Renoprotection persists after cessation of treatment with very low doses of perindopril in Lyon hypertensive rats
Introduction
Since an increase in blood pressure (BP) is a direct determinant of progression of renal failure[1], one of the goals of hypertension treatment is to prevent a decline in renal function, especially of proteinuria, which is the most important risk factor for the prediction of renal outcome[2]. Originally, the renoprotective effects of antihypertensive agents were attributed solely to their BP-lowering potential. However, blockers of the renin-angiotensin system (RAS), such as angiotensin-converting enzyme (ACE) inhibitors, seem able to provide renoprotection even without modifying BP[3–11]. However this concept of BP-independent renoprotection by RAS blockers was recently challenged on the basis that episodic measurements of BP did not allow an accurate assessment of the pressure load to the kidneys. In effect, authors using a telemetric measurement of BP in a remnant kidney model of hypertensive rats have observed that renoprotection was closely (r≥0.80) related to continuously monitored BP[12,13]. In addition, clinical studies which indicated that RAS blockers were more protective for the kidneys than other antihypertensive agents were also associated with a better control of BP[14,15].
Using the Lyon model of genetically-hypertensive (LH) rats, which associates spontaneous hypertension and increased proteinuria[16], we observed that hypertension fully disappeared after an early RAS blockade[17], and that after cessation of the treatment, while BP levels were rapidly restored to pretreatment levels, proteinuria levels remained decreased[18].
Therefore, the aim of the present work was to determine whether in LH rats low non-antihypertensive doses of perindopril could afford significant renoprotection not only during treatment, but also after its cessation.
Material and methods
Forty-eight 3-week-old male LH rats were used in 2 successive experiments. The rats were maintained under a constant temperature (21±1 °C), humidity (60%±10%), and light (8:00–20:00 hours), and had free access to a standard rat chow (UAR A03; Epinay-sur-Orge, France) and tap water. The rats served as controls (no treatment) or received different doses of perindopril, which was included in the drinking water for rats aged 3–14 weeks, that is, from the time of weaning up until the stabilization of hypertension. Systolic blood pressure (SBP) was measured using tail-cuff plethysmography (Narco Biosystems, Houston, TX, USA) in unrestricted and preheated (38 °C for 10 min) rats at 16 and 23 weeks of age. The rats were then placed into metabolic cages, and their urine was collected at 24 h, and stored at –20 °C after centrifugation. The day after, the 23 week-old rats were killed, the kidneys were dissected, halved, and fixed in alcohol, formalin, and acetic acid for the semiquantitative evaluation of glomerular and interstitial lesions. The histological score was the sum of the number of glomeruli exhibiting focal and segmental sclerosis among the 50 examined, as well as signs of interstitial fibrosis and inflammation. These 2 latter parameters were graded from 0 to 3: (0=normal, 0.5=minimal, 1=slight, 2=moderate, and 3=severe). The urinary creatinine (Cr) concentration was measured using Jaffe’s reaction, and the urinary protein (Uprot) was determined using the pyrogallol red method and N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) with a previously-described enzyme immunoassay[19,20].
Protocols In the first experiment, 4 groups of 8 LH rats were randomly assigned to receive tap water (controls), 0.04 (P0.04), 0.1 (P0.01), or 0.4 mg·kg-1·d-1 (P0.4) of perindopril, an ACE inhibitor.
In the second experiment, 4 LH rats served as controls, and 2 groups of 6 animals received 0.01 (P0.01) or 0.04 mg·kg-1·d-1 (P0.04) perindopril. The doses were chosen according to a previous study[17] and unpublished preliminary trials. These protocols followed our institutional guidelines for animal care and experiments.
Statistical analysis Data are mean±SEM. Comparisons between groups used one-way ANOVA followed by Fisher’s exact test. P<0.05 was considered as significant.
