Preparation of recombinant human bone morphogenetic protein-2 loaded dextran-based microspheres and their characteristics1
Introduction
Tissue engineering is one of the biomedical technologies used to induce tissue regeneration or repair body defects. The goal is to isolate adult stem cells in the tissue, then grow them in experimental devices into types of tissue that can be placed into injured regions to promote regeneration of tissue in and around injured areas of the body[1,2]. Drug delivery is an area in which chemical engineers have had a major impact, particularly for controlled delivery of pharmaceuticals to specific target sites such as tumors. Gene therapy is really metabolic engineering combined with drug delivery. It is a quantitative problem requiring a systems analysis. The right genes need to be delivered to the desired tissues, and proteins from that gene need to be made at the right time in the right amount. The lack of success with gene therapy is, at least in part, due to the inability of medical scientists to deal with these issues of well-controlled gene delivery and gene expression. This is a broad area that includes materials that are produced using biological processes and biologically compatible materials that are used for drug delivery and other medical applications. However, questions pertaining to the scarce resources and the potential risk of seed cells, the biomaterials of scaffold and the methods used to design the cell and scaffold complex remain unresolved, which have resulted in their limited clinical application [3–5]. So far, several research approaches – both in animal experiments and in clinical treatment – have been attempted to promote tissue regeneration by bioactive agents, including members of the bone morphogenic protein, transforming growth factor, and insulin-like growth factor families, which are important to the maintenance and repair of most tissue defects[6–8]. Since periodontal tissue defects typically remain unhealed and often lead to further tissue degeneration and the loss of teeth, understanding the physiological role of these proteins in the wound-healing cascade and tissue regeneration is of critical importance in advancing the treatment of periodontal tissue loss and in enhancing periodontal tissue regeneration. Most of the research in this field has centered on examining the therapeutic effects that these molecules have on hard or soft tissues with the presence of 1 or more growth factors[9–16]. However, as the fields of tissue engineering and biomaterials merge with molecular and cellular biology, new drug delivery vehicles can be utilized to study the in vivo effect of these molecules on periodontal tissue regeneration or periodontal defect repair. Accordingly, this paper details the design and development of a genus of new composite scaffolds with the sustained delivery property of recombinant human bone morphogenetic protein-2 (rhBMP2).
Bone morphogenetic proteins (BMPs) have been shown to modulate the wound-healing response in both hard and soft tissues. During the past decade, many investigators have demonstrated the anabolic effects of these wound-healing molecules on the promotion of periodontal attachment structures, namely alveolar bone, periodontal ligament and tooth root cementum[6–8,10–13]. However, because of their drawbacks such as short-term BMP retention and losable BMP biological activity in vivo, using agents for BMPs sustained release is of great importance . Although many agents such as calcium phosphate cement (CPC), collagen, bioactive glass ceramic (BGC), hydroxyapaite (HA) and more recently, some hydrogels have been used to enhance tissue regeneration and have gained good results. The actual effect is still uncertain considering the time BMP bioactivity might keep in vivo, as those materials themselves could not preserve BMPs from humoral dilution, metabolized and degraded action. There is evidence that the continuous presence of some growth factors at the periodontal tissue interface to provide a tangible effect is essential, which can accelerate the soft and hard tissue regeneration[6–8,17]. Therefore, it is rather inviting to search for new pharmaceutical forms that can sustain elevated growth factor levels and increase or improve tissue regeneration in periodontal diseases treatment or periodontal defect repair[18–20].
Microspheres as drug carriers have the advantage of sustained or controlled release, passive or active drug targeting to specific tissues, which notably reduce the side effects of drugs and improve their bioavailability. Therefore, microspheres as a drug delivery system have drawn much attention in the pharmaceutical field and have been successfully used in tumor chemotherapeutics and in the treatment of diabetes. Dextran-based hydrogels are widely used polymers in pharmaceutical products. The good biocompatibility, the degradation to non-toxic and readily excreted products were the main attractive characteristics of dextran-based hydrogels, which suggest its use in the drug delivery field[21]. In addition, dextran is relatively cheap and dextran-co-gelatin hydrogels have putative bioadhesive properties that allow the drug delivery systems, designed in different forms, to meet the need of periodontal tissues and mucosal tissues, as well as other especial tissues, such as the eye, nasal, gastrointestinal and urinary epithelial tissues[22−24]. In order to improve the therapeutic efficiency and to prolong BMP retention in periodontal bone defects, and to explore the possibility of preparing targeted rhBMP2 delivery devices, this study was aimed at developing a new kind of rhBMP2 delivery system: rhBMP2-loaded dextran-based microspheres (rhBMP2-MPs). The results indicated that using this delivery system could result in a long-term release of rhBMP2 and could enhance periodontal tissue regeneration continually when compounded with tissue engineering scaffold materials.
Materials and methods
Materials Dextran (Mr 69 800 with 5% branches) was purchased from Xia-si Biomaterial Inc (Beijing, China), Gelatin G-6650 was obtained from Sigma (Sigma Chemicals, Saint-Louis, MO, USA) and dried at 60 ºC in a vacuum oven for 2 d. Span-80 and Dextranase (400–800 U/mg protein) was also purchased from Sigma. Triethylamine, 2,20-dimethoxy-2-phenyl acetophenone (DMAP), dimethyl formamide (DMF), N-methyl pyrrolidone (NMP) and N,N,N',N'- tetramethyl-ethylene diamine (TEMED) were obtained from E Merk (Mumbai, India). Glycidyl methacrylate (GMA) was purchased from Bioengineering Inc (Boston, MA, USA). All these materials were dried overnight at 70 ºC in a vacuum oven. RhBMP2 were obtained from the Academy of Military Medical Science (Beijing, China), acrylic acid (AA, Merck) was used after vacuum distillation. Water was double distilled. All other chemicals and solvents were used without further purification.
