Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR
Introduction
Although progress has been made in the treatment of patients with gastric cancer over the last two decades, a significant percentage of these patients fail to achieve complete remission or they relapse due to the phenomenon of multidrug resistance (MDR). In tumor cell lines, MDR is often associated with the overexpression of ATP-dependent drug efflux proteins belonging to the superfamily of ATP-binding cassette (ABC) transporters: the 170 kDa P-glycoprotein (P-gp) encoded by the MDR1 gene and the 190 kDa multidrug resistance-associated protein-1 encoded by the MRP-1 gene[1,2]. These proteins bind to and transport various structurally unrelated compounds to maintain their intracellular concentrations below cytotoxic levels.
Over the last two decades, considerable efforts have been made to circumvent the problem with multidrug resistance. One approach is to use various resistance modulators to reverse MDR mechanisms and thus sensitize MDR tumor cells to anticancer drugs. Several compounds can reverse MDR, such as calcium channel blockers, CsA, MK571, buthionine sulfoximine (BSO) and genistein. However, the use of effective doses of these agents for systemic chemotherapy has been difficult because of their side effects and dose-limiting toxicity. Therefore, it is necessary to find new reversing agents that do not have the undesirable toxicological effects[3].
Curcumin is a natural phenolic coloring compound that is found in the rhizomes of Curcuma longa L, commonly called turmeric. It has been widely used as a spice, to color cheese and butter, as a cosmetic[4], and in some medicinal preparations[5]. Curcumin has a wide range of biological and pharmacological activities, including antioxidant properties[6], anti-inflammatory properties[5], anti-mutagenic activity[7], and anti-carcinogenic[8], hypocholesterolemic[9] and hypoglycemic effects[10]. The safety of C longa and its derivatives has been studied in various animal models[11], and it is clear that turmeric is not toxic even at high doses in laboratory animals. A single feeding of a 30% turmeric diet to rats did not produce any toxic effects. In a 24-h acute toxicity study, mice were fed doses of 0.5 g/kg, 1.0 g/kg, and 3.0 g/kg of turmeric extract daily. There was no increase in the mortality rate when compared with the controls in either study.
Because of its wide range of biological and pharmacological effects, and a lack of toxicity in animal models, curcumin has been examined to determine whether it interacts with MDR. In vitro studies have demonstrated that curcumin is able to modulate both the expression and function of P-gp in multidrug-resistant KB cells[12,13] and primary cultures of rat hepatocytes[14]. It is not clear whether curcumin can reverse the P-gp-mediated MDR of resistant gastric cancer cells. This led us to evaluate the effect of curcumin on the MDR of a vincristine (VCR)-resistant gastric cancer cell line (SGC7901/VCR). We found that treatment of drug-resistant SGC7901/VCR cells with curcumin increased their sensitivity to vincristine, which was consistent with decreased P-gp function and expression. In addition, curcumin was able to promote VCR-stimulated caspase-3 activation in SGC7901/VCR cells. It is possible that curcumin could be useful in the treatment of drug resistant gastric cancer cells as a MDR modulator.
Materials and methods
Reagents Curcumin, MTT, ethidium bromide (EB), acridine orange (AO) and rhodamine123 (Rh123) were obtained from Sigma (St Louis, MO, USA); vincristine (VCR) was obtained from Farmitalia Carlo Erba (Milan, Italy); the anti-cleaved caspase-3 antibody was purchased from Cell Signaling Technology (Beverly MA); the anti-P-gp antibody and FITC-conjugated goat antimouse IgG were bought from Beijing Zhongshan Biological Technology Co (Peking, China).
Cells and cell cultures Gastric cancer cell line SGC7901 and VCR-resistant gastric cancer SGC7901/VCR cells were kindly provided by the Department of Gastroenterology, Xijing Hospital, the Fourth Military Medical University (Xi’an China). The MDR subline SGC7901/VCR was developed by exposing the parental SGC7901 cells to increasing concentrations of the anticancer drug VCR. All cells were cultivated in RMPI-1640 supplemented with 10% heat-inactivated fetal bovine serum at 37 °C in a 5% CO2 atmosphere. The medium for SGC7901/VCR cells was further supplemented with VCR (0.25 µmol/L). Before their use in experiments, the SGC7901/VCR cells were cultured in a drug-free medium for 2 weeks.