Results
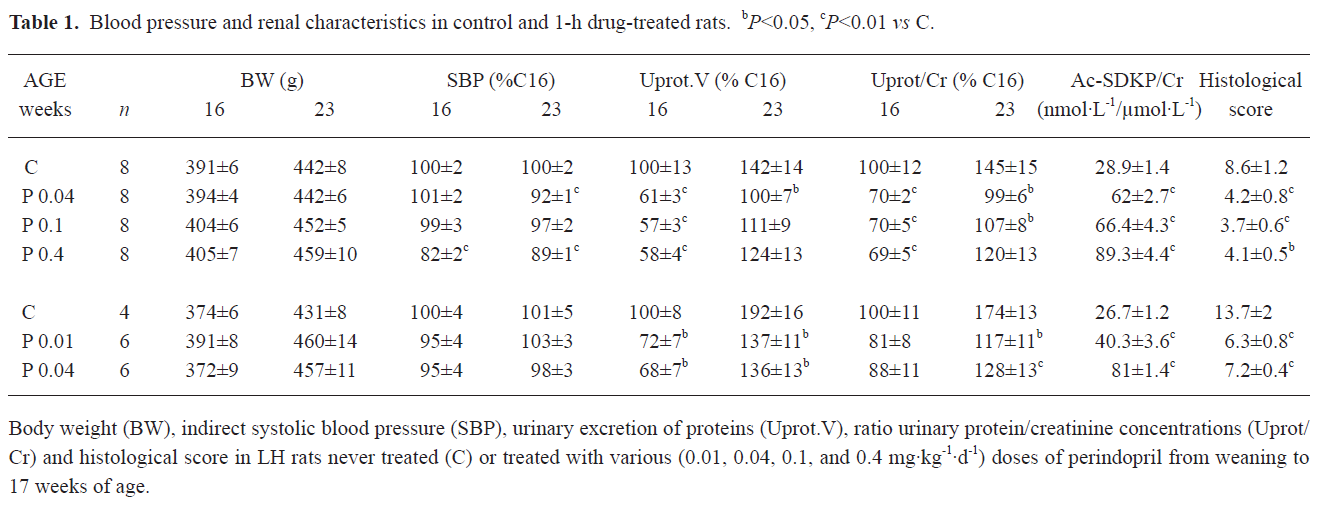
As indicated in Table 1, perindopril did not change the body weight of the rats. In order to facilitate the comparison between the 2 sets of experiments, the SBP values were expressed as the percentage of those measured in the 16 week-old controls (169±5 and 157±7 mmHg for experiments 1 and 2, respectively). However, for the correlation studies, only absolute values were used. At the end of the treatment period, only the highest dose of perindopril used (0.4 mg·kg-1·d-1) induced a significant decrease in SBP, which persisted 6 weeks after the cessation of treatment. The lower doses of perindopril were devoid of effects on SBP.
Full table
Proteinuria was expressed as the ratio Uprot/Cr so as to be independent of any mistake during the urines collection period. As well as for SBP, data are percentages of the values obtained in 16 week-old controls. Baseline proteinuria was as usual in LH rats, high and increasing from 16 to 23 weeks of age (182±24 to 260±27 and 191±13 to 370±32 mg per 24 h in experiments 1 and 2, respectively). At the end of the treatment, perindopril dose-dependently decreased Uprot/Cr. A maximum was reached with the 0.1 mg·kg-1·d-1 dose. The ratio Ac-SDKP/Cr, an index of ACE inhibition, was significantly and dose dependently increased with all the doses of perindopril used.
Six weeks after the cessation of the treatment Uprot/Cr remained significantly below the values observed in the control LH rats, but not in those that received the highest dose of perindopril (0.4 mg·kg-1·d-1).
Considering the histological data, the score summarizing the lesions of focal and segmental glomerular sclerosis, interstitial fibrosis, and inflammation was significantly reduced in all of the treated animals. Again, the 0.1 mg·kg-1·d-1 dose seemed the most efficient.
