Expression of feeding-related peptide receptors mRNA in GT1-7 cell line and roles of leptin and orexins in control of GnRH secretion1
Introduction
Reproduction including mating, pregnancy, and lactation is an energy-demanding process. In normal circum-stances, energy intake and expenditure from food is used for essential life activities such as maintenance of basal body temperature, cellular metabolism, fertility and storage of energy in fat tissue. When the food intake is limited or when an inordinate fraction of the available energy is diverted to other uses such as exercise or fattening, reproductive attempts are sacrificed in favor of more critical life activities[1,2]. The negative energy balance, as typified by fasting, anorexia nervosa or exercise-induced amenorrhea, is associated with a suppression of reproductive function and ovarian cyclicity. Appropriate regulation of reproduction, energy intake and energy expenditure, and thus maintenance of body weight and fertility, relies on complex hypothalamic neurocircuitry, which serve as key signals to integrate and/or coordinate the status of energy balance and the neuroendocrine reproductive axis. Many neuropeptides that have been shown to play a role in the regulation of food intake have overlapping functions in the regulation of reproductive function[3].
Successful reproduction and thus survival of the species in mammals is dependent on the GnRH pacemaker. The network of neurons that controls GnRH secretion is not yet defined, but it is thought to be the final common pathway through which many factors influence gonadal activity, including metabolic status[4]. GT1-7 cells, a subclone of the GT1 cell line, were developed by targeting expression of the potent oncogene, SV40 T-antigen, with the regulatory region of GnRH gene, has been shown to faithfully exhibit many of the known characteristics of GnRH neurons. To date, the GT1 cells have proven to be the best characterized cell model available to study biology of the hypothalamic GnRH neurons[5].
In the last few years, the receptors for several families of neurotransmitters known to modify GnRH secretion have been identified on GT1 cells, and the stimulatory or inhibitory activity of the corresponding ligands on GnRH secretion has been documented[6–8]. Here we used the GT1-7 cells to investigate the expression of some feeding-related peptide receptors mRNA and the effects of leptin and orexins on GnRH secretion.
Materials and methods
Cell culture and reagents GT1-7 cells kindly provided by Dr KNOBIL (University of Texas-Houston Medical School, Houston, Texas, USA), were maintained in high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum at 37 °C in an atmosphere of 5% CO2+95% air. GnRH antiserum was the generous gift of Dr KNOBIL. GnRH, chloramine T, sodium pyrosulfite, leptin, bacitracin, orexin A, and orexin B were purchased from Sigma-Aldrich (St Louis, MO, USA). TRIZOL Reagent was purchased from GIBCO-BRL (Gaithersburg, Maryland, USA). SuperScript II reverse transcriptase was obtained from Life Technologies (Rockville, USA). All oligodeoxynucleotides were synthesized from Biosia Co Ltd (China).
RT-PCR analysis Total RNA was isolated from GT1-7 cells and rats hypothalamus by the TRIZOL extraction method. The first-strand cDNA was synthesized from 1−10 μg deoxyribonuclease treated RNA using SuperScript II reverse transcriptase. The RT reaction was primed with oligo. The specificity of each amplification reaction was monitored by control reactions with RT omission reaction. Because all these receptors have previously been reported in the hypo-thalamus, we used the rat hypothalamic tissue as a positive control. Polymerase chain reaction (PCR) amplifications were performed with 1.25 U Taq polymerase from Sangon in a 50-μL reaction for 35 cycles (1 min at 94 °C, 45 s at 55–60 °C, 1 min at 72 °C). The primers selected were shown in Table 1. PCR products were electrophoresed in 1.5% agarose and visualized by ethidium bromide staining.
Full table
Static incubation For GnRH secretion studies, GT1-7 cells were plated in 24-well multiwell plates at a density of 2×105 cells/well and grown in culture for 3–4 d, the medium was replaced by DMEM with 0.2% BSA 16 h before the experiments. Confluent cells were washed three times with Locke’s mediums (NaCl 154 mmol/L, KCl 5.6 mmol/L, CaCl2 2.2 mmol/L, MgCl2 1 mmol/L, NaHCO3 6 mmol/L, glucose 10 mmol/L, HEPES 2 mmol/L) containing 0.2% bovine serum albumine (BSA), 20 µmol/L bacitracin and then were treated with or without leptin, orexin A and B at a cohort of concentrations for 15, 30, and 60 min in Locke’s medium. At the end of incubation period, media were stored at -80 ºC until radioimmunoassay (RIA) for GnRH.
