Adeno-associated virus-mediated bone morphogenetic protein-7 gene transfer induces C2C12 cell differentiation into osteoblast lineage cells1
Introduction
Currently, the repair of massive segmental bone defects and non-healing fractures remains a challenging problem in orthopedic surgery. Bone morphogenetic proteins (BMP) are known to possess strong osteoinductive properties and BMP gene therapy plays an important role in modulating bone regeneration[1]. However, more efficient and safe delivery vectors must be obtained before clinical trials can be carried out successfully. Previous work with recombinant adeno-associated virus (AAV) vector for gene therapy has shown some outstanding advantages and has made it an attractive candidate for clinical trials in recent years[2]. How-ever, there have only been a small number of experiments using AAV vectors carrying BMP to induce bone healing[3–5]. Previous studies have indicated that the C2C12 myoblast cell line may be a useful model to investigate osteoblast differentiation during bone formation in muscular tissues[6–8]. Although it has been reported that continuous exposure of C2C12 cells to BMP7 protein could inhibit myotube formation and induce osteoblastic differentiation[6], it is not known whether transfer of the BMP7 gene into C2C12 cells using a BMP7-harboring AAV could also produce consistent results. This committed osteoblastic differentiation would be important for AAV-BMP7 in vivo gene therapy for bone healing. In addition, because recombinant AAV does not cause disease in humans and does not contain coding sequences necessary to trigger inflammatory or immune responses[2–4], we speculate that the AAV-BMP7 vectors could be injected into the fracture sites or segmental bone defects to induce bone formation by direct local gene therapy. To investigate the feasibility of BMP7 gene transfer into C2C12 cells using the AAV vector, we constructed an AAV vector carrying the BMP7 gene, designated AAV-BMP7. C2C12 cells were infected with AAV-BMP7 and their committed differentiation was examined in vitro. This study provides fundamental understanding and a platform for future applications of AAV-BMP7 local gene therapy.
Materials and methods
Cells HEK293, BHK-21 and C2C12 cells (all from ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and penicillin/streptomycin at 37 °C in humidified 5% CO2 atmosphere.
Plasmids The recombinant AAV2 packaging plasmid pSNAV[9] was constructed by deleting all viral open reading frames and introducing the sequences for human cytomegalovirus promoter/enhancer, a multiple-cloning site, a neomycin-resistant gene, a simian virus 40 promoter and a polyadenylation signal, retaining the 2 inverted terminal repeats (ITR), which contain the palindromic sequences necessary in cis for replication of the intact viral genome. The AAV plasmid pSNAV-BMP7 (Figure 1) was constructed by inserting full-length human BMP7 (hBMP7) cDNA (1.3 kb) into the restriction sites between KpnI and BgIII of the multiple-cloning site. The BMP7 cDNA was cloned by reverse transcription-polymerase chain reaction from HEK293 cells using the following set of primers: forward, 5´-GT

Adeno-associated virus vector packaging, purification and titration BHK-21 cells were transfected with the purified pSNAV-BMP7 plasmid according to a standard calcium phosphate precipitation method. The cells were then cultured in selection media containing 500 µg/mL G418 (Gibco/BRL). G418-resistant BHK-21 cell clones were isolated and the integrity of hBMP7 gene was determined by polymerase chain reaction (PCR) using the above PCR primers. To package the virus, stably transfected BHK-21 cells were subsequently infected with recombinant herpes simplex virus type 1 (rHSV-1)[10], which can express the AAV-2 Rep and Cap genes of wild-type AAV and possesses packaging functions for recombinant AAV. For large-scale recombinant AAV production and purification, BHK-21 cells were incubated in 6 roller bottles (Ø 110 mm×480 mm; Wheaton, Millville, NJ, USA) at 37 °C at 1 roll/min. Confluent cells in a volume of 10 mL medium were infected with helper virus rHSV-1 at multiplicities of infection (MOI) of 0.1 for 2 h. The collected cells were processed by chloroform treatment, PEG8000/NaCl precipitation and chloroform extraction for purification[11]. The titer was determined using quantitative DNA dot blots[12] and the purity was examined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis[11]. Titers averaged approximately 2×1012 vector genomes (v g) per mL and purity was >97%. Recombinant AAV-enhanced green fluorescence protein (EGFP) was also constructed using the same procedure.
