Chronic morphine exposure induces degradation of receptive field pro-perties of LGN cells in cats1
Introduction
The brain is rich in opiate receptors, and significant concentrations of opiate receptors are observed in the visual systems of cats[1], macaques[2], and rats[3]. It has been suggested that the visual system is subject to opiate modulation. Previous studies have shown that morphine-like drugs decrease visual sensitivity in humans[4], affect visual discrimination performance in rats[5], and evoke cortical potentials by stimulating the optic chiasm in cats[6].
Repeated use of addictive drugs leads to multiple adaptive neuronal responses[7,8]. Increasingly, research suggests that chronic exposure to opiates significantly changes glutamatergic synaptic transmission and neuronal plasticity in many brain regions[9,10]. Similarly, GABAergic synaptic transmission is also influenced by opiates[11–13]. However, normal excitatory and inhibitory synaptic transmission is crucial for the development and maintenance of visual function. These findings indicate that opiates might influence the performance of neurons in the lateral geniculate nucleus (LGN). In the present study, we tested this possibility by using extracellular single-unit recording techniques to examine the stimulus sensitivity of LGN neurons in chronic morphine-treated cats.
Materials and methods
Subjects and drug exposure The experiments were performed on 8 healthy adult male cats (2–3 kg), 4 of which were allocated to the morphine treatment group and 4 of which were used as controls. All animals were treated strictly in accordance with the Chinese National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. The method of morphine treatment was similar to that used by other researchers[9]. Morphine HCl (10 mg/kg) was administered by cervical subcutaneous injection twice per day at 9:00 and 21:00 for 10 d before the electrophysiological experiments. Control cats were treated similarly with saline instead of morphine.
All cats were examined ophthalmoscopically before the experiment to confirm that they had no optical problems or obvious retinal problems that would impair the visual function.
Preparation for extracellular recording On the 11th day of morphine administration, animals were prepared for extracellular single-unit recording as described previous-ly[14,15]. A typical recording session lasted for 3 d. During recording, morphine or saline was injected in the same way as described in the previous section.
Visual stimulation The receptive fields of cells were first plotted by using manually controlled stimuli displayed on the tangent screen. Computer-controlled visual stimuli consisting of drifting sinusoidal gratings were presented on a CRT (cathode-ray tube) monitor (1024×768, 85 Hz), placed 57 cm away from the animals’ eyes. The program to generate the stimulus was written in MATLAB, using the extensions provided by the high-level Psychophysics Toolbox[16] and the low-level Video Toolbox[17]. The time frequency of drifting gratings was set at 3 Hz. At first, a tuning curve of spatial frequency was plotted. We selected a high spatial frequency where cells’ responses were approximately half of that at peak frequency to compile the orientation and direction tuning curves. Before each stimulus presentation, 5 s spontaneous activities were obtained while the mean luminance was shown on the display. Additional stimuli were used to identify a cell as X- or Y-type, and on- or off-center. The contrast of the stimulus was set at 80%. The mean luminance of the display was 19 cd/m2, and the environment luminance on the cornea was 0.1 lux.
Data collection and analysis After the neuronal signal was amplified with a microelectrode amplifier (Nihon Kohden, Tokyo, Japan) and a differential amplifier (FHC, Bowdoinham, USA), action potentials were fed into a window discriminator with an audio monitor. The original voltage traces were digitized using an acquisition board (National Instruments, Austin, USA) controlled by IGOR software (WaveMetrics, Portland, USA). The original data were saved for online and offline analysis. The post-stimulus time histograms (PSTH) of neuronal responses with a bin width of 10 ms were obtained for further analysis. The responses of cells to the sinusoidal gratings were treated using a fast Fourier transform and the amplitude was defined as the resultant fundamental Fourier component (FFT1) of the PSTH. The FFT1 value of each stimulus orientation (direction) was used to draw the spatial frequency and orientation (direction) tuning curves. The method for calculation of orientation bias (OB) and direction bias (DB) has been described elsewhere[18,19]. A cell with bias=0.1 was considered significantly biased for orientation or direction. A cell with bias=0.2 was considered strongly biased for orientation or direction. A cell’s signal-to-noise ratio (STN) was defined as the ratio of the cell’s visual evoked response (FFT1 value in preferred orientation and direction) to spontaneous response. To avoid data skewing or overestimation, all spontaneous activities below 1 spike per second were set equal to 1 spike per s for signal-to-noise analysis.