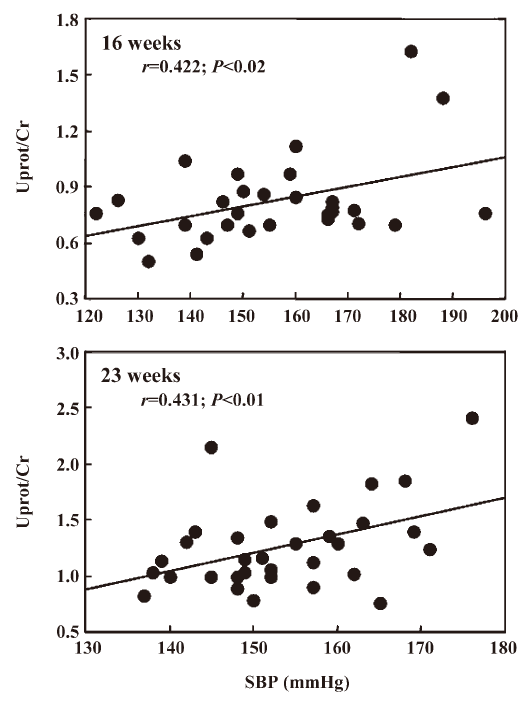
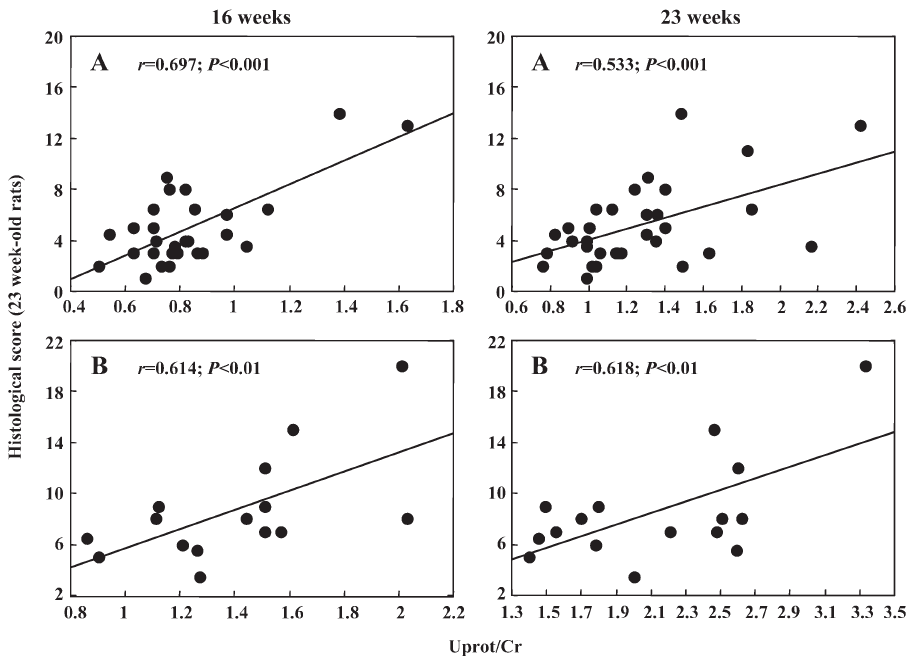
Interestingly, in the first experiment Uprot/Cr was related to SBP at the end of the treatment (r=0.422, n=27, P<0.02) and 6 weeks after its cessation (r=0.431, n=32, P<0.01, Figure 1). Such a relationship could not be disclosed in the second experiment, probably due to the narrow range of SBP values. As shown in Figure 2, in both experiments Uprot/Cr was closely related to the histological score. Similarly, Ac-SDKP/Cr of 16 weeks-old rats was inversely related with Uprot/Cr (r=–0.40) and with the histological score measured 6 weeks later (r=–0.45). When the data of the 2 successive experiments were pooled, the relationships between the histological score and the Uprot/Cr ratio reached highly significant (P<0.001) values of r=0.73 (n=45) and 0.67 (n=48) at 16 and 23 weeks of age, respectively.
Discussion
The present work demonstrates that in LH rats, an early and chronic treatment with very low, non-antihypertensive doses of perindopril, an ACE inhibitor induces a significant decrease in the proteinuria which is closely related to the state of renal lesions, and this renoprotection persists at least 6 weeks after the cessation of treatment.
SBP was measured using the tail-cuff method, which obviously does not allow to exclude that, even very low doses of perindopril, may have blunted short lasting peaks in SBP which could have been deleterious for the kidneys as suggested by Kurtz[21]. However this seems unlikely to be of importance since renal blood flow is well protected against increases in SBP as observed in sinoaortic denervated rats[22], and the dose of perindopril used in the second series of experiments is 40 times lower than the lowest dose exhibiting modest antihypertensive activity.
