Curcumin synergistically augments bcr/abl phosphorothioate antisense oligonucleotides to inhibit growth of chronic myelogenous leukemia cells1
Introduction
Chronic myelogenous leukemia (CML) is characterized by a reciprocal t (9; 22) chromosomal translocation, known as the Philadelphia chromosome, which fuses parts of the c-abl gene, located on chromosome 22, and is the hallmark of chronic myeloid leukemia[1–3]. Today, P210bcr/abl, the gene product of the resultant bcr/abl hybrid gene, is one of the most intensively studied signaling proteins in cancer research[4]. P210bcr/abl-initiated signaling such as NF-κB decreases the ability of a variety of stimuli to induce apoptosis in vitro[5,6]. Therefore, it is a new and attractive therapeutic strategy to target P210bcr/abl and P210bcr/abl signaling.
In recent years, antisense therapy with phosphorothioate oligodeoxynucleotides has emerged as a potentially useful strategy for inhibiting CML[7]. Bcr/abl phosphorothioate antisense oligonucleotides (PS-ASODN) might be synthesized for uses as therapeutic agents. The idea is that PS-ASODN would block disease processes by blocking the synthesis of a protein encoded by bcr/abl. The binding of the PS-ASODN to the bcr/abl mRNA would normally result in the downregulation of that protein, thus preventing malignant transformation and development of resistance to apoptosis. However, their use as therapeutic agents is limited by inefficient cellular uptake, scare nuclear internalization, and oligonucleotide self-aggregation[8].
Curcumin (cur) is a diferuloylmethane derived from the plant Curcuma longa. It is a potent antioxidant that possesses both anti-inflammatory and antitumor activities, and has been regarded as one of the prosperous antitumor agents[9]. In our previous works, cur has been proved to inhibit the proliferation of K562 cells correlates with the downregulation of the P210bcr/abl protein[10]. Therefore, we supposed that cur might synergistically inhibit the growth of CML with PS-ASODN. If there is a combination, what would the action of cur after bcr/abl mRNA be blocked by PS-ASODN?
We selected NF-κB to investigate whether the combination of cur and PS-ASODN exerted a synergistic effect on the downstream of P210bcr/abl signaling, thus affecting cell growth. NF-κB, a transcription factor with a critical role in promoting inflammation and which is connected with multiple aspects of oncogenesis and cancer cell survival, appears to be involved in the regulation of cell proliferation, control of apoptosis, promotion of angiogenesis, and the stimulation of invasion/metastasis[5,11]. For further analysis of the inhibitory mechanism of PS-ASODN and cur, we studied the molecular chaperone heat shock protein 90 (Hsp90), which is important in maintaining the conformation, stability, and function of P210bcr/abl[12].
In this study, the P210bcr/abl-positive K562 cell line was used as a cellular model of CML for drug screening (Figure 1).
Materials and methods
Drugs and antibodies Cur was purchased from Shanghai N
Cell culture The human leukemia cell line K562 (purchased from Shanghai Institute of Biochemistry and Cell Biology, Shanghai, China) was cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum and gentamicin (80 U/100 mL) in a humidified atmosphere with 5% CO2 at 37 ºC.
Cell growth inhibition assay The K562 cells were plated in 0.1 mL in triplicate at a density of (10–20)×104 cells/mL in 96-well plates and were exposed to the tested agents: PS-ASODN, cur, and a combination of both, which were added at the selected concentrations to the culture medium with a final volume of 0.2 mL per well for 48 h. Nonsense was used as a control. At the indicated time, 20 µL of 5 mg/mL MTT solution in phosphate-buffered saline (PBS pH 7.2) was added to each well for 4 h. After removal of the medium, 170 µL of DMSO was added to each well to dissolve the formazan crystals. The absorbance at 490 nm was determined using a Biokinetics plate reader (Bio-Rad Laboratories, Hercules, CA, USA). Triplicate wells were assayed, and standard deviations were determined.