Cytotoxicity assay by MTT Cells were seeded into 96-well plates at densities of 1×104 SGC7901/VCR cells per well and 0.5×104 SGC7901 cells per well, and incubated in a humidified atmosphere of 5% CO2+95% air overnight, then normal cell medium containing either test compounds or solvents at the desired concentration were added. After 72 h incubation, 20 µL MTT (5 g/L in phosphate buffered saline,PBS) were added. The plates were incubated for 4 h and the blue dye formed was dissolved in 100 µL dimethyl sulfoxide (DMSO or Me2SO). The absorbance at 570 nm was recorded using an ELISA reader. The survival rate was calculated as follows:
Survival rate (%)=(T–B)/(U–B)×100%
where T is the absorbance of treated tumor cells when exposed to drugs, U is the absorbance of untreated cells, B is the absorbance when neither the drug nor MTT was added (blank).
The 50% inhibitory concentration (IC50) of 3 d exposure for a particular agent was defined as the drug concentration that causes in a 50% reduction in the number of cells compared with the untreated control. The IC50 values were determined by Bliss software.
Apoptosis assay by flow cytometer using propidium iodide staining Cells were treated with various concentrations of VCR and curcumin for 24 h, and then harvested with 0.25% trypsin and washed with PBS. Cells (2×105) were fixed in 70% ice-cold EtOH/PBS for 20 min on ice, and then washed with PBS and incubated in propidium iodide (PI) solution (69 mmol/L PI, 388 mmol/L sodium citrate, 100 µg/mL RNase A) for 15 min at 37 °C. Cells were immediately analyzed with FAC scan flow cytometry (Becton Dickinson, San Jose, CA).
Apoptosis detection by morphological observation after AO/EB staining Cells were treated with various concentrations of VCR and curcumin for 24 h, and then harvested with 0.25% trypsin and resuspended in RPMI-1640 medium. After staining for 10 min with 4 mL of an AO (100 mg/mL)/EB (100 mg/mL) dye mixture, cells were visualized immediately under a fluorescence microscope.
Accumulation and efflux of Rh123 as measured by flow cytometry The measurement of Rh123 accumulation was performed as previously described[15]. Briefly, cells (5×105 per sample) were incubated with 1 µg/mL of Rh123 in the dark at 37 °C in 5% CO2 for 120 min. Curcumin (dissolved in 0.1% DMSO) was added to cultures at the same time as Rh123. A final concentration of 0.1% Me2SO (v/v) was used for all experiments and controls. Following Rh123 accumulation, cells were washed twice with ice-cold Hanks’ Balanced Salt (HBSS) (without phenol red), placed in HBSS with 10% fetal bovine serum on wet ice. The green fluorescence of Rh123 was measured by using a FAC scan flow cytometer (Becton Dickinson).
For determination of Rh123 efflux, cells were loaded for 60 min with Rh123 in the absence of curcumin, and then the medium was replaced with Rh123-free medium containing curcumin, or the vehicle (Me2SO). Following efflux intervals of 60 min, the medium was removed, and the cells were washed twice with ice-cold HBSS and prepared for flow cytometry as described earlier. As measured by Trypan blue exclusion, the cells remained viable during the Rh123 accumulation and efflux studies with curcumin.
Flow-cytometric analysis of the expression of P-gp and the activation of caspase-3 The cells were collected and adjusted to a concentration of 1×106 cells per mL and centrifuged at 1500×g for 5 min at 20 °C. The cell pellet was resuspended in 20 mL anti-P-gp or anti-active caspase-3 antibody (1:400), and then incubated at 4 °C for 30 min while being protected from light. After three washes with cold PBS containing l % fetal calf serum (FCS), cells were incubated while being protected from light at 4 °C for 30 min with FITC-conjugated goat antimouse IgG (l:1000). Then cells were centrifuged at 1500×g for 5 min at 20 °C and were resuspended with PBS. The fluorescence intensity of cells was then analyzed by using a flow cytometer (Becton Dic-kinson).
Statistical analysis Data were expressed as mean±SD. Student’s t-test was used to assess the statistical significance of differences. P<0.05 was considered to be statistically significant.