Preparation of rhBMP2-MPs Using dextran and GMA, we synthesized the dex-GMA precursor as the method we reported recently with some modifications[25]. The synthesized procedure was as follows: Dextran was dissolved in a dimethyl sulphoxide(Me2SO) solvent system (60 mL with Span-80 0.4 mL and TEMED 0.2 mL, DMAP 1.5 g, DMF 0.2 g and NMP 0.2 g) at 60 ºC under N2 gas and stirred for 15 min. After dissolving, the solution was cooled down to 35 ºC and GMA was then slowly added to the dextran solution. The reaction was conducted at 35 ºC for 72 h under N2 gas. The reaction product was precipitated with cold isopropyl alcohol, filtered, washed several times with isopropyl alcohol, and then freeze-dried in a vacuum oven. The degree of substitution (DS) of the dex-GMA precursor was estimated by the 1H-NMR method. There is an anomeric proton attached to the C1 position of the dextran glucose ring that appears at 4.5–5.5 ppm in the NMR spectrum, where the protons of the hydroxyl groups appear. This proton does not react during the GMA substitution reaction, while some of the other protons of other hydroxyl groups are substituted by GMA. So, we can use the ratio of the normalized, integrated intensities of the sum of the hydroxyl group peaks to the normalized, integrated intensities of the anomeric proton peak to estimate the DS. For unsubstituted pure dextran, the ratio should be 3; while for GMA-substituted dextran, this ratio should be less than 3, and the magnitude would depend on the number of substitution. Thus, the DS could be calculated from the ratio: 3×(difference of the NMR proton intensity between dextran and dextran derivatives)/dextran. The dex-GMA precursor synthesized in this study had a DS of 2.6, that is, 2.6 hydroxyl groups per dextran glucose ring were substituted by GMA. Using dex-GMA, we synthesized a species of biodegradable poly (dex-GMA-co-gelatin) hydrogel microspheres, which were used as the carrier of rhBMP2. The rhBMP2-MPs were prepared by double-phase emulsified condensation polymerization method and optimal preparation parameters were obtained by the orthogonal design. The technique was as follows: 3 cuvettes of equivalent homogeneous aqueous dex-GMA and gelatin solution (10%, w/v) of 6 mL, each containing 0.6 g dex-GMA and 0.15 g gelatin, were preheated to 30 ºC and dropped into 3 different reactor systems comprising of 30 mL paraffin liquid, 0.2 mL Span-80 and 0.01 mL, 0.02 mL, or 0.03 mL TEMED. The 3 reactor systems were at 30 ºC in a water bath, forming a water-in-oil emulsion by stirring with a two-paddle stirrer. Ten minutes later, as the emulsion was obtained, concentrated rhBMP2 solution (containing 0.08 g rhBMP2) was dropped into the reactor systems. The resulting microspheres were washed 3 times with cool isopropanol, ethyl ether to remove the residual paraffin, washed with rhBMP2 saturation water and then lyophilized, sized by passing through sieves of different apertures. The collected loaded micro-spheres were preserved in a desiccator. Unloaded micro-spheres were synthesized by the same procedure, with the exception of the concentrated rhBMP2 solution added and rhBMP2 saturation water washing.
Morphology, size, and swelling analysis Freeze-dried microspheres were sprinkled onto a piece of electric-glue paper, gold-sprinkled in a vacuum and then examined by scanning electron microscopes (SEM,S-520, Hitachi, Japan). Size and size distribution were calculated by 500 micropar-ticles during SEM and the roundness of the microspheres were determined by a particle size analyzer (BI-90, Brook-haven Co, California, USA). At the same time, freeze-dried microspheres (5 mg) were suspended in aqueous solution (4 mL) for 5 min using an ultrasonic bath and then dropped onto a sheet glass. The morphology of swelling microspheres was observed by photomicroscope (XI 70, Olympus, Tokyo, Japan). Another solidified rhBMP2-MPs powder (weight Wo of 5 mg) was dipped in aqueous solution for approximately 30 min, then using filtered paper absorbed the water and weighed the weight of the swollen microspheres (Ws). The swelling ratio (Rs) of the microspheres was calculated by the following formula:
Rs (%) = (Ws-Wo)/ Wo×100%
Determination of drug loading and drug encapsulation efficiencies Drug encapsulation efficiencies(DE) and drug loading (DL) are 2 main indexes of pharmaceutical forms. DE reflects the pharmaceutical form’s ability to encapsulating drugs and high DE means little drug wasted during preparation, while DL reflects the effectual drug component in a pharmaceutical form and high DL means high therapeutic component available. They are all of great importance. The determination methods included an exact weighed amount of rhBMP2-MPs (100 mg); unloaded microspheres (100 mg) were treated with 50 mL NaOH (10%, w/v) water solution at 80 ºC for 20 min. Fully degraded dex-GMA/gelatin solution were adjusted to neutral solution with 3 mol·L-1 HCl and centrifugated for 5 min. The supernatants were mixed with total-ionic-strength adjusting buffer (TISAB II)and made into 50 mL of solution. Drug content in the solution were conducted by means of High Performance Liquid Chromatography (HPLC) (94-09SC, Orion Research Inc, Boston, MA, USA). The actual weight of rhBMP2 found loaded should be equal to the remainder of the rhBMP2 contents between rhBMP2-loaded and unloaded microspheres.