Assessment of apoptosis The K562 cells were plated in 0.1 mL in triplicate at a density of (10–20)×104 cells/mL in 96-well plates and were exposed to the tested agents: PS-ASODN, cur, or a combination of both. They were added at the selected concentrations to the culture medium, with a final volume of 0.2 mL per well for 48 h. After the indicated time, 100 cells were counted under a microscope after AO/EB fluorescent staining to determine the apoptotic rate[14]. One hundred micrograms of AO and EB were dissolved, respec-tively, in 1 mL PBS (pH 7.2) as stock solution. To further confirm the synergistic apoptosis, the K562 cells were treated with selected concentrations of 10 µmol/L PS-ASODN, 10 µmol/L cur, or a combination of both for 48 h. After the indicated time, the apoptotic cells were detected by nuclear morphologic changes using AO/EB fluorescent staining. Four µL of AO/EB mixture was added with 100 µL of K562 cells on the cover slip, and then the stained nuclei were viewed under an Olympus microscope (Hatagaya, Tokyo, Japan) to evaluate the extent of apoptosis (ie cell shrinkage, nuclear condensation, formation of apoptotic bodies etc).
Protein extraction and Western blotting After treatment with drugs for 48 h, the cells were collected by centrifugation and washed 3 times in ice-cold PBS (pH 7.2). The cell pellets were resuspended in lysis buffer (Tris-HCl 50 mmol/L, pH 8.0, NaCl 150 mmol/L, dithiothreitol 1 mmol/L, EDTA 0.5 mmol/L, NP40 0.1% (v/v), sodium dodecyl sulfate 0.1% (w/v) containing protease inhibitors (aprotinin 1 mg/L, leupeptin 2 mg/L, sodium orthovanadate 100 µmol/L and phenylmethyl-sulfonly fluoride 10%). The protein concentration of each sample was estimated using the Coomassie brilliant blue kit (Nanjing Jiancheng Biotechnology, Jiangsu, China). After boiling for 5 min, the lysates were subjected to electrophoresis on 8% polyacrylamide gels and transferred to Hybond-C membranes (Amersham, Arlington Heights, IL, USA) in transfer buffer [Tris 25 mmol/L, glycine1 90 mmol/L, methanol 20% (v/v)] using a transfer apparatus at 150 mA for 2 h. The membranes were blocked with blocking-buffer [5% (g/v) non-fat milk] at 4 °C overnight and were incubated with mouse anti-primary antibodies: c-Abl, Hsp90, NF-κB, and β-actin for 2 h at room temperature. They were washed 4 times in TBST [Tris-buffered saline supplemented with 0.03% (v/v) Tween-20], and incubated with horse anti-mouse antibody (1:500) for 1.5 h at room temperature; they were washed 4 times with Tris-HCl (pH 7.2), and the membranes were detected with substrate according to the product kits (Gene Lab, Singapore). Intensities of the different proteins were quantified by densitometric scanning. All experiments were repeated at least 3 times and yielded similar results.
Statistical analysis Data were expressed as mean±SD. Jin’s formula was used to evaluate the synergistic effects between the drugs[15]. The formula is: Q=Ea+b/(Ea+Eb-Ea×Eb); Q is the combination index; Ea+b which represents the cell proliferative inhibition rate of the combined drug; Ea and Eb are signs of the cell proliferative inhibition rate of each drug. After calculation: Q=0.85-1.15, means indication of simple addiction (+); Q=1.15-2.0 → synergism (+ +); Q>2.0 → significant synergism (+ + +); Q=0.85-0.55 → antagonistic effect (–); Q<0.55 → significant antagonistic effect (– –). The differences between treatment and control groups were evaluated with Student’s t-test. The differences were considered significant at P<0.05.
Results
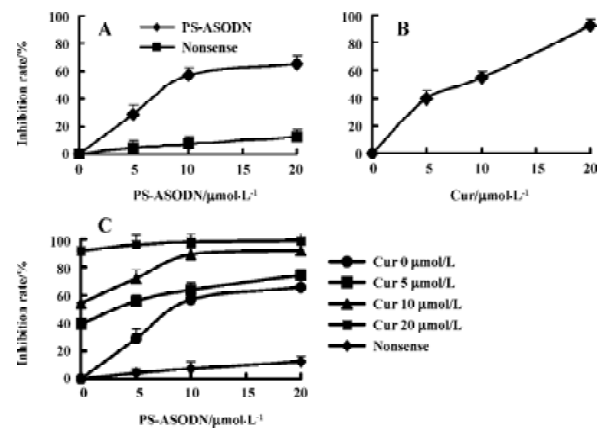
Effects of cur and/or PS-ASODN on cell growth Treatment with either cur (5–20 µmol/L) or PS-ASODN (5–20 µmol/L) resulted in an inhibition of cell growth of 40%, 55%, 90%, 30%, 45%, and 65%, respectively (Figure 2A,B). In combination, the cell number was reduced greatly (Figure 2C), cur (5–20 µmol/L) and PS-ASODN (5–10 µmol/L) showed synergism (Q>1.15), that is, a combination of 10 µmol/L cur and 10 µmol/L PS-ASODN induced an inhibition of cell growth of 90%, compared to cur (55%) or PS-ASODN (45%), and showed synergism (Q=1.19).