For practical reasons the study included 2 sets of experiments. This induced an additional variability, which is why we expressed the data as percentages of the values observed as means in the control 16 week-old rats of each set of experiments. However, it may represent an advantage since it gives more power to the findings when they are consistent among the 2 experiments.
As expected, hypertension was fully established at 16 weeks of age and did not vary in the control rats up to 23 weeks of age. On the contrary, despite this stable SBP, proteinuria increased with age, and this increase was more marked in the rats used in the second set of experiments than in those of the first. This more severe spontaneous evolution of proteinuria was in good accordance with more marked renal lesions in these animals although they did not display higher SBP levels.
In the treated LH rats, when the dose of perindopril was high enough to lower SBP (0.4 mg·kg-1·d-1), there was a significant relationship between SBP and proteinuria either at the end of the treatment or 6 weeks after its cessation. However, the strength of this correlation is low, which suggests that SBP is not the only factor determining proteinuria. This partial pressure independency of proteinuria is fully confirmed when much lower doses of perindopril significantly decreased proteinuria without affecting SBP. It may also find its roots in genetics since urinary albumin excretion is controlled by chromosomal loci, which for a vast majority, differ from those linked to SBP[23]. In addition, since angiotensin II plays a unique role in the pathophysiology of proteinuria[24–31], it can be hypothesized that even very low doses of perindopril are able to lower the intrarenal formation of angiotensin II, and consequently slow the development of renal lesions. This hypothesis is strengthened by the fact that all the doses of perindopril used elevated the excretion of Ac-SDKP, which demonstrates a blockade of renal ACE[32].
The other prominent observation made in the present work is that the renoprotective effect of very low non-antihypertensive doses of perindopril weakens, but remains highly significant 6 weeks after the end of the treatment, as shown by the values of proteinuria and the score of histological lesions.
In conclusion, this work demonstrates for the first time that, at least in LH rats, early and chronic treatment with an ACE inhibitor exerts renoprotective effects, which can be fully independent of BP decrease and persist after withdrawal of the treatment.
Acknowledgement
The authors are grateful to Dr Ming LO for his invaluable help.
References
- Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. Am J Kidney Dis 2000;36:646-61.
- Keane WF, Brenner BM, De Zeeuw D, Grunfeld JP, McGill J, Mitch WE, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL STUDY. Kidney Int 2003;63:1499-507.
- Remuzzi A, Imberti O, Puntorieri S, Malanchini B, Macconi D, Magrini L, et al. Dissociation between antiproteinuric and anti-hypertensive effect of angiotensin converting enzyme inhibitors in rats. Am J Physiol 1994;267:F1034-F1044.
- Opie L. Renoprotection by angiotensin-receptor blockers and ACE inhibitors in hypertension. The Lancet 2001;358:1829-31.
- Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 2002;106:672-8.
- Isogai S, Kameyama M, Iso K, Yoshino G. Protective effects of a small dose of captopril on the reduction of glomerular basement membrane anionic sites in spontaneously hypertensive rats with streptozocin-induced diabetes. J Diabetes complications 1998;12:170-5.
- Linz W, Becker R, Scholkens B, Wiemer G, Keil M, Langer KH. Nephroprotection by long-term ACE inhibition with ramipril in spontaneously hypertensive stroke prone rats. Kidney Int 1998;54:2037-44.
- Keilani T, Danesh F, Schlueter W, Molteni A, Batlle D. A subdepressor low dose of ramipril lowers urinary protein excretion without increasing plasma potassium. Am J Kidney Dis 1999;33:450-7.
- Suyama K, Muso E, Yashiro M, Sasayama S. Significant suppressive effect of low-dose temocapril, an ACE inhibitor with biliary excretion, on FGS lesions in hypertensive rats. Nephron 2000;86:491-8.
- Toto RD, Adams-Huet B, Fenvers AZ, Mitchell HC, Mulcahy W, Smith RD. Effect of ramipril on blood pressure and protein excretion rate in normotensive nondiabetic patients with proteinuria. Am J Kidney Dis 1996;28:832-42.
- Rostoker G, Ben-Maadi A, Remy P, Lang P, Lagrue G, Weil B. Low-dose angiotensin converting enzyme inhibitor captopril to reduce proteinuria in adult idiopathic membranous nephropathy: A prospective study of long-term treatment. Nephrol Dial Transplant 1995;10:25-9.