Statistical comparisons between morphine-treated and saline-treated cat data were carried out using t-test. All mean values were presented as mean±SEM.
Results
The results described here were obtained from 168 cells in 4 morphine-treated cats (abbreviated to MCs) and 173 cells in 4 saline-treated cats (abbreviated to control) in LGN. Our chief findings were that chronic morphine exposure led to a decrease in the signal-to-noise ratio and a degradation in the orientation and direction sensitivity of LGN cells, which was accompanied by an increase in spontaneous and evoked response. Our analysis revealed that there was a consistent effect of chronic morphine exposure on different types of LGN cells (X or Y; on or off), so comparisons of data between two groups ignore the type of cell.
Orientation and direction sensitivity As an example, we show here the response of an LGN cell to a drifting sinusoidal grating (Figure 1A) and its PSTH (Figure 1B). Figure 1C and 1D illustrate the tuning curve of a cell from controls (OB=0.2, DB=0.01) and MCs (OB=0.1, DB=0.08), respectively. MCs have a decrease in orientation and direction sensitivity relative to controls. The average OB and DB values varied individually in both MCs and controls. The OB were significantly smaller in MCs (0.103±0.068) than in control cats (0.135±0.084, P<0.01; Figure 1E). Sixty-three percent of LGN cells in controls and 50% of cells in MCs had significant orientation sensitivity (OB=0.1). In MCs, the percentage of cells (10%) with strong orientation sensitivity (OB=0.2) was half of that in controls (20%). Similarly, the direction sensitivity of LGN cells was also affected by chronic morphine exposure. The DB of LGN cells were significantly less for MCs (0.074±0.059) than for controls (0.100±0.064, P<0.01; Figure 1F). As with the orientation bias, the percentages of cells having significant and strong direction bias in MCs (30% and 6%, respectively) were less than those in controls (47% and 10%, respectively).

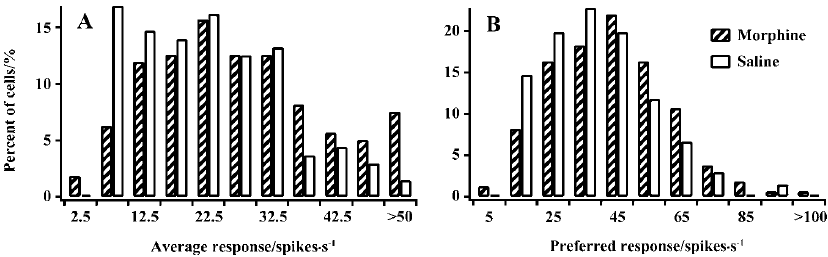
Visually evoked activity To explore whether the sensitivity decrease of cells in MCs resulted from an increase of responsiveness to previously non-preferred orientations and directions or from a reduction of responsiveness to the previously preferred orientations and directions, or both, we compared the average response (AR) to all stimulating grating orientations of LGN cells in MCs and controls. Cells in MCs showed a tendency for an increase in the AR compared with controls (Figure 2A). Half of the cells (50.3%) in MCs had an AR value of more than 25 spikes per second. In contrast, most cells (63.2%) in controls had an AR value of less than 25 spikes per second. A statistical analysis showed that the mean AR value in MCs (28.1±16.1) was significantly larger than that in controls (22.3±11.3, P<0.01). Moreover, we also compared the response of LGN cells to their preferred orientation and direction in MCs and controls. For convenience, we call this the preferred response (PR). Similar to the AR, the PR value in MCs was also increased relative to that in controls (Figure 2B). The t-test indicated that the mean PR value (42.2±19.1) for MCs was significantly higher compared with that for controls (38.1±16.2, P<0.05). Therefore, LGN cells in MCs have increased responsiveness to both preferred and non-preferred stimuli.