Results
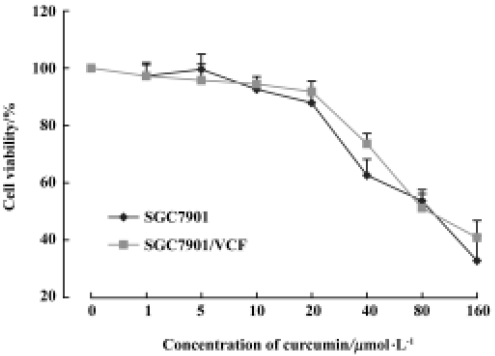
Cytotoxic effect of curcumin on SGC7901 and SGC7901/VCR cells We found that 1–20 µmol/L curcumin was not obviously cytotoxic to gastric cancer cell line SGC7901 or VCR-resistant gastric cancer SGC7901/VCR cells (survival rate >85%). However, 40–160 µmol/L curcumin caused significant cytotoxicity in SGC7901 and SGC7901/VCR cells (Figure 1). Because treatment of the cells with 5 µmol/L, 10 µmol/L, and 20 µmol/L curcumin had no significant effect on cell viability, we used these concentrations for further analysis.
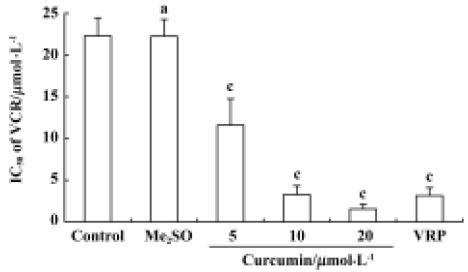
Effect of curcumin on VCR resistance in SGC7901/VCR cells The IC50 values of VCR were 0.49 µmol/L and 22.27 µmol/L in the sensitive and resistant lines, respectively, which indicated that the SGC7901/VCR cell line was 45 times more resistant to VCR than the parent SGC7901 cell line. As shown in Figure 2, when 5 µmol/L, 10 µmol/L, or 20 µmol/L curcumin was added, the IC50 value of VCR in the SGC7901/VCR cell line significantly decreased from 22.27 µmol/L to 11.60 µmol/L, 3.28 µmol/L, and 1.53 µmol/L, respectively, in a dose-dependent manner. After treatment with 20 µmol/L verapamil (positive control), the IC50 value of VCR in the SGC7901/VCR cell line only decreased from 22.27 µmol/L to 3.13 µmol/L.
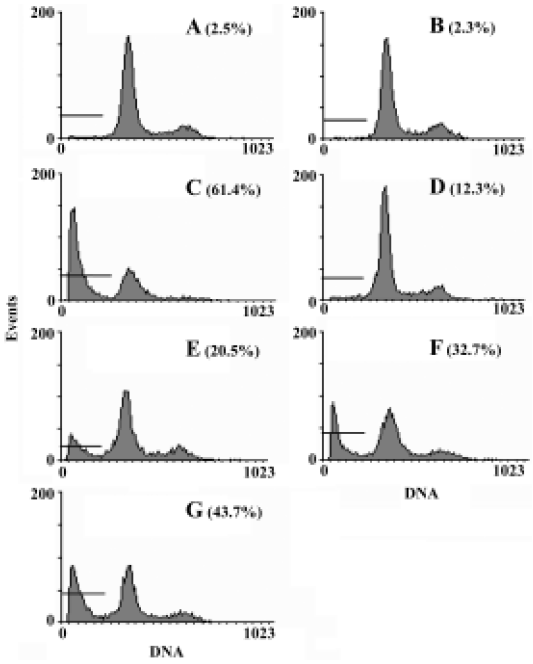
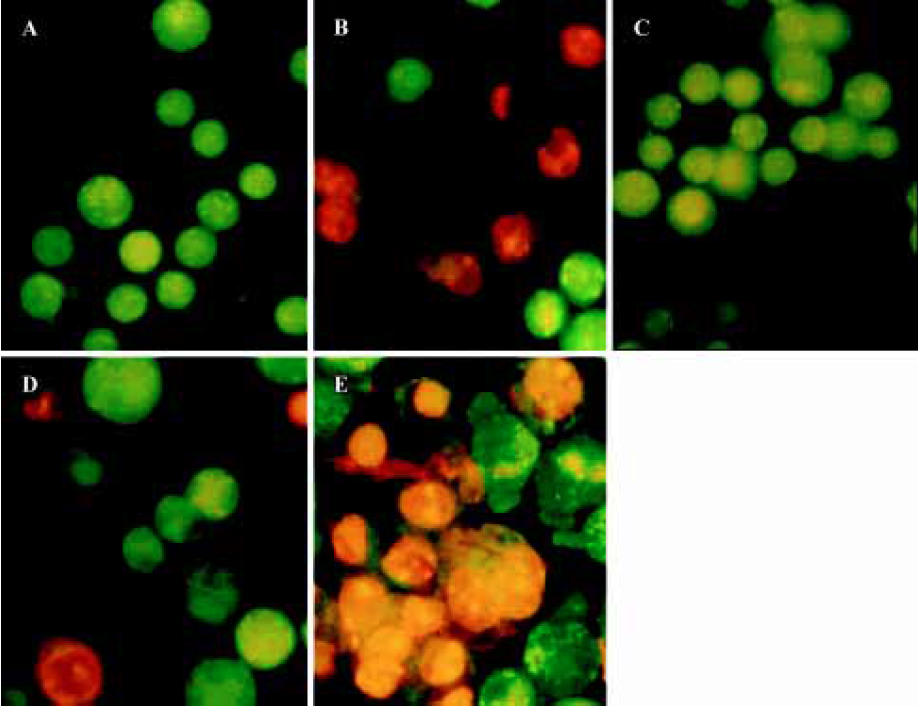
Effect of curcumin on the apoptotic resistance of SGC7901/VCR cells Apoptotic cells were quantified as the proportion of cells that had a DNA content of less than 2N (sub-Gl DNA content)[16]. As shown in Figure 3, after treatment with 1 µmol/L VCR for 24 h, the apoptosis of SGC7901/VCR cells was only 12.5%, but significant apoptosis was