DE (%)=(Weight of rhBMP2 found loaded)/(Weight of rhBMP2 input)×100%
DL (%)=(Weight of rhBMP2 found loaded)/(Weight of rhBMP2-loaded microspheres)×100%
Biodegradation of rhBMP2-MPs Reports have been found to show that dextran-based biomaterials could be biodegraded in any liquid with presence a very tiny quantity of dextranase. Thirty-six exact weighed amounts of rhBMP2-MPs (100 mg) were dipped in 0.9% physiological saline (containing 0.05 μg/mL,0.1 µg/mL and 0.2 µg/mL dextranase, respectively) and divided into 4 groups at certain time intervals of 10 d, 20 d, 30 d, and 40 d; three of each group were collected and freeze-dried. The morphology of the biodegraded microspheres was observed by SEM and weight loss was also studied.
Stability and biological activities of rhBMP2-MPs Dry rhBMP2-MPs were sealed and deposited in a 4 ºC refrigerator or at room temperature for 6 months respectively. Their appearance, morphology, DE and DL were checked as routine procedure. The bioactivity of the microspheres were evaluated by the biological effects they had on the cultured cells in vitro, and at the same time, we investigated the biological effects of loaded microspheres compared to the equivalent concentrated rhBMP2 solution. Human periodontal ligament cells (PDLCs) were obtained from premolars extracted for orthodontic reasons from a 14-year-old patient, using cultured methods as previously described by Somer-man et al[26]. The protocol was approved by the Ethical Committee in Research from the Fourth Military Medical University. The cell growth assay was performed by MTT (purchased from Sigma, Saint-Louis, MO, USA) methods[27]. Generally, PDLCs were placed in a 96-well culture plate (Nunc A/S, Roskilde, Denmark) at a density of 30,000 cells/well in 2 µL of DMEM containing 10% FBS and antibiotics. After 16 h, the cells were washed with PBS and cultured in serum-free (CT)-FBS/DMEM supplemented with 100 µg/mL rhBMP2 (group I) or 782 µg/mL rhBMP2-MPs synthesized within 3 d (equal to 100 µg/mL rhBMP2; group II), or 782 µg/mL rhBMP2-MPs deposited in a 4 ºC refrigerator for 6 months (group III), or at room temperature for 6 months (group IV), the control group (group IV) served as the control supplemented with nothing. After 1, 2, 3, 5, 7, 10, 12 d, absorbance was read at 570 nm according to routine MTT methods. The cellular proliferation was calculated as the amount of MTT uptake.
Drug release study in vitro In vitro drug release profiles were obtained by a dynamic dialysis method simulating the temperature in vivo. Briefly, 3 groups of rhBMP2-MPs (50 mg), according to different quantity of TEMED (0.01 mL, 0.02 mL and 0.03 mL) when prepared, were poured into a dialysis tube (IL61105, Rockford, USA), then placed at 37.0±0.5 ºC into phosphate buffer solution (PBS) of 150 mL (pH=7.0), respectively, and continuously stirred with a magnetic stirrer at 200 r/min. At specific time intervals of 5 h, 20 h, 30 h, 60 h, 90 h, 120 h, 150 h, 190 h, and 250 h, 10 mL of samples were removed from the release medium and the same volume and temperature of PBS was added back to the release medium. The samples were then assayed for drug content according to intraday standard curve. Results of triplicate test data were used to calculate accumulated drug release. Cumulative release profiles of different rhBMP2-MPs were studied respectively.
CPC/rhBMP2-MPs scaffolds preparation The calcium phosphate ceremic (CPC) was purchased from Rui Bang Biomaterial Inc (Shanghai, China) and CPC/rhBMP2-MPs was obtained by a physical concoction mechanism using CPC, PBS, and rhBMP2-MPs with the inverse proportion being 1:3:0.01, and then solidified in a vacuum desiccator. Human PDLCs cultured in vitro were collected and seeded on the concreting compound, the cell growth in the scaffolds and CPC/rhBMP2-MPs framework were observed by SEM.