RIA The concentration of GnRH released into the medium (1 mL/well) was measured by RIA in duplicate. GnRH was iodinated by chloramine T technique as described[9]. Purification was achieved on a sephadex G25 column, using 0.1 mol/L acetic acid with 0.25% BSA as eluent. Aliquots of medium (150 µL) were cultured with 50 µL polyclonal antiserum (1: 24 000) for 24 h at 4 °C. The antigen-antibody complex was precipitated with a goat anti-rabbit g-globulin and ethanol. The limit of detection was 0.5 pg/tube, and the intra-assay coefficient of variation was 6.7%.
Statistical analysis For assessment of statistical significance, data were analyzed using analysis of variance. Comparisons between individual pairs were determined using Student’s t-test. Statistical significance was defined as P<0.05.
Results
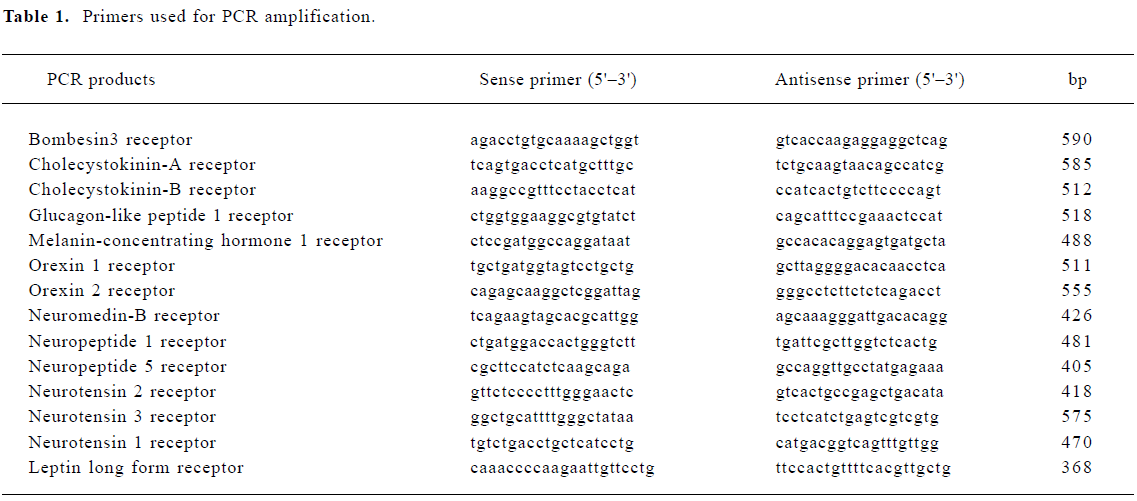
RT-PCR analysis of the feeding-related peptide receptors transcript in GT1-7 cells Feeding-related peptide is known to have a profound effect on the regulation of reproductive function through the GnRH neuron, however, it is not yet known whether the GnRH neuron itself contains receptors for them. The expression of orexin, neurotensin, neuropeptide Y (NPY), glucagon-like peptide 1 (GLP-1), melanin-concentrating hormone (MCH), cholecystokinin (CCK), leptin, neuromedin B and bombesin receptor mRNA were analyzed. Our results confirmed that bombesin3, CCK-B, GLP1, MCH1, orexin1, neuromedin-B, NPY1, NPY5, NT1, NT3 and leptin long form receptor mRNA were expressed in GT1-7 cells, the amplified fragments were identical in size to those amplified by total RNA derived from the rat hypothalamus, which was used as the positive control. No amplification fragment was obtained from RNA samples when the incubation with RT was omitted, a fact that excludes contamination of the samples with genomic DNA and the pseudo-positive bands, in which, GLP1, neuromedin-B, NPY1, and NT3 receptor were highly expressed in GT1-7 cell line. Additionally, though the primers designed for orexin2, NT2, CCK-A receptor cDNA amplified the fragments of the expected size with rat hypothalamic RNA (data not shown), no amplified fragment was generated with GT1-7 RNA, indicating that the GT1-7 cells did not express mRNA for them (Figure 1).