Spontaneous activity and signal-to-noise ratio LGN cells in controls trended to have a small spontaneous response (SR) value, whereas cells in MCs had a more extensive distribution. Most cells (63.9%) in controls had an SR value of less than 20 spikes per second. In contrast, approximately half of the cells (53.4%) in MCs had an SR value of more than 20 spikes per second. The average SR value (27.4±22.5) in MCs was significantly higher than that in controls (17.5±14.3, P<0.01; Figure 3A).
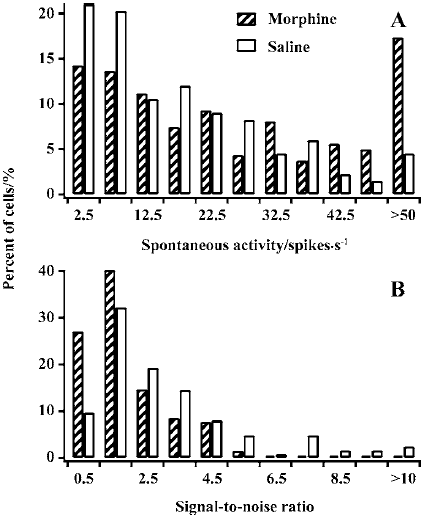
Figure 3B presents the distribution of various signal-to-noise ratios (STN=PR/SR) for cells in MCs and controls. The number of cells with lower STN was much more for MCs than for controls. Almost all cells (98.4%) in MCs had an STN value of less than 5. However, quite a number of cells (16.1%) had an STN value of more than 5 in controls. A comparison of the averaged STN for controls (3.1±2.3) versus MCs (1.9±1.2) showed that the difference was statistically significant (P<0.01). Taken together, these data suggest that chronic morphine exposure results in an increase in spontaneous activity and a decrease in signal-to-noise ratio in LGN cells in MCs.
Discussion
We found significant degradation in the receptive field properties of LGN neurons in MCs. The morphine-derived degradation in orientation and direction sensitivity in LGN neurons was accompanied by an unspecific increase in responsiveness. The average response across all orientations of stimulus gratings for MCs increased by 26.0% compared with controls, whereas the response to preferred stimuli increased by only 11.2%. Thus, the increased responsiveness to non-preferred orientations and directions appears to be an important mechanism mediating the reduction in cells’ sensitivity to stimuli in MCs. On the other hand, the spontaneous activity of LGN neurons in MCs increased by 56.6% compared with controls. This increase was much higher than the increase in preferred response. As a result, the ratio of preferred response to spontaneous activity (signal-to-noise ratio) in MCs was much lower than in controls. The decrease in signal-to-noise ratio in MCs thus appears to mainly reflect the large increase in spontaneous activity.
Orientation and direction sensitivity is a well-known receptive field property of neurons in the mammalian visual cortex[20]. However, it is quite controversial whether orientation and direction sensitivity exists in LGN neurons. Many tests beyond the peak spatial frequency have shown that a lot of LGN cells are sensitive to stimulus orientation or direction[14,21]. The results of the present study also show this. It has been proposed that an elliptical shape of the receptive-field center and asymmetry in the inhibitory surrounds contribute to the orientation and direction sensitivity of LGN cells. Previous investigations suggest that microionto-phoresis of bicuculline (an antagonist of the GABAA receptor) affects the orientation and direction sensitivity of neurons in LGN of cats[22,23]. Moreover, LGN cells also have increased spontaneous and evoked activity when GABAergic inhibition is blocked[22,24]. In the present study, LGN cells in MCs had decreased orientation and direction sensitivity accompanied by increased spontaneous and evoked activity. Furthermore, research on drug abuse suggests that the decline of inhibitory neurotransmission actually happens in many brain regions[11–13]. In view of the extensive expression of opiate receptors throughout the visual system, reduced GABAergic inhibition might be a reasonable explanation for the degradation of receptive field properties of LGN cells in MCs. Nevertheless, some studies have shown that drug abuse also affects glutamatergic and other transmitter systems, indicating that these transmitter systems might affect the sensitivity of LGN cells to visual stimuli following chronic morphine exposure. Additionally, LGN neurons reflect the properties of the center-surround organized retinal receptive fields and convey them to the cortex in a way that does not significantly alter their spatial structure[24]. Therefore, the action of chronic morphine exposure on the retina might be one factor resulting in the degradation of the receptive field properties of LGN cells in MCs.