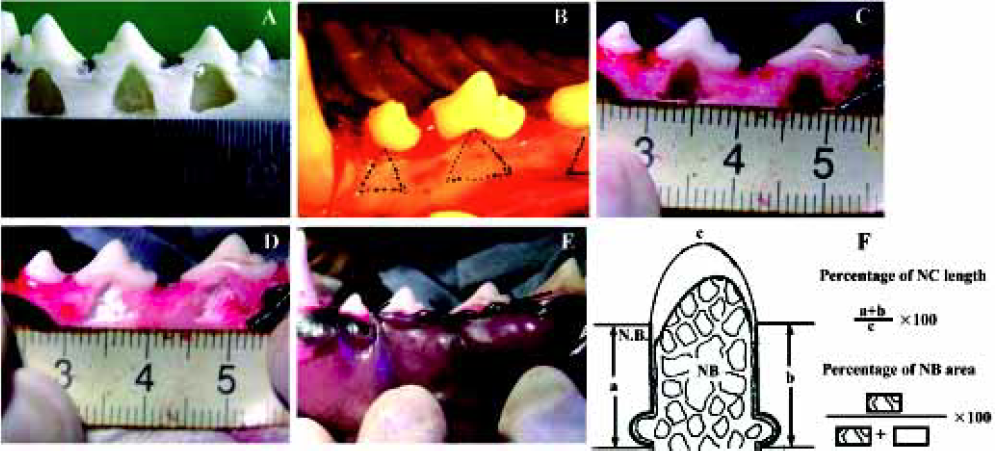
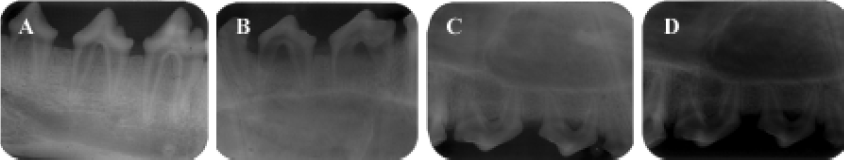
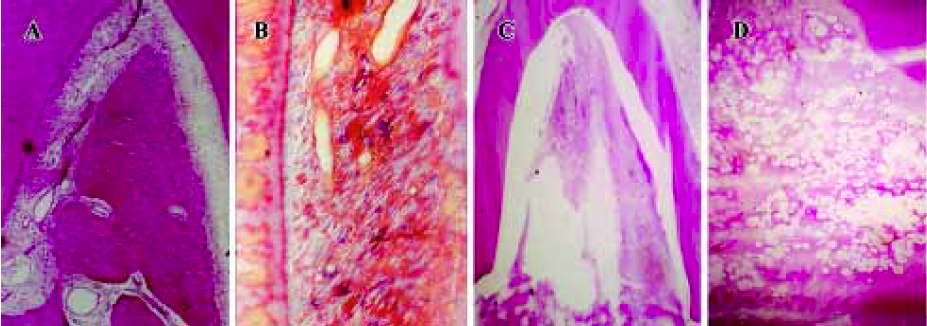
Animal experiment After receiving approval from the Committee of Research Facilities for Laboratory Animal Science, Fourth Military Medical University, 12 female beagle dogs weighing 10 to 14 kg and aged 12 to 20 months were used in this study. Good oral health was established by scaling and mechanical tooth-brushing. All surgical procedures were performed under general anesthesia with sodium pentobarbital (40 mg/kg), and local infiltrated anesthesia with 2% lidocaine with 1:80 000 noradrenaline. Experimental Class III furcation defects prepared in this study were based on the model described by Lindhe et al[28]. The second, third, and fourth premolars (P2, P3, and P4) in each dog were selected for experimentation (Figure 1A). Following sulcular incisions, mucoperiosteal flaps were raised, and Class III furcation defects were created surgically at P2, P3, and P4. The Class III defect height from the cemento-enamel junction to the reduced alveolar crest was 5.00±0.20 mm. Denud-ed root surfaces were prepared to remove all periodontal ligament and cementum. The roots were denuded only in the area within the furcation and extending to the mesial line angle for the mesial root and to the distal line for the distal root. Reference notches were placed around the circumference of the mesial and distal roots at the bottom of the bone level. All teeth were divided into 4 sub areas: maxillary left and right, submaxilla left and right, and then divided into 2 groups stochastically (to avoid the interaction of defects in one subarea): the experimental group and the control group. The experimental group was transplanted with CPC/rhBMP2-MPs scaffolds while the control group was transplanted with CPC concreting compound soaked with the commensurate quantity of concentrated rhBMP2 compared with rhBMP2-MPs. In another words, the actual rhBMP2 quantity transplanted into each Class III furcation defects was exactly same (Figure 1B, 1C, 1D, 1E). One or 2 months after transplantation, anesthetized animals were perfused with 1% glutaraldehyde in a sodium cacodylate buffer containing 0.05% calcium chloride (pH 7.3). The periodontal tissue regeneration was evaluated by unaided eye and X-ray. After that, the mandibles and maxillas were dissected and immersed in the same fixative. After decalcification with hydrochloride for 3 to 5 d, the mandibles and maxillas were dehydrated in paraffin. Serial section (5 µm) were cut in the mesial-distal plane throughout the buccal-lingal extension of the tooth. The sections were stained with hematoxylin and eosin (HE) or the Azan method, and observed using a light microscope. The center-most section and the immediate section on either side were subjected to morphometric analysis. The percentage of new cementum (NC) length and percentage of new bone (NB) area were measured on digitized photomicrographs captured in a computer. The lengths of NC formed along the denuded root surface on each specimen were added, and the percentage of the lengths to the total root surface length from one notch to the next notch was calculated. The area of NB on each specimen was calculated as a percentage of the area surrounded with reference notches at mesial and distal root surfaces space is present in normal periodontal tissue (Figure 1F). All data were statistically analyzed using the Mann-Whitney U test.