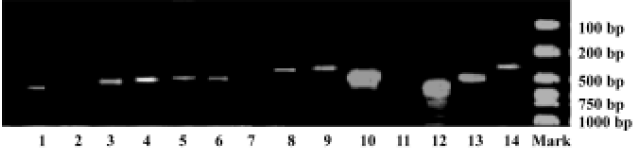
Dose-response effect of leptin on GnRH release from GT1-7 cells In experiments conducted under static conditions, GT1-7 cells were treated with leptin for 15, 30, and 60 min, and GnRH peptide levels in the medium were measured by RIA (Figure 2). Exposure of GT1-7 cells to leptin resulted in dose-dependent stimulation of GnRH release at concentrations of 0.1, 1, 10, and 100 nmol/L with rapid effect. The most profound changes of GnRH release were induced at the dose of 0.1 nmol/L after 15 min, with an increased extension of 1.5 fold (vs basal release, 2.69±0.49 pg/tube vs 1.87±0.33 pg/tube). However, as mentioned above, the release of GnRH was routinely measured after 30 and 60 min, extending leptin treatment in GT1-7 cells did not further increase the stimulation of GnRH secretion.
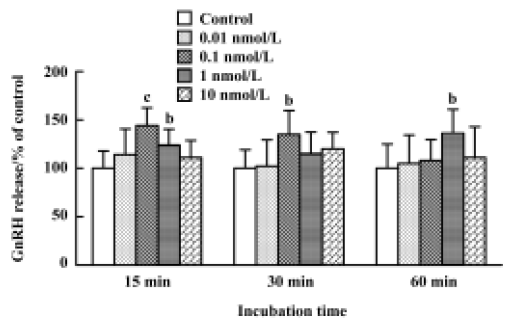
Effect of orexin A and B on release of GnRH from GT1-7 cells Using RT-PCR, we observed readily detectable orexin 1 receptor mRNA in GT1-7 cells. To verify whether orexin receptors play a physiological role in these cells, the effects of orexin A and B on the release of GnRH were subsequently investigated. GnRH secretion has previously been shown to be stimulated by orexin A from hypothalamic explants in male rats and females at proestrus. However, in GT1-7 cells, we were unable to detect a similar stimulated effect, neither orexin A nor orexin B affected basal GnRH secretion over a wide range of concentrations ranging from 1 nmol/L to 500 nmol/L for 15, 30, and 60 min. The positive control (56 mmol/L KCl) significantly increased GnRH release in this study (Figure 3).
Discussion
In mammals, hypothalamic control of food intake involves counter-regulation of appetite by orexigenic peptides and anorexigenic peptides, which are produced in the central nervous system and periphery. Several major orexigenic signals have been identified including NPY, the orexins, MCH, endorphins, galanin, growth hormone-releasing hormone, γ-aminobutyric acid, and agouti-related protein, similarly, a few major anorexigenic signals of recent interest include bombesin, glucagon-like peptide-1 (GLP-1), corticotropin-releasing hormone, CCK, cocaine and amphetamine-regulated transcript, serotonin, neurotensin, neuromedin α-melanocyte-stimulating hormone, leptin, ciliary neurotrophic factor, urocortin and dopamine[2], these essential messenger molecules serve as a communication bridge between neural processes that regulate reproduction and energy homeostasis.
The expression of feeding stimulant receptors mRNA of MCH, NPY, the orexins were detected first. Previous studies have shown that the GnRH-releasing effect of MCH is estrous-cycle stage dependent and it takes place only in proestrous rats[10]. MCH has been shown to bind and activate two G-protein-coupled receptors, called MCH receptor 1 and MCH receptor 2, expressed in the human brain and other tissues, but several non-human species (ie, rat, mouse, hamster, guinea pig, and rabbit) did not have functional MCH receptor 2 receptors, or encode a nonfunctional MCH receptor 2 pseudogene while retaining MCH receptor 1 expression[11]. We detected MCH receptor 1 mRNA in the GT1-7 cell line by RT-PCR, suggesting that MCH released from the median eminence might act directly on GnRH nerve terminals through MCH receptor 1. The NPY1 and NPY5 receptor subtypes are believed to play a role in appetite control[12,13]. There was evidence that NPY stimulated GnRH release from GT1-7 cells through a direct, Y1-like receptor-mediated action on the GnRH neuron itself[14], further research is needed to verify whether the NPY5 receptor exerts its effects at the hypothalamic level by regulating the release of GnRH.