- Bidani A, Griffin K, Bakris G, Picken M. Lack of evidence of BP-independent protection by renin-angiotensin system blockade after renal ablation. Kidney Int 2000;57:1651-61.
- Griffin K, Abu-Amarah I, Picken M, Bidani A. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure-dependent. Hypertension 2003;41:201-6.
- Lasaridis AN, Sarafidis PA. Diabetic nephropathy and antihypertensive treatment: What are the lessons from clinical trials? Am J Hypertens 2003;16:689-97.
- Vaur L, Gueret P, Lievre M, Chabaud S, Passa P. DIABHYCAR Study Group. (type 2 DIABetes, Hypertension, CARdiovascular Events and Ramipril) study. Development of congestive heart failure in type 2 diabetic patients with microalbuminuria or proteinuria: Observation from the DIABHYCAR (type 2 DIABetes, Hypertension, Cardiovascular events and ramipril) study. Diabetes Care 2003;26:855-60.
- Vincent M, Sassard J. Lyon strains. In: Swales JD, editor. Textbook of hypertension. Oxford: Blackwell Scientific; 1994. p455–7.
- Lantelme P, Lo M, Luttenauer L, Sassard J. Pivotal role of the renin–angiotensin system in Lyon hypertensive rats. Am J Physiol Regul Integr Comp Physiol 1997;273:R1793-R1799.
- Bertram D, Blanc-Brunat N, Sassard J, Lo M. Differential evolution of blood pressure and renal lesions after RAS blockade in Lyon hypertensive rats. Am J Physiol Regul Integr Comp 2002;283:R104-15.
- Ezan E, Carde P, Le Kerneau J, Ardouint T, Thomas F, Isnard F, et al. Pharmacokinetics in healthy volunteers and patients of NAc-SDKP (seraspenide), a negative regulator of hematopoiesis. Drug Metab Dispos 1994;22:843-8.
- Blanc-Brunat N, Vincent M, Sassard J. Evolution des lésions rénales précoces chez trois souchess de rats génétiquement hypertendus, normotendus et normotendus bas. Arterial Wall 1980;6:145-56. (in French).
- Kurtz TW. False claims of blood pressure-independent protection by blockade of the renin angiotensin aldosterone system. Hypertension 2003;41:193-6.
- Pires S, Julien C, Chapuis B, Sassard J, Barrès C. Spontaneous renal blood flow autoregulation curves in conscious sinoaortic denervated rats. Am J Physiol Renal Physiol 2002;282:F515-8.
- Garret MR, Dene H, Rapp JP. Time-course genetic analysis of albuminuria in Dahl salt-sensitive rats on low-salt diet. J Am Soc Nephrol 2003;14:1175-87.
- D’Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int 2003;63:809-25.
- Florin M, Lo M, Liu KL, Sassard J. Salt sensitivity in genetically hypertensive rats of the Lyon strains. Kidney Int 2001;59:1865-72.
- Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, et al. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 2003;42:31-8.
- Franco M, Tapia E, Santamaria J, Zafra I, García-Torres R, Gordon KL, et al. Renal cortical vasoconstriction contributes to development of salt sensitive hypertension after angiotensin II exposure. J Am Soc Nephrol 2001;12:2263-71.
- Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 2002;161:1679-93.
- Del Prete D, Gambaro G, Lupo A, Anglani F, Brezzi B, Magistroni R, et al. Precocious activation of genes of the renin-angiotensin system and the fibrogenic cascade in IgA glomerulonephritis. Kidney Int 2003;64:149-59.
- Ruiz-Ortega M, Ruperez M, Lorenzo O, Anglani F, Brezzi B, Magistroni R, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int 2002.Suppl 82:12-22.
- Kuno A, Yamada T, Masuda K, Ogawa K, Sogawa M, Nakamura S, et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology 2003;124:1010-9.
- Wang D, Yang XP, Rhaleb NE, Beierwaltes WH, Carretero OA. Renoprotective effect of Ac-SDKP in Dahl salt-sensitive rats. Hypertension 2003;42:418. (abst).