Results
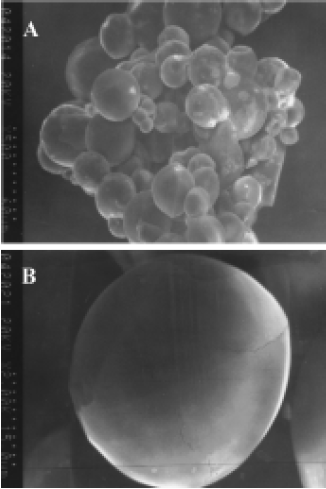
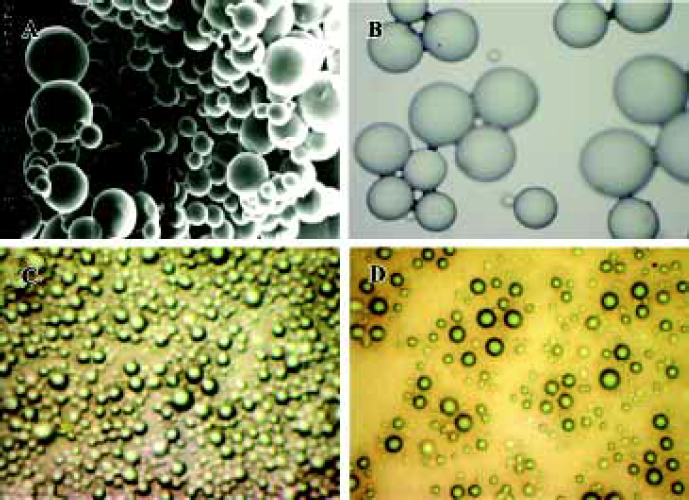
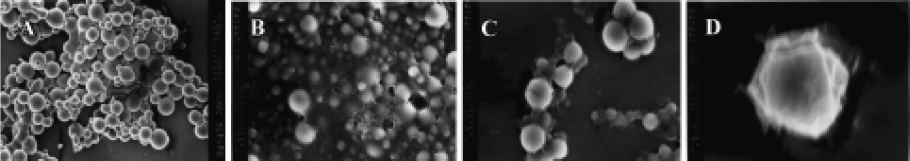
Morphology and particle size The SEM photomicrographs of dried rhBMP2-MPs showed a smooth and uniform surface (Figure 2A). The roundness of microspheres was 1.020±0.005. The average diameter was (30.33±4.32) µm with 75.4% ranging from 20 µm to 40 µm, The swelling ratio for solidified and dried microspheres was 4.18±0.06, and the size of the soggy microspheres size was approximately 5–10 times larger (Figure 2B). The morphology and particle size analysis showed no difference between loaded and unloaded freeze-dried or swelling microspheres, but when the swelling microspheres were sprinkled onto a piece of glass, half freeze-dried and then observed through a photomicroscope (XI 70, Olympus), the loaded microspheres were found to be a little fuscous. The unloaded microspheres were briefly translucent (Figure 2C, 2D), which was perhaps caused by the increase of the microspheres’ density when drugs were encapsulated into the microspheres. Thus, we could presume that drug encapsulation was the primary drug association mechanism of dex-GMA-co-gelatin microspheres.
DL and DE The calculated results of DL and DE were7.82% and 82.25%, respectively. In the present study, we achieved optimal preparation parameters using the orthogonal design, which was rhBMP2 (0.5 mmol·L-1), dex-GMA/gelatin solution (20%, m/v), emulsifying agent (1%, m/v), and 1:5 (v/v) of water phase to oil phase. During the experiment we discovered that the concentration of rhBMP2 affected the quality of the microspheres. When the content of rhBMP2 exceeded 1 mmol/L, the microspheres’ morphology was not uniform, and was prone to sticking. When the concentration was lower than 0.1 mmol/L, the drug content was also too low and thus, unfit for practical application. In order to reduce the loss of rhBMP2 during the process of preparation, and improve the encapsulation efficiencies, presolidification was introduced and microspheres were washed with concentrated rhBMP2 solution. Glutaraldehyde (25%, v/v) was used to solidify; the solidification time was over 24 h. The encapsulation efficiencies of rhBMP2-MPs reached 86.73%, which was much higher than other biomaterials that have been reported[29].
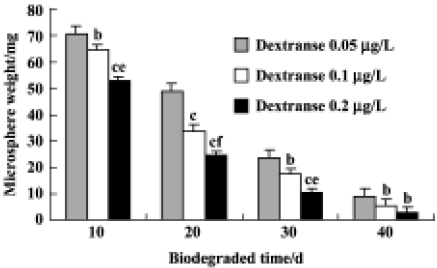
Biodegradation of rhBMP2-MPs rhBMP2-MPs could completely degrade within 30–40 d by dextranase action (Figure 3) . The weight at specific time intervals was shown in Figure 4, and the microspheres eventually dissolved. However, Figure 4 also shows that the degraded speed interrelated closely to the consistence of dextranase. This might have important clinical meanings. In periodontal diseases such as periodontitis, especially rapidly progressive periodontitis, when the prolific nosogenetic bacteria and organisms in local environment (which cause excessive dextranase biosynthesis and the biodegradation of rhBMP2-MPs more rapidly), rapid releasing rhBMP2 from microspheres is beneficial for tissue repair and at the same time, can prevent tissue loss. It could be hypothesized that when rhBMP2-MPs are topically applied, their presence in the periodontal environment, even at low concentrations, can prevent demineralization as well as promote remineralization of incipient lesions through rhBMP2 release which are quickened under more dextranase conditions.