Orexin A and B were first discovered in rats and found to increase feed intake in these animals when administered directly into the brain. Orexins orchestrate their actions by orexin-1 receptor and orexin-2 receptor. Orexin-2 receptor is a nonselective receptor that binds both orexin A and B, and orexin-R1 is highly selective for orexin A[15]. It was found that about 75%–85% of GnRH neurons contacted by orexin fibers and approximately 85% of GnRH neurons were colocalized with the orexin receptor 1, which provided the basis for a functional neuroanatomical pathway[16]. In whole hypothalamic tissue, orexin A stimulated GnRH release harvested from male rats and from females at proestrus, with no effect at estrus or metestrus[17]. To further investigate the mechanism underlying the orexins stimulating the release of GnRH, we used the GnRH immortalized cell line GT1-7 cells. However, we were unable to detect a similar stimulating effect of orexin A and B. the different effects observed in hypothalamic explants and GT1-7 cells could be explained by differences between GnRH neurons from animal tissue and cultured GnRH neurons.
The receptors of feeding suppressant including CCK, bombesin, GLP-1, neuromedin, neurotensin and leptin were also observed. CCK is involved in the modulation/control of multiple central functions, these actions are mediated by at least two distinct receptors (CCK-A and CCK-B). The CCK-B receptor antagonist L-365,260, but not the CCK-A receptor antagonist L-364,718 infused into the medial preoptic area of recently mated females blocked pregnancy. These findings implicated CCK acting on CCK-B receptors in the medial preoptic area as a mediator of olfactory influenced on reproductive physiology[18], and was further supported by our findings that provided the first evidence of the presence of CCK-B receptor but not CCK-A receptor mRNA in total RNA isolated from GT1-7 cells.
The central GLP-1 system has been implicated in the control of feeding behavior. GLP-1 receptor has been identified in the medial and lateral preoptic areas, regions rich in GnRH neurons. The GLP-1 receptor knock-out mouse had delayed puberty[19]. We showed that GLP-1 receptor was highly expressed in GT1-7 cells, suggesting that GLP-1 may have a permissive role in the regulation of the reproductive axis.
Bombesin, neuromedin, and neurotensin are known to be essential to the regulation of feeding behavior. Neuro-tensin exerts its effects through at least three receptors that have been cloned and designated NT1 (high affinity), NT2 and NT3[20]. Dual-label in situ hybridization revealed that NT1 mRNA was expressed in some GnRH neurons in the OVLT/rPOA region, the percentage of dual-labeled neurons varied significantly depending on the stage of the cycle surge[21]. We demonstrated that NT1 and NT3 but not NT2 receptor sub-types were in fact expressed in GT1-7 cells. Bombesin and its structurally related peptide neuromedin-B belonged to negative regulators of appetite[22,23], the present study provided the first evidence of the presence of bom-besin receptor 3 and neuromedin-B receptor mRNA in GT1-7 cells, strongly supporting the possibility that hypothalamic LHRH neurons in situ might also express these receptors. Studies are continuing to determine whether the receptors encoded by these mRNA mediate the actions of their neuropeptides on GnRH release from GT1-7 cells.
Leptin, a fat-derived anorexigenic hormone, has been described to be an important peripheral signal that indicates body fat stores to the hypothalamus and thus links nutrition and reproductive processes. Leptin treatment corrected infertility, hyperinsulinemia, and hyperglycemia of ob/ob mice, however, diet restriction alone is ineffective in restoring fertility to either female or male ob/ob mice, suggesting that obesity is not the sole cause of infertility and that leptin may be essential for normal reproductive function[24]. Our results seem to agree with others studies, reporting that treatment of GT1-7 cells with leptin stimulated the release of GnRH. Moreover, we found a rapid stimulation of leptin after 15 min of incubation, at concentrations of 0.1–100 nmol/L with moderate, but significant increments, which suggested a more direct and immediate modulation of GnRH release.
In summary, our results suggested that feeding-related peptide receptors of bombesin3, CCK-B, GLP1, MCH1, orexin1, neuromedin-B, NPY1,NPY5, NT1, NT3 and leptin long form mRNA were expressed in GT1-7 cells, in which, receptor of GLP1, neuromedin-B, NPY1 and NT3 were highly expressed. Additionally, no amplified fragments of orexin2, NT2 or CCK-A receptor cDNA were generated, indicating that the GT1-7 cells did not express mRNA for them. Leptin showed a rapid stimulating effect on GnRH release after 15 min incubation, and neither orexin A nor orexin B affected basal GnRH secretion from GT1-7 cells. The different effects of orexin A observed in hypothalamic explants and GT1-7 cells could be explained by differences between GnRH neurons from animal tissue and cultured GnRH neurons. These results, in accordance with previous findings, suggest that in addition to any indirect actions, feeding-related peptides may also exert their effects directly on hypothalamic GnRH neurons as a results of expression of their receptors mRNA. Feeding and reproductive function are closely linked.