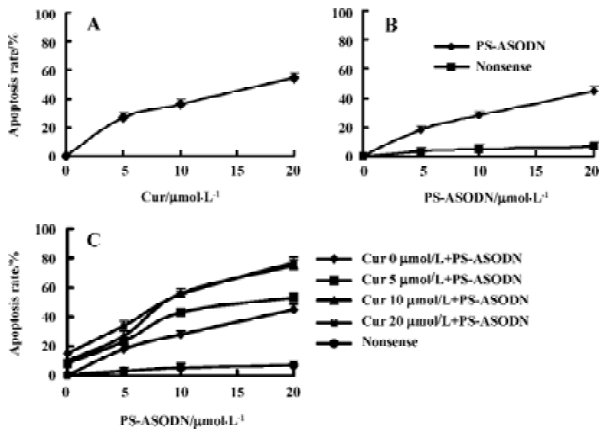
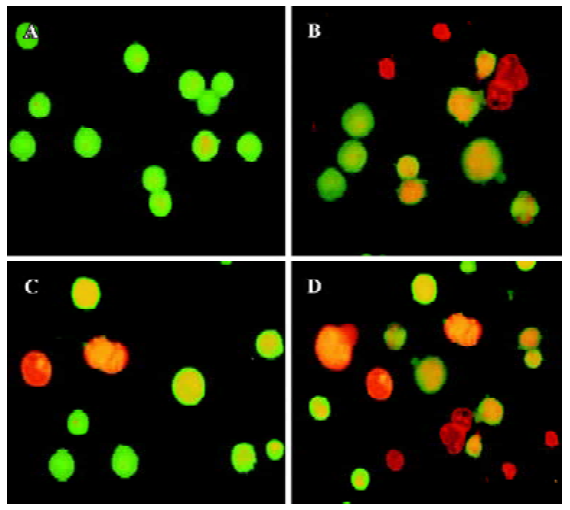
Effects of cur and/or PS-ASODN on cell apoptosis The extent of apoptosis was more significant when the K562 cells were treated with the combination of cur and PS-ASODN than each treated alone. Exposure of cells for 48 h to cur (5–20 µmol/L) or PS-ASODN (5–20 µmol/L) induced apoptosis of 25%–55% and 18%–45% (Figure 3A, 3B). However, when exposed to the combination of cur and PS-ASODN, it significantly increased the percentage of the hallmark of apoptosis, that is, when 20 µmol/L cur (55%) and 20 µmol/L PS-ASODN (45%) were treated in combination, a marked increase (80%) in apoptosis was induced (Q=1.06), corresponding to a synergistic interaction (Figure 3C). The drug-treated K562 cells were examined for morphologic evidence of apoptosis using AO/EB fluorescent staining under an Olympus microscope (×400). Typical apoptotic features of chromatin condensation and nuclear fragmentation were observed in the treated cells when they were exposed to both drugs in combination (Figure 4).
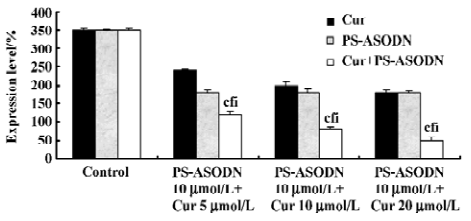
Cur and PS-ASODN synergistically downregulated P210bcr/abl, NF-κB, and Hsp90 expression To determine whether the potentiality of apoptosis in the K562 cells treated with PS-ASODN combined with cur would be associated with the downregulation of P210bcr/abl and selected P210bcr/abl signaling- NF-κB, Western blot was performed to evaluate their expression. As a result, treatment with a combination of PS-ASODN and cur resulted in a marked reduction of P210bcr/abl compared to each drug treated alone (Figures 5, 8). When the cells were exposed for 48 h to 10 µmol/L PS-ASODN in combination with increasing concentrations of cur (5–20 µmol/L), the P210bcr/abl level decreased by 75%, 80%, and 90%, respectively, compared to the treatment of PS-ASODN (50%) or cur (20%, 40%, and 60%) alone.