observed in the parental cells (61.4%, P<0.01) at the same drug concentration, indicating that SGC7901/VCR cells had apoptotic resistance to VCR. Curcu-min, at concentrations of 5 µmol/L, 10 µmol/L, and 20 µmol/L for 24 h, promoted the VCR-mediated apoptosis of SGC7901/VCR cells from 12.3% to 20.5%, 32.7%, and 43.7%, respectively, in a dose-dependent manner. Moreover, AO/EB staining also revealed that curcumin promoted VCR-induced apoptosis in SGC7901/VCR cells (Figure 4). These results demonstrate that curcumin is able to overcome the apoptotic resistance of SGC7901/VCR cells to VCR.
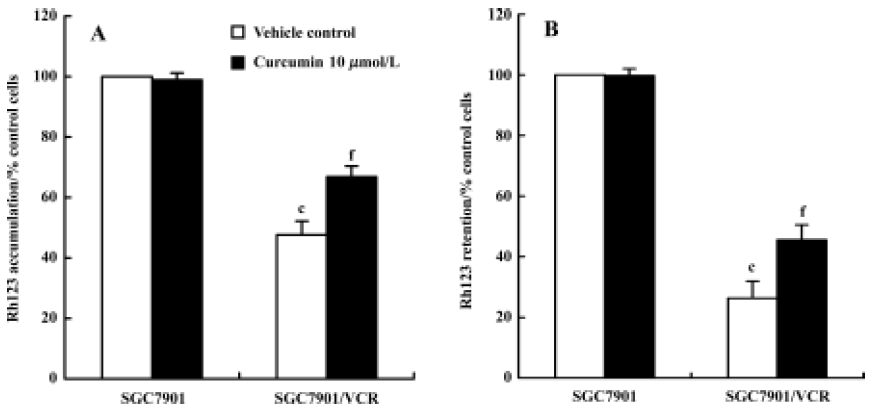
Effect of curcumin on P-gp functions To examine the function of P-gp on the surface of viable cells, Rh123 accumulation and efflux studies were chosen because they appear to be sensitive indicators of P-gp activity when assayed by FAC scans[17,18]. As shown in Figure 5A, Rh123 accumulation in SGC7901/VCR cells was obviously less than that in SGC7901 cells after being incubated with Rh123 for 120 min (P<0.01). After treatment with curcumin (10 µmol/L), Rh123 accumulation in SGC7901/VCR cells was increased by 40%; however, there was no change in SGC7901 cells. The effect of curcumin on the P-gp-mediated efflux of Rh123 in SGC7901 and SGC7901/VCR cells was also examined. As shown in Figure 5B, Rh123 retention in SGC7901/VCR cells was obviously less than that in SGC7901 cells. When SGC7901/VCR cells were treated with curcumin (10 µmol/L) for 60 min, Rh123 retention increased by 80% compared with the vehicle control (0.1% Me2SO), indicating that curcumin could cause a significant decrease in Rh123 efflux. However, in SGC7901 cells, there was no change in Rh123 retention in the presence of curcumin.