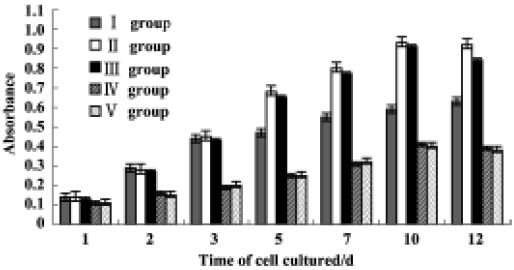
Stability of rhBMP2-MPs The rhBMP2-MPs were stable when sealed and stored below 4 ºC for 6 months without obvious physical and chemical characteristics change. However, when stored at room temperature, rhBMP2-MPs biomorphic characteristic could not keep unchangeable and with a configuration of conglutination and abnormity (Figure 5). The proliferation curves of PDLCs in different groups are shown in Figure 6. Cytology studies showed that there was no significant differences between groups II and III (P>0.05), indicating no significant bioactivity loss of rhBMP2-MPs when stored below 4 ºC for 6 months. On the other hand, after 6 months of being stored at room temperature, rhBMP2-MPs would no longer have any biological activity (P<0.01). In addition, the rhBMP2-MPs could enhance the proliferation of PDLCs for more than 12 d continuously, while the pute rhBMP2 bioactivity could function only in 3 d, which also showed the sustained delivery property of rhBMP2-MPs (Figure 6).
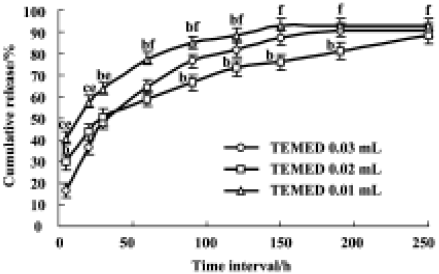
In vitro release studies The release profiles of rhBMP2 from microspheres as a function of time showed that rhBMP2-releasing kinetics in vitro fitted to first-order and Higuchi equations. From Figure 7 we could see that the drug release from rhBMP2-MPs mainly consisted of 2 components with an initial rapid release followed by a slower exponential stage. The release profile in vitro was in accord with 2-phase kinetics law and more than 80% of the drugs were released during the first 10 d. Furthermore, changing the quantity of TEMED could influence drug release; rhBMP2 release from rhBMP2-MPs was slower when the quantity of TEMED was increased during preparation, which could be caused by the DS of microspheres increase followed with TEMED increased. During the initial stages, the microspheres were absorbed and the sphere enlarged rapidly; the drugs were rapidly released from the microspheres through the exoteric micro-aperture; When swelling was counterpoise, the drug release slowed down and was determined by drug pervasion and microsphere biodegradation. Thus, we can speculate that rhBMP2 release from rhBMP2-MPs might be controlled by some preparation technique change.
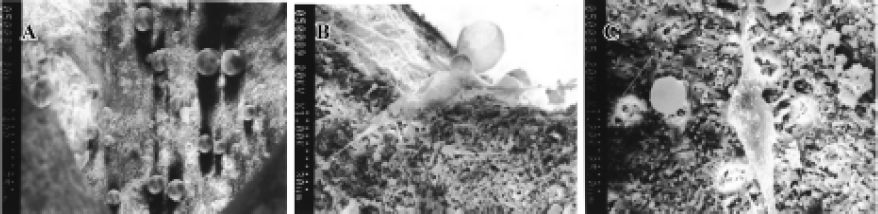
Cells growth on CPC/rhBMP2-MPs scaffolds A scanning electron microscope showed that the porous structure of CPC/rhBMP2-MPs compound and rhBMP2-MPs could be adhered in the porous structure (Figure 8A). After 3 d, human PDLCs could grow on CPC/rhBMP2-MPs compound both in the porous structure (Figure 8B) and on the material’s surface (Figure 8C).
Animal experiment When observed with unaided eye in the experimental group, the defect was well regenerated after 1 month (Figure 9A) and was almost completely regenerated after 2 months (Figure 9B). The control group gained less tissue regeneration than the experimental group even after 2 months (Figure 9C). The X-ray observation results were shown in Figure 10. In the experimental group, a significant amount of new bone and an adequate width of periodontal ligament were observed (Figure 11A). The denuded root surface was almost completely covered with NC, and regenerated periodontal ligament separated the NB from the cementum. On the denuded root surfaces of the furcation area, newly formed cementum covered the surface, and the Sharpey’s fibers inserted into the cementum were frequently observed (Figure 11B). In the control group, no cementum regeneration was observed (Figure 11C) and epithelial cells invaded into the top of the furcation in the area (Figure11D). Less bone regeneration was observed in this group compared to the experimental group. The percentages of NC length in the experimental group after 1 and 2 months were 93.9%±14.2% and 96.7%±5.29%, respectively, compared to 70.4%±12.1% and 72.8%±9.2% in the control group. The results showed significant difference (P<0.01). Accordingly, the percentages of NB were 62.6%±13.5% and 68.7%±9.73% in the experimental group and 54.7%±10.73% and 57.3%±11.2% in the control group also showed significant difference (P<0.01).