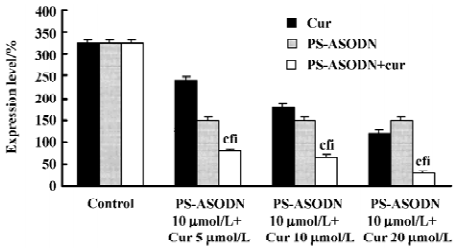
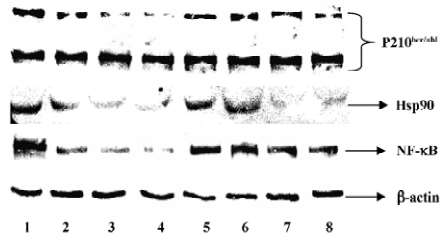
The synergistic inhibition was observed in the inhibition of NF-κB expression (Figures 6, 8), that is, the individual effect of 10 µmol/L PS-ASODN or (5–20 µmol/L) cur caused a 48%, 31%, 42%, and 48% decrease of NF-κB expression, respectively. However, when both of the drugs were combined, the NF-κB level markedly decreased by 66%, 77%, and 86%. This was consistent with the downregulated proportion of P210bcr/abl.
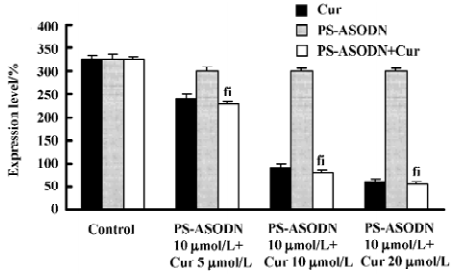
Individual treatment of PS-ASODN had little effect on Hsp90 (P>0.05 vs control), whereas (5–20 µmol/L) cur caused a 26%, 72%, and 81% decrease of Hsp90. The Hsp90 treated with cur, or the combination of PS-ASODN and cur, had no significant difference (P>0.05). This suggested that the downregulation of Hsp90 was through the effect of cur, but not PS-ASODN (Figures 7, 8).
Discussion
ASODN is the high degree of specificity, without altering the expression of genes with closely-related sequences. Theoretically, it is one of the ideal therapeutic agents of CML[9,16]. However, they are very poorly soluble, exhibit low affinity for their target complementary RNA sequences, and target RNase-H poorly. The solubility of PS-linked ASODN may be improved in that it can direct RNase-H-mediated destruction of the target RNA, but this may result in non-antisense inhibition of growth[13]. What’s more, PS-ASODN are very poorly transported into human cells; liposomes may enhance cellular ASODN uptake, but it seems, can not meet its initial high expectations. In this study, treatment with PS-ASODN (5–20 µmol/L) alone resulted in an inhibition of cell growth (30%–60%).
Recently, cur has been commonly studied as a prosperous drug in its anticancer effect. It can inhibit the proliferation and induce the apoptosis of many kinds of cancer cells in vitro, and cur is safe, with human clinical trials indicating no dose-limiting toxicity when administered at doses up to 10 g/d[9,17–19]. In this study, cur synergistically augmented the growth inhibitory effects of PS-ASODN in human leukemic cells in vitro. This observed synergistic effect may be clinically important, and the combinatorial strategies in cancer therapy can provide dramatic improvements in safety and efficacy over monotherapy regiments.
Our current results show that the combination of low concentrations of cur (5–20 µmol/L) and PS-ASODN (5–20 µmol/L) were sufficient to inhibit cell growth by inhibiting proliferation and inducing apoptosis, downregulating P210bcr/abl, NF-κB, and Hsp90. The consistent downregulation of NF-κB with P210bcr/abl showed that the effect of the combination of PS-ASODN and cur also exerted a synergistic inhibition on the downstream signals of P210bcr/abl, thus affecting the inhibition of cell growth. The possible rationales for combining PS-ASODN and cur were that both drugs inhibit P210bcr/abl by different mechanisms: PS-ASODN might downregulate the P210bcr/abl protein by blocking bcr/abl mRNA, while cur might accelerate the degradation of Hsp90[12] which is important in maintaining the conformation, stability, and function of P210bcr/abl, thus downregulating P210bcr/abl after bcr/abl mRNA was blocked by PS-ASODN.