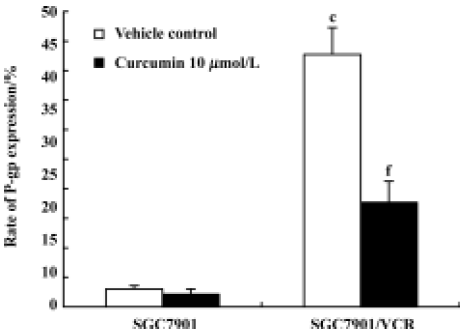
Effect of curcumin on P-gp expression Cells were plated at a concentration of 6×104 cells per mL in supplemented RPMI-1640. At 24 h after plating, the medium was replaced with fresh medium containing 0.1% Me2SO (vehicle control) or 10 μmol/L curcumin. After 24 h, cells were collected to examine the expression of P-gp by flow cytometry. The expression rate of P-gp was 42.73% in SGC7901/VCR cells and 2.03% in SGC7901 cells. Treatment with curcumin (10 µmol/L) for 24 h led to downregulation of P-gp expression in MDR cells. The expression rates of P-gp in SGC7901/VCR cells decreased from 42.73% to 17.69% after curcumin treatment (Figure 6).
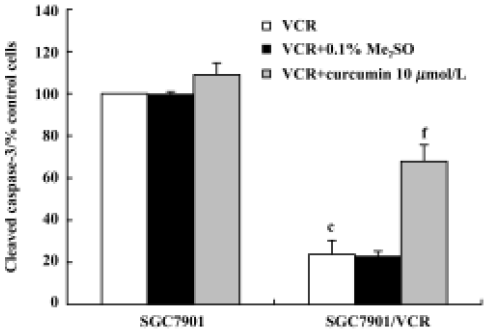
Effect of curcumin on the activation of caspase-3 in SGC7901/VCR cells The activation of caspase-3 was assessed by examining the expression of cleaved caspase-3 by flow cytometry. SGC7901/VCR cells treated with VCR (1 µmol/L) alone for 24 h showed 77% lower levels of caspase-3 activation than did SGC7901 cells (Figure 7). When cells were treated with VCR (1 µmol/L) in combination with curcumin (10 µmol/L) for 24 h, the activation of caspase-3 was increased by 44% (P<0.01) in the SGC7901/VCR cell line.
Discussion
The development of P-gp-dependent multidrug resistance in tumors is a major obstacle for successful chemotherapy[19]. For this reason, there is an increasing search for new reagents that inhibit the expression and/or function of P-gp to overcome MDR. Recent studies have shown that curcumin is able to modulate the expression and function of P-gp, and therefore it might be an possible new agent for the chemosensitization of cancer cells[12–14]. VCR-resistant gastric cancer SGC7901/VCR cells have been shown to express P-gp at a high level, but P-gp was not expressed in the drug-sensitive cells (SGC7901)[20]. Thus, in the present study, we wanted to demonstrate the possibility of using curcumin in gastric cancer as an MDR modulator.
We showed that curcumin enhanced the toxicity of VCR in the MDR cell line SGC7901/VCR. The SGC7901/VCR cell line was 45 times more resistant than the parental SGC7901 cell line. Curcumin, at concentrations of 5.0 µmol/L, 10.0 µmol/L and 20.0 µmol/L, is able to decrease the IC50 of VCR to SGC7901/VCR cells in a dose-dependent manner. These data indicate that curcumin is able to reverse MDR in the SGC7901/VCR cell line.
The potent MDR-reversing capacity of curcumin in tumor cells was further confirmed by its ability to enhance VCR-induced apoptosis in MDR SGC7901/VCR cells. Apoptotic and anti-apoptotic character are also strongly related to drug sensitivity and resistance. A number of tumor cells have been reported to undergo apoptotic cell death when treated with such chemotherapeutic agents as etoposide, camptothecin, cisplatin, Ara-C, mitomycin C, adriamycin, and vincristine. These findings indicate that apoptosis in tumor cells plays a critical role in chemotherapy-induced tumor cell killing, and also suggest that blockade of the apoptosis-inducing pathway could be another mechanism for MDR to chemotherapy[21]. VCR, at a concentration of 1 µmol/L, did not induce apoptosis in SGC7901/VCR cells, but significantly induced apoptosis in the parental cells, which indicated that the MDR SGC7901/VCR cell line was also apoptosis-resistant. In our study, we found that curcumin could promote the VCR-mediated apoptosis of MDR SGC7901/VCR cells, which suggests that curcumin could overcome the apoptosis-resistance of MDR cells.