References
- Judd SJ. Disturbance of the reproductive axis induced by negative energy balance. Reprod Fertil Dev 1998;10:65-72.
- Ordog T, Chen MD, O’Byrne KT, Goldsmith JR, Connaughton MA, Hotchkiss J, et al. On the mechanism of lactational anovulation in the rhesus monkey. Am J Physiol 1998;274:E665-76.
- Smith MS, Grove KL. Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol 2002;23:225-56.
- Blache D, Chagas LM, Blackberry MA, Vercoe PE, Martin GB. Metabolic factors affecting the reproductive axis in male sheep. J Reprod Fertil 2000;120:1-11.
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 1990;5:5-10.
- Wetsel W. Immortalized hypothalamic luteinizing hormone-releasing hormone (LHRH) neurons: a new tool for dissecting the molecular and cellular basis of LHRH physiology. Cell Mol Neurobiol 1995;15:43-78.
- Cui H, Lin SY, Belsham DD. Evidence that dehydroepiandro-sterone, DHEA, directly inhibits GnRH gene expression in GT1-7 hypothalamic neurons. Mol Cell Endocrinol 2003;203:13-23.
- Beltran-Parrazal L, Noris G, Clapp C, Martinez de la Escalera G. GABA inhibition of immortalized gonadotropin-releasing hormone neuronal excitability involves GABA(A) receptors negatively coupled to cyclic adenosine monophosphate formation. Endocrine 2001;14:189-95.
- Clemens LE, Kelch RP, Markovs M, Westhoff MH, Dermody WC. Analysis of the radioimmunoassay for gonadotropin-releasing hormone (GnRH): studies on the effect of radioiodinated GnRH. J Clin Endocrinol Metab 1975;41:1058-64.
- Chiocchio SR, Gallardo MG, Louzan P, Gutnisky V, Tramezzani JH. Melanin-concentrating hormone stimulates the release of luteinizing hormone-releasing hormone and gonadotropins in the female rat acting at both median eminence and pituitary levels. Biol Reprod 2001;64:1466-72.
- Tan CP, Sano H, Iwaasa H. Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics 2002;79:785-92.
- Kanatani A, Mashiko S, Murai N, Sugimoto N, Ito J, Fukuroda T, et al. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology 2000;141:1011-6.
- Criscione L, Rigollier P, Batzl-Hartmann C, Rueger H, Stricker-Krongrad A, Wyss P, et al. Food intake in free-feeding and energy-deprived lean rats is mediated by the neuropeptide Y5 receptor. J Clin Invest 1998;102:2136-45.
- Besecke LM, Wolfe AM, Pierce ME, Takahashi JS, Levine JE. Neuropeptide Y stimulates luteinizing hormone-releasing hormone release from superfused hypothalamic GT1-7 cells. Endocrinology 1994;135:1621-7.
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998;92:573-85.
- Campbell RE, Grove KL, Smith MS. Gonadotropin-releasing hormone neurons coexpress orexin 1 receptor immunoreactivity and receive direct contacts by orexin fibers. Endocrinology 2003;144:1542-8.
- Russell SH, Small CJ, Kennedy AR, Stanley SA, Seth A, Murphy KG, et al. Orexin A interactions in the hypothalamo-pituitary gonadal axis. Endocrinology 2001;142:5294-302.
- Li CS, Kaba H, Saito H, Seto K. Cholecystokinin: critical role in mediating olfactory influences on reproduction. Neuroscience 1992;48:707-13.
- MacLusky NJ, Cook S, Scrocchi L, Shin J, Kim J, Vaccarino F, et al. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology 2000;141:752-62.
- Rostene WH, Alexander MJ. Neurotensin and neuroendocrine regulation. Front Neuroendocrinol 1997;18:115-73.
- Smith MJ, Wise PM. Neurotensin gene expression increases during proestrus in the rostral medial preoptic nucleus: potential for direct communication with gonadotropin-releasing hormone neurons. Endocrinology 2001;142:3006-13.
- Maekawa F, Quah HM, Tanaka K, Ohki-Hamazaki H. Leptin resistance and enhancement of feeding facilitation by melanin-concentrating hormone in mice lacking bombesin receptor subtype-3. Diabetes 2004;53:570-6.
- Williams G, Cardoso HM, Lee YC, Ball JM, Ghatei MA, Stock MJ, et al. Hypothalamic regulatory peptides in obese and lean Zucker rats. Clin Sci (Lond) 1991;80:419-26.
- Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 1997;138:1190-3.