Discussion
Biodegradable hydrogel microspheres are attractive devices for drug release because they combine good tissue biocompatibility with the possibility of manipulating the permeability for solutes. In particular, their use in pharmaceutics shows promise since recent advancements in biotechnology have led to a great variety of pharmacologically active peptides and proteins that are not adequately released from systems that are not biodegradable. Degradation of the hydrogel microspheres not only allows a tailored release of entrapped molecules, but also circumvents the removal of the empty device from the body[30–32]. Although the use of biodegradable hydrogel microspheres related to prolonged and/or controlled drug delivery has been widely investigated, the need for the development of hydrogels using simpler and more feasible methods still exists. Among the starting materials, polysaccharides and polyaminoacids represent good candidates to prepare biodegradable hydrogels by photocrosslinking reactions. Besides their biocompatibility and biodegradability, these polymers can also have functional groups incorporated into their structures. These functional groups include acrylic or methacrylic ones, which can easily lead to the formation of inter- and intra-chain bonds by UV irradiation. In this context, the synthesis and characterization of dextran-co-gelatin hydrogel microspheres show good biocompatibility and biodegradability. Dextran is a natural polysaccharide that is widely used in the pharmaceutical field[33–37]. It is water soluble, inert in biological systems and does not affect cell viability. The characteristic α-1,6-glucosidic linkage is hydrolyzed by dextranases, enzymes produced by various molds and certain bacteria as well as by mammalian cells. It has also been found that dextranases are produced by anaerobic Gram-negative intestinal bacteria[38]. The use of hydrogels based on dextran represents a strategy to release drug molecules in the colon after hydrolysis of the polysaccharide microspheres[39]. After sucking and swelling in vivo, rhBMP2-MPs can keep rhBMP2 concentrations over prolonged periods of time, which might be explained by the sustained-release of rhBMP2 from rhBMP2-MPs and dextran-based microspheres’ bioadhesive properties to mouth parenchyma. Experiments conducted using rhBMP2-MPs and rhBMP2 solution as control revealed that rhBMP2-MPs maintained rhBMP2 elevation above baseline for about 10 d, but rhBMP2 maintained for less than 3 d. This indicated the possibility that rhBMP2-MPs could meet clinical intermittent administration by prolonging rhBMP2 retention time significantly.
Many biomaterials such as Chitosan, polylacticacid (PLA), polyglycolicacid (PGA), PGA-co-PLA polymers (PLGA), poly (ethylene glycol) (PEG), gelatin, and polybutylcyano-acrylate (PBCA) were all reported to prepare growth factor-sustained release systems recently, but a lot of questions remain unanswered. The outstanding obstacle in the clinical application of growth factor carriers to promote tissue regeneration and control release of growth factors is still unrealized, which results in the growth factors not being able to maintain enough time or concentration during the tissue regenerated period. To meet the need of periodontal regeneration, new biomaterials used to prepare controlled release system of growth factors have become increasingly important. From this study, we prepared a new carrier for the sustained release of rhBMP2, which can be used to synthesize guided tissue regeneration biomembranes and build functional scaffolds in tissue engineering. In summary, for the first step in developing a new type of rhBMP2 agent, we chose dextran-based micropsheres as sustained release carriers for topical rhBMP2 applications. We proved the ability of rhBMP2-MPs in prolonging rhBMP2 release and their potential in prohibiting demineralization and enhancing the rhBMP2 retention both in vitro and in vivo. This work offered a new method in contributing growth factors, not only BMPs, but also other growth factors for clinical use, which can be popularized with further evaluation both in animal experiment and in clinical cases. However, some limitations of using the microsphere-carrier combination, including the potential difficulty in manufacturing the carrier, and the expensive price considering the costly price of growth factors themselves. Moreover, the manufacture procedure and the application of this pharmaceutical form still require further study both experimentally and clinically.
References
- Yu TT, Shoichet MS. Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials 2005;26:1507-14.
- Cheng MH, Brey EM, Allori A, Satterfield WC, Chang DW, Patrick CW Jr, et al. Ovine model for engineering bone segments. Tissue Eng 2005;11:214-25.
- Choo AB, Padmanabhan J, Chin AC, Oh SK. Expansion of pluripotent human embryonic stem cells on human feeders. Biotechnol Bioeng 2004;88:321-31.
- Hsu SH, Tsai CL, Tang CM. Evaluation of cellular affinity and compatibility to biodegradable polyesters and type-II collagen-modified scaffolds using immortalized rat chondrocytes. Artif Organs 2002;26:647-58.
- Kim SS, Vacanti JP. The current status of tissue engineering as potential therapy. Semin Pediatr Surg 1999;8:119-23.
- Sorensen RG, Wikesjo UM, Kinoshita A, Wozney JM. Periodontal repair in dogs: evaluation of a bioresorbable calcium phosphate cement (Ceredex) as a carrier for rhBMP-2. J Clin Periodontol 2004;31:796-804.
- Wikesjo UM, Sorensen RG, Kinoshita A, Jian Li X, Wozney JM. Periodontal repair in dogs: effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol 2004;31:662-70.
- Hanisch O, Sorensen RG, Kinoshita A, Spiekermann H, Wozney JM, Wikesjo UM. Effect of recombinant human bone morphogenetic protein-2 in dehiscence defects with non-submerged immediate implants: an experimental study in Cynomolgus monkeys. J Periodontol 2003;74:648-57.
- Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. A reentry study on the use of bovine porous bone mineral, GTR, and platelet-rich plasma in the regenerative treatment of intrabony defects in humans. Int J Periodontics Restorative Dent 2005;25:49-59.
- Wikesjo UM, Qahash M, Thomson RC, Cook AD, Rohrer MD, Wozney JM, et al. rhBMP-2 significantly enhances guided bone regeneration. Clin Oral Implants Res 2004;15:194-204.
- Wikesjo UM, Xiropaidis AV, Thomson RC, Cook AD, Selvig KA, Hardwick WR. Periodontal repair in dogs: space-providing ePTFE devices increase rhBMP-2/ACS-induced bone formation. J Clin Periodontol 2003;30:715-25.
- Wikesjo UM, Xiropaidis AV, Thomson RC, Cook AD, Selvig KA, Hardwick WR. Periodontal repair in dogs: rhBMP-2 significantly enhances bone formation under provisions for guided tissue regeneration. J Clin Periodontol 2003;30:705-14.