The experiment for evaluating the effect of curcumin treatment on P-gp expression determined that curcumin clearly inhibited P-gp expression in SGC7901/VCR cells. The uptake and/or efflux of Rh123 is frequently used for assays of P-gp in tumor cells[22,23]. In the present study, we also investigated the effects of curcumin on the P-gp function of SGC7901/VCR cells. Curcumin caused a substantial increase in the accumulation of Rh123 in SGC7901/VCR cells, and inhibited the efflux of Rh123, but had no effect on drug-sensitive cells (SGC7901), which do not overexpress P-gp. Because Rh123 is known to be a good substrate for P-gp, we suppose that curcumin modulates intracellular Rh123 level by inhibiting P-gp. It is unlikely that curcumin acts by downregulating P-gp expression because the time of exposure of cells to curcumin in these experiments was short (1–2 h). Taken together, our data indicate that treatment of drug-resistant SGC7901/VCR cells with curcumin increased their sensitivity to VCR, which is consistent with an increase in intracellular drug concentration by decreasing P-gp function and expression.
Although P-gp is best known for its ability to efflux a variety of amphiphilic substances across plasma membranes, new functions are emerging. Transfected MDR1 in Chinese hamster ovary fibroblasts results in a decrease in growth factor withdrawal-induced apoptosis[24]. P-gp has been shown to inhibit apoptosis induced by anti-fas, ultraviolet radiation, and tumor necrosis factor in both lymphoid and myeloid cell lines[25,26], to resist spontaneous apoptosis in acute myeloid leukemia (AML) blasts[27,28], and is implicated in the inhibition of ceramide-induced apoptosis in TF-1 cells[29]. P-gp has also been shown to protect kidney proximal tubule cells from cadmium- and oxygen species-induced apoptosis[30]. Therefore we suggest that, besides exporting a wide range of xenobiotics from cells, the multidrug transporter P-gp may also confer resistance to apoptosis from different stimuli. Our results suggest that the ability of curcumin to promote VCR-induced apoptosis in drug-resistant SGC7901/VCR cells might be associated with a decrease in P-gp function and expression.
Recent studies have shown that functional P-gp plays a role in regulating cell death not only by removing drugs from the cell, but also by inhibiting the activation of proteases involved in apoptotic signaling (caspase-8)[25] and execution (caspase-3)[25,26]. In agreement with this, our data indicate that SGC7901/VCR cells inhibited VCR-induced caspase-3 activation. However, treatment of drug-resistant SGC7901/VCR cells with curcumin promoted VCR-induced caspase-3 activation. The results of a previous study have demonstrated that curcumin overcomes P-gp-mediated multidrug resistance through induction of caspase-3 activation[31]. Our results indicate that the MDR reverse function of curcumin may be associated with promoting anticancer agent-induced caspase-3 activation.
Summing up, our results suggest that curcumin could be considered a promising chemosensitizer of MDR in gastric cancer.
References
- Lehne G. P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr Drug Targets 2000;1:85-99.
- Hamilton KO, Topp E, Makagiansar I, Siahaan T, Yazdanian M, Audus KL. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J Pharmacol Exp Ther 2001;298:1199-205.
- Silva KL, Vasconcelos FC, Marques-Santos LF, Kwee JK, Maia RC. CPT-11-induced cell death in leukemic cells is not affected by the MDR phenotype. Leukemia Res 2003;27:243-51.
- Suhaimi H, Ahmad FB, Friberg SE. Curcumin in a model skin lotion formulation. J Pharm Sci 1995;84:376-80.
- Ammon HPT, Wahl MA. Pharmacology of Curcuma longa. Planta Med 1991;57:1-7.
- Mishra B, Priyadarsini KI, Bhide MK, Kadam RM, Mohan H. Reactions of superoxide radicals with curcumin: probable mechanisms by optical spectroscopy and EPR. Free Radic Res 2004;38:355-62.