In summary, PS-ASODN and cur exhibited a synergistic inhibitory effect on the cell growth of K562. The synergistic growth inhibition was mediated through different mechanisms that involved the inhibition of P210bcr/abl. The synergistic effect is clinically important and may provide the combinatorial strategies in cancer therapy.
Acknowledgements
We thank Dr Qun-hao ZHANG from Harvard Medical School who helped in our analysis of Western blot, and Dr Dan LU from Ohio State University for language support.
References
- Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science 1986;233:212-4.
- Heisterkamp N, Stam K, Groffen J, Groffen J, de Klein A, Grosveld G. Structural organization of the bcr gene and its role in the Ph1 translocation. Nature 1985;315:758-61.
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood 2000;96:3343-56.
- Warmuth M, Danhauser-Riedl S, Hallek M. Molecular pathogenesis of chronic myeloid leukemia: implications for new therapeutic strategies. Ann Hematol 1999;78:49-64.
- Piva R, Gianferretti P, Ciucci A, Taulli R, Belardo G, Santoro MG. 15-Deoxy-delta 12,14-prostaglandin J2 induces apoptosis in human malignant B cells: an effect associated with inhibition of NF-kappa B activity and down-regulation of antiapoptotic proteins. Blood 2005;105:1750-8.
- Nimmanapalli R, Porosnicu M, Nguyen D, Worthington E, O’Bryan E, Perkins C, et al. Cotreatment with STI-571 Enhances tumor necrosis factor α-related apoptosis-inducing ligand (TRAIL or Apo-2L)-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Clin Cancer Res 2001;7:350-7.
- Jahagirdar BN, Miller JS, Shet A, Verfaillie CM. Novel therapies for chronic myelogeous leukemia. Exp Hematol 2001;29:543-56.
- Rapozzi V, Cogoi S, Spessotto P, Risso A, Bonora GM, Quadrifoglio F, et al. Antigene effect in K562 cells of a PEG-conjugated triplexforming oligonucleotide targeted to the bcr/abl oncogene. Biochemistry 2002;41:502-10.
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcuma: preclinical and clinical studies. Anticancer Res 2003;23:363-98.
- Wu LX, Xu JH, Wu GH, Chen YZ. Inhibitory effect of curcumin on proliferation of K562 cells involves down-regulation of P210bcr/abl initiated Ras signal transduction pathway. Acta Pharmacol Sin 2003;24:1155-60.
- Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ 2006;13:738-47.
- An WG, Schulte TW, Neckers LM. The heat shock protein 90 antagonist geldanamycin alters chaperone associaton with P210bcr/abl and v-src proteins before their degradation by the proteasome. Cell Growth Differ 2000;11:355-60.
- Bhatia R, Vertaillie CM. Inhibition of BCR-ABL expression with antisense oligodeoxynucleotides restores β1 integrin-mediated adhesion and proliferation inhibition in chronic myelogenous leukemia hematopoietic progenitors. Blood 1998;91:3414-22.
- Chen LJ, Shen RL, Wang CY, Zhu GR. Ara-C induced apoptosis of HL-60 by AO/EB fluorescent staining. Chin J Hematol 1998;19:41-2.
- Jin ZJ. Additionin drug combination. Acta Pharmacol Sin 1980;1:70-6.
- Clark RE. Antisense therapeutics in chronic myeloid leukaemia: the promise, the progress and the problems. Leukemia 2000;14:347-55.
- Jiang MC, Lin JK, Chen SS. Inhibition of HIV-1 tat-mediated transactivation by quinacrine and chloroquine. Biochem Biophys Res Commun 1996;226:1-7.
- Sokoloski JA, Shyam K, Sartorelli AC. Induction of the differentiation of HL-60 promyelocytic leukemia cells by curcumin in combination with low levels of vitamin D3. Oncol Res 1997;9:31-9.
- Xu JH, Chen YZ, Huang XW. Curcumin inducing apoptosis and inhibiting expression of P210bcr/abl protein in human chronic myelogenous leukemia K562 cells. FASEB J 2000;14:1516-9.