- Ripamonti U, Reddi AH. Tissue engineering, morphogenesis, and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit Rev Oral Biol Med 1997;8:154-63.
- Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol 2003;74:1282-92.
- Park YJ, Ku Y, Chung CP, Lee SJ. Control release of platelet-derived growth factor from porous poly (L-lactide) membranes for guided tissue regeneration. J Control Release 1998;12:201-11.
- Wikesjo UM, Razi SS, Sigurdsson TJ, Tatakis DN, Lee MB, Ongpipattanakul B, et al. Periodontal repair in dogs: effect of recombinant human transforming growth factor-beta1 on guided tissue regeneration. J Clin Periodontol 1998;25:475-81.
- Rimondini L, Nicoli-Aldini N, Fini M, Guzzardella G, Tschon M, Giardino R. In vivo experimental study on bone regeneration in critical bone defects using an injectable biodegradable PLA/PGA copolymer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:148-54.
- Hench LL, Xynos ID, Polak JM. Bioactive glasses for in situ tissue regeneration. J Biomater Sci Polym Ed 2004;15:543-62.
- Schmokel HG, Weber FE, Seiler G, von Rechenberg B, Schense JC, Schawalder P, et al. Treatment of nonunions with nonglycosylated recombinant human bone morphogenetic protein-2 delivered from a fibrin matrix. Vet Surg 2004;33:112-8.
- Holland TA, Tessmar JK, Tabata Y, Mikos AG. Transforming growth factor-beta 1 release from oligo (poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Release 2004;94:101-14.
- Vandelli MA, Rivasi F, Guerra P, Forni F, Arletti R. Gelatin microspheres crosslinked with D,L-glyceraldehyde as a potential drug delivery system: preparation, characterisation, in vitro and in vivo studies. Int J Pharm 2001;215:175-84.
- Brime B, Ballesteros MP, Frutos P. Preparation and in vitro characterization of gelatin microspheres containing levodopa for nasal administration. J Microencapsul 2000;17:777-84.
- Morimoto K, Katsumata H, Yabuta T, Iwanaga K, Kakemi M, Tabata Y, et al. Evaluation of gelatin microspheres for nasal and intramuscular administrations of salmon calcitonin. Eur J Pharmacol Sci 2001;13:179-85.
- Nakase H, Okazaki K, Tabata Y, Uose S, Ohana M, Uchida K, et al. Development of an oral drug delivery system targeting immune-regulating cells in experimental inflammatory bowel disease: a new therapeutic strategy. J Pharmacol Exp Ther 2000;292:15-21.
- Chen FM, Wu ZF, Jin Y, Wang QT, Wu H, Wang GF, et al. Development of a hydrogel microsphere delivery system for rhBMP2. J Pract Stomatol 2005;21:174-7. Chinese..
- Someman MJ, Foster RA, Vorsteg GM, Progebin K, Wynn RL. Effects of minocycline on fibroblast attachment and spreading. J Periodontal Res 1988;23:154-9.
- Coletta RD, Almeida OP, Graner E, Page RC, Bozzo L. Differential proliferation of fibroblast cultured from hereditary gingival fibromatosis and normal gingival. J Periodontal Res 1998;33:469-75.
- Lindhe J, Pontoriero R, Berglundh T, Araujo M. The effect of flap management and bioresorbable occlusive devices in GTR treatment of degree III furcation defects. An experimental study in dogs. J Clin Periodontol 1995;22:276-83.
- Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm 2002;249:165-74.
- Stenekes RJ, Franssen O, van Bommel EM, Crommelin DJ, Hennink WE. The preparation of dextran microspheres in an all-aqueous system: effect of the formulation parameters on particle characteristics. Pharm Res 1998;15:557-61.
- Kamath KR, Park K. Biodegradable hydrogels in drug delivery. Adv Drug Delivery Rev 1993;11:59-84.
- Kuijpers AJ, van Wachem PB, van Luyn MJ, Brouwer LA, Engbers GH, Krijgsveld J, et al. In vitro and in vivo evaluation of gelatin-chondroitin sulphate hydrogels for controlled release of antibacterial proteins. Biomaterials 2000;21:1763-72.
- Chourasia MK, Jain SK. Polysaccharides for colon targeted drug delivery. Drug Deliv 2004;11:129-48.
- Harada M, Murata JI, Sakamura Y, Sakakibara H, Okuno S, Suzuki T. Carrier and dose effects on the pharmacokinetics of T-0128, a camptothecin analogue-carboxymethyl dextran conjugate, in non-tumor- and tumor-bearing rats. J Control Release 2001;71:71-86.
- Kojima T, Hashida M, Muranishi S, Sezaki HJ. Mitomyc in C dextran conjugate: a novel high molecular weight pro-drug of mitomycin C. J Pharm Pharmacol 1980;32:30-4.
- Williams AS, Taylor G. Synthesis, characterization and release of cromoglycate from dextran conjugates. Int J Pharm 1992;83:233-9.
- Kim IS, Jeong YI, Kim DH, Lee YH, Kim SH. Albumin release from biodegradable hydrogels composed of dextran and poly (ethylene glycol) macromer. Arch Pharm Res 2001;24:69-73.
- Sery TW, Herhe EJ. Degradation of dextrans by enzymes of intestinal bacteria. J Bacteriol 1956;71:373-80.
- Brondsted H, Andersen C, Hovgaard L. Crosslinked dextran—a new capsule material for colon targeting of drugs. J Control Release 1998;53:7-13.