- Nagabhushan M, Bhide SV. Nonmutagenicity of curcumin and its antimutagenic action versus chili and capsaicin. Nutr Cancer 1986;8:201-10.
- Limtrakul P, Anuchapreeda S, Lipigorngoson S, Dunn FW. Inhibition of carcinogen induced c-Ha-ras and c-fos proto-oncogenes expression by dietary curcumin. BMC Cancer 2001;1:1-7.
- Asai A, Miyazawa T. Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J Nutr 2001;131:2932-5.
- Suryanarayana P, Krishnaswamy K, Reddy GB. Effect of curcumin on galactose-induced cataractogenesis in rats. Mol Vis 2003;9:223-30.
- Qureshi S, Shah AH, Ageel AM. Toxicity studies on Alpinia galanga and Curcuma longa. Planta Med 1992;58:124-7.
- Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul PN. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol 2002;64:573-82.
- Limtrakul P, Anuchapreeda S, Buddhasukh D. Modulation of human multidrug-resistance MDR-1 gene by natural curcuminoids. BMC Cancer 2004;4:13.
- Romiti N, Tongiani R, Cervelli F, Chieli E. Effects of curcumin on P-glycoprotein in primary cultures of rat hepatocytes. Life Sci 1998;62:2349-58.
- Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell 1991;66:85-94.
- Maxwell SA, Davis GE. Biological and molecular characterization of an ECV-304-derived cell line resistant to p53-mediated apoptosis. Apoptosis 2000;5:277-90.
- Efferth T, Lohrke H, Volm M. Reciprocal correlation between expression of P-glycoprotein and accumulation of rhodamine 123 in human tumors. Anticancer Res 1989;9:1633-7.
- Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell 1991;66:85-94.
- Arceci RJ. Tumor cell survival and resistance to therapy. Curr Opin Hematol 1996;3:279-287.
- Cao JG, Tang XQ, Shi SH. Multidrug resistance reversal in human gastric carcinoma cells by neferine. World J Gastroenterol 2004;10:3062-4.
- Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci 2003;94:15-21.
- Chen LM, Liang YJ, Ruan JW, Ding Y, Wang XW, Shi Z, et al. Reversal of P-gp mediated multidrug resistance in-vitro and in-vivo by FG020318. J Pharm Pharmacol 2004;56:1061-6.
- Liu ZL, Hirano T, Tanaka S, Onda K, Oka K. Persistent reversal of P-glycoprotein-mediated daunorubicin resistance by tetrandrine in multidrug-resistant human T lymphoblastoid leukemia MOLT-4 cells. J Pharm Pharmacol 2003;55:1531-7.
- Robinson LJ, Roberts WK, Ling TT, Lamming D, Sternberg SS, Roepe PD. Human MDR1 protein overexpression delays the apoptotic cascade in Chinese hamster ovary fibroblasts. Biochemistry 1997;36:11169-78.
- Johnstone RW, Cretney E, Smyth MJ. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood 1999;93:1075-85.
- Smyth MJ, Krasovskis E, Sutton VR, Johnstone RW. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc Natl Acad Sci USA 1998;95:7024-9.
- Pallis M, Russell N. P-glycoprotein plays a drug-efflux-independent role in augmenting cell survival in acute myeloblastic leukemia and is associated with modulation of a sphingomyelin-ceramide apoptotic pathway. Blood 2000;95:2897-904.
- Pallis M, Turzanski J, Grundy M, Seedhouse C, Russell N. Resistance to spontaneous apoptosis in AML blasts is associated with P-glycoprotein exression and function, but not with the presence of FLT3 internal tandem duplications. Br J Haematol 2003;120:1009-16.
- Turzanski J, Grundy M, Shang S, Russell N, Pallis M. P-glycoprotein is implicated in the inhibition of ceramide-induced apoptosis in TF-1 acute myeloid leukemia cells by modulation of the glucosylceramide synthase pathway. Exp Hematol 2005;33:62-72.
- Thevenod F, Friedmann JM, Katsen AD, Hauser IA. Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-kappaB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem 2000;275:1887-96.
- Bielak-Mijewska A, Piwocka K, Magalska A, Sikora E. P-glycoprotein expression does not change the apoptotic pathway induced by curcumin in HL-60 cells. Cancer Chemother Pharmacol 2004;53:179-85.
