentration-dependent manner. And the elevating effects of Str on calcium transient or the contractility of FC were more potent than NC. That is to say, FC is more sensitive to Str than NC. But why?
Evidence suggests that there is an endogenous ouabain-like factor in the heart tissue, which possesses hormone-like properties and can relay messages through various signal transduction pathways[5,13,14]. Yin et al[3] proved that low concentrations of dihydroouabain increased [Ca2+]i by Ca2+ influx via L-type Ca2+ channels, TTX-sensitive Na+ channels, or/and by directly triggering intracellular calcium release in guinea pig cardiac myocytes, suggesting that the effects of CS might involve more complex mechanisms.
Xie et al reported that in neonatal and adult rat cardiac myocytes, ouabain caused a partial inhibition of Na+/K+-ATPase and increased [Ca2+]i through the MAPK pathway[5]. They found that the increase of contractility in cardiac myocytes does not only rely on [Ca2+]i, but also on ROS generation. These effects induced by ouabain may involve many signaling pathways, but mainly involve the MAPK and ROS pathways[8]. In cardiac myocytes, the binding of CS to Na+/K+-ATPase results in the activation of ROS and MAPK pathway. So is the signal-transducing function of Na+/K+-ATPase that increases [Ca2+]i involved in mediating positive inotropic actions?
Our experiments detected whether the inhibitors of the MAPK or ROS signaling pathway of Na+/K+-ATPase affect the positive inotropic response induced by Str in isolated NC and FC from guinea pigs. These results indicated that in NC, the MAPK signal transduction pathway of Na+/K+-ATPase is involved in the increase the of calcium transient, and the ROS pathway is involved in the increase in the positive inotropic effects induced by Str. In FC, both the Na+/K+-ATPase MAPK and ROS signal transduction pathways are involved increasing the calcium transient induced by Str, and only the ROS pathway is involved in the increase of the positive inotropic effects of Str.
In NC, Str may affect contractility through directly activating the ROS signaling pathway, because in the embryonic myocardium, the increase of ROS production through the opening of the mitoKATP channel improves the postanoxic recovery of ventricular E–C coupling[15]. In addition, the other factor also affected the contractility such as calcium sensitization, except [Ca2+]i[16]. We had found that Str increased calcium sensitivity only in NC[17].
Like NC, in FC, the Na+/K+-ATPase MAPK and ROS pathways were involved in [Ca2+]i induced by Str, but only the ROS pathway affected myocyte contractility. This indicates that in FC, the positive inotropic effects induced by Str may rely on: (1) increased [Ca2+]i via the inhibition of the Na+/K+-ATPase ion pumping function; (2) increased [Ca2+]i through the activation of the ROS signal transduction pathways; and (3) increased ROS products through the opening of the mitoKATP channel.
The Na+/K+-ATPase ROS signal transduction pathway was involved in increasing the calcium transient induced by Str in FC, but not in NC. In FC, Str may mediate [Ca2+]i via the activation of the ROS pathway, and also by simultaneously activating the ROS and MAPK pathways, which form a signal amplification loop in cardiac myocytes. However, this mechanism needs to be further explored.
In NC and FC, Str produces the positive inotropic effects directly through the activation of the ROS signal transduction pathway. Earlier studies[18] have suggested that ROS products increase after heart failure, which results in a reduction of the antioxidative capacity and a reduction in myocardial contractility[19,20], but Str increases myocardial contractility via the ROS signaling pathway; this mechanism may be due to the increase of ROS products[21].
In short, we demonstrated here that the signal-transduc ing function of Na+/K+-ATPase is involved in the effects of Str on [Ca2+]i and contractility in NC and FC. However, the Na+/K+-ATPase signaling pathways involved in the positive inotropic effects of Str in NC and FC are consistent, but the Na+/K+-ATPase signaling pathways involved in the increase of [Ca2+]i in NC and FC differ.
References
- Blaustein MP, Juhaszova M, Golovina VA. The cellular mechanism of action of cardiotonic steroids: a new hypothesis. Clin Exp Hypertens 1998;20:691-703.