In summary, our results suggest that chronic morphine exposure can lead to the degradation of the receptive field properties of LGN cells in cats. Morphine-derived decreases of GABAergic inhibition together with other factors might contribute to the functional degradation of LGN cells in MCs. All in all, much remains to be learned about the role of opiate receptors in the visual system and the morphine-derived decay of receptive field properties of LGN neurons.
References
- Walker JM, Bowen WD, Thompson LA, Frascella J, Lehmkuhle S, Hughes HC. Distribution of opiate receptors within visual structures of the cat brain. Exp Brain Res 1988;73:523-32.
- Wise SP, Herkenham M. Opiate receptor distribution in the cerebral cortex of the Rhesus monkey. Science 1982;218:387-9.
- Lewis ME, Pert A, Pert CB, Herkenham M. Opiate receptor localization in rat cerebral cortex. J Comp Neurol 1983;216:339-58.
- Rothenberg S, Peck EA, Schottenfeld S, Betley GE, Altman JL. Methadone depression of visual signal detection performance. Pharmacol Biochem Behav 1979;11:521-7.
- Grilly DM, Genovese RF, Nowak MJ. Effects of morphine, xd-amphetamine, and pentobarbital on shock and light discrimination performance in rats. Psychopharmacology (Berl) 1980;70:213-7.
- Wilkison DM, Hosko MJ. Selective augmentation of visual pathways by morphine in alpha-chloralose-anesthetized cats. Exp Neurol 1982;77:519-33.
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 1997;17:8491-7.
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse 1999;33:160-2.
- Pu L, Bao GB, Xu NJ, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci 2002;22:1914-21.
- Martin G, Ahmed SH, Blank T, Spiess J, Koob GF, Siggins GR. Chronic morphine treatment alters NMDA receptor-mediated synaptic transmission in the nucleus accumbens. J Neurosci 1999;19:9081-9.
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature 1997;390:611-4.
- Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci 2004;7:160-9.
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Luscher C. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci 2004;7:153-9.
- Zhou Y, Leventhal AG, Thompson KG. Visual deprivation does not affect the orientation and direction sensitivity of relay cells in the lateral geniculate nucleus of the cat. J Neurosci 1995;15:689-98.
- Shou T, Li X, Zhou Y, Hu B. Adaptation of visually evoked responses of relay cells in the dorsal lateral geniculate nucleus of the cat following prolonged exposure to drifting gratings. Vis Neurosci 1996;13:605-13.
- Brainard DH. The psychophysics toolbox. Spat Vis 1997;10:433-6.
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 1997;10:437-42.
- Levick WR, Thibos LN. Analysis of orientation bias in cat retina. J Physiol 1982;329:243-61.
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent Rhesus monkeys. Nat Neurosci 2000;3:384-90.
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol 1968;195:215-43.
- Thompson KG, Leventhal AG, Zhou Y, Liu D. Stimulus dependence of orientation and direction sensitivity of cat LGNd relay cells without cortical inputs: a comparison with area 17 cells. Vis Neurosci 1994;11:939-51.
- Hu B, Li X, Zhou Y, Shou T. Effects of GABA antagonist bicuculline on the orientation tuning character of relay cells in dorsal geniculate nucleus (dLGN). Acta Biophys Sin 1994;10:121-7.
- Hu B, Li X, Zhou Y, Shou T. Effects of bicuculline on direction-sensitive relay cells in the dorsal lateral geniculate nucleus (LGNd) of cats. Brain Res 2000;885:87-93.
- Holdefer RN, Norton TT, Godwin DW. Effects of bicuculline on signal detectability in lateral geniculate nucleus relay cells. Brain Res 1989;488:341-7.