- Su SW, Wang YL, Li JX, Mei HS, Yin JX. Relationship between cardiotonic effects and inhibition on cardiac sarcolemmal Na+,K+-ATPase of strophanthidin at low concentrations. Acta Pharmacol Sin 2003;24:1103-7.
- Yin JX, Wang YL, Li Q, Shang ZL, Su SW, Cheng YP, et al. Effects of low concentration of dihydroouabain on intracellular calcium in guinea pig ventricular myocytes. Acta Physiol Sin 2002;54:385-9.
- Xie Z. Molecular mechanisms of Na/K-ATPase-mediated signal transduction. Ann NY Acad Sci 2003;986:497-503.
- Tian J, Gong X, Xie Z. Signal-transducing function of Na+/K ATPase is essential for ouabain’s effect on [Ca2+] i in rat cardiac myocytes. Am J Physiol Heart Circ Physiol 2001;281:H1899-907.
- Fitzgerald EM. Regulation of voltage-dependent calcium channels in rat sensory neurones involves a Ras-mitogen-activated protein kinase pathway. J Physiol 2000;527:433-4.
- Tian J, Liu J, Garlid KD, Shapiro JI, Xie Z. Involvement of mitogen-activated protein kinases and reactive oxygen species in the inotropic action of ouabain on cardiac myocytes. A potential role for mitochondrial KATP channels. Mol Cell Biochem 2003;242:181-7.
- Xie Z. Ouabain interaction with cardiac Na/K-ATPase reveals that the enzyme can act as a pump and as a signal transducer. Cell Mol Biol 2001;47:383-90.
- Kiss E, Ball N, Kranias E, Walsh R. Differential changes in cardiac phospholamban and sarcoplasmic reticular Ca2+-ATPase protein levels: effects on Ca2+ transport and mechanics in compensated pressure overload hypertrophy and congestive heart failure. Circ Res 1995;77:759-64.
- Wang Y, Gao J, Mathias RT, Cohen IS, Sun X, Baldo GJ. α-Adrenergic effects on Na+-K+ pump current in guinea pig ventricular myocytes. J Physiol 1998;509:116-28.
- Ren J, Wold LE. Measurement of cardiac mechanical function in isolated ventricular myocytes from rats and mice by computerized video-based imaging. Biol Proced Online 2001;3:43-53.
- Sipido KR, Callewaert G. How to measure intracellular [Ca2+] in single cardiac cells with Fura-2 or indo-1. Cardiovasc Res 1995;29:717-26.
- Ferrandi M, Manunta P. Ouabain-like factor: is this the natriuretic hormone? Curr Opin Nephrol Hypertens 2000;9:165-71.
- Ke YS. Endoxin: a major factor regulating cardiovascular system. Acta Pharmacol Sin 2001;22:201-9.
- Sarre A, Lange N, Kucera P, Raddatz E. mitoKATP channel activation in the postanoxic developing heart protects E-C coupling via NO-, ROS-, and PKC-dependent pathways. Am J Physiol Heart Circ Physiol 2005;288:H1611-9.
- Huang TY, Sakurai K, Sugawara H, Watanabe T, Norota I, Endoh M. Role of Na+/Ca2+ exchange in endothelin-1-induced increases in Ca2+ transient and contractility in rabbit ventricular myocytes: pharmacological analysis with KB-R7943. Br J Pharmacol 1999;126:1785-95.
- Qi YJ, Li JH, Li SM, Wang YL. The effects of strophanthidin on the contractility and calcium transient of a single normal or failure ventricular myocyte. Chin Pharmacol Bull 2008;24:333-7.
- Cheng XS, Shimokawa H, Momii H, Oyama J, Fukuyama N, Egashira K, et al. Role of nitric oxide and peroxynitrite in the cytokine-induced sustained myocardial dysfunction in dogs in vivo. J Clin Invest 1998;101:2207-14.
- Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol 1996;28:506-14.
- Schrier GM, Hess ML. Quantitative identification of superoxide anion as a negative inotropic species. Am J Physiol 1988;255:138-43.
- Liu J, Tian J, Haas M, Shapiiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na/K-ATPase initiates signal cascades independent of changes in intracellular Na and Ca concentrations. J Biol Chem 2000; 275: 27 838–44.