Adeno-associated virus vector transduction C2C12 cells were placed in monolayer culture in 6-well plates at a density of 2×105 cells per well in DMEM containing 10% FBS. Sub-confluent cells were incubated with either AAV-BMP7 or AAV-EGFP at an MOI of 1×105 v.g. per cell, or as a control, left alone in a total volume of 500 µL serum-free medium for 1 h at 37 °C. The medium was then aspirated and 1 mL growth medium (DMEM supplemented with 5% FBS, 50 mmol/L sodium butyrate) was added. An MOI of 1×105 was chosen as a result of pilot studies demonstrating that an MOI of 1×105 produced the highest level of EGFP transgene expression. Morphological changes were monitored with a phase contrast microscope. All experiments were carried out in triplicate.
Determination of bone morphogenetic protein-7 production To quantify BMP7 levels, culture medium of C2C12 cells was collected for enzyme-linked immunosorbent assay (ELISA) every 2 d up to 28 d after transduction. ELISA was carried out according to the manufacturer’s recommendations (ADL, San Antonio, TX, USA). Briefly, standards and culture media were incubated at room temperature with sample buffer in 96-well plates for 90 min and then with biotin-labeled anti-human BMP7 detection antibody for 60 min. Finally, a streptavidin-horseradish peroxidase conjugate was added at room temperature for 30 min. Bound BMP7 was detected by adding tetramethylbenzidine substrate solution for 15 min and the plates were read at 450 nm.
Gene expression of Cbfal and MyoD A total of 2 µg cellular RNA from C2C12 cells was reverse transcribed using Mouse Moloney murine leukemia virus reverse transcriptase and oligo(dT) 15 primer (both from Promega, Madison, Wisconsin, USA). PCR were carried out for 25 cycles (94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min) for MyoD and glyseraldehyde-3-phosphate dehydrogenase (GAPDH), and 30 cycles for Cbfal (annealing temperature 62 °C). Primers were: MyoD (expected product size 275 bp): upstream, 5´-TCCAACTGCTCTGATGGCA-3´; downstream, 5´-GTTCCC-TGTTCTGTGTCGCT-3´; Cbfal (expected product size 330 bp)[13]: upstream, 5´-CTTCATTCGCCTCACAAAC-3´; downstream, 5´-CACGTCGCTCATCTTGCCGG-3´. GAPDH was used as an internal control (expected product size 413 bp): upstream, 5´-GGAAAGCTGTGGCGTGATGG-3´; downstream, 5´-GTAGGCCATGAGGTCCACCA-3´. The PCR products were subjected to electrophoresis on 1.5% agarose gels, scanned, and semiquantitated using Image-Quant software (Kodak I D V3.53; Kodak, Tokyo, Japan).
Alkaline phosphatase activity assay and osteocalcin production C2C12 cells were rinsed twice with phosphate-buffered saline, then collected into 50 mmol/L Tris-HCl, 0.1% Triton X-100, pH 7.5, and sonicated for 10 s at 4 °C. The samples were centrifuged for 5 min at 13400×g. Alkaline phosphatase (ALP) activity was measured using an ALP assay kit (Zhongsheng Biochemical, Beijing China) at 37 °C. The enzyme activity was normalized against the protein concentration and expressed as U·g-1·L-1 [14]. Protein concentration was measured using the bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA) using bovine serum albumin as a standard. The amount of osteocalcin secreted into the culture medium was determined by radioimmunoassay using a mouse osteocalcin assay kit following the manufacturer’s recommendations (Biomedical Technologies, Stoughton, MA, USA).
Statistical analysis Data (mean±SD) were analyzed using two-tailed Student’s t-test with a level of significance of 0.05.
Results
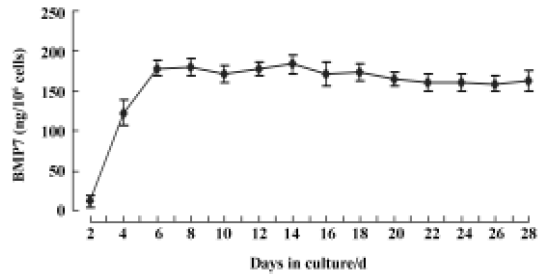
Bone morphogenetic protein-7 expression in C2C12 cells The in vitro release kinetics of BMP7 from AAV-BMP7-infected C2C12 cells was evaluated over the course of 28 d using ELISA. No detectable BMP7 was produced by the uninfected and AAV-EGFP-treated C2C12 cells. However, cells transduced with AAV-BMP7 produced low levels of BMP7 by 48 h (12±8 ng/106 cells per 48 h), followed by an increase in production with a mean of 165±10 ng/106 cells per 48 h from d 6 to d 28 (Figure 2).
Effect of bone morphogenetic protein-7 on C2C12 cell morphology After cultured in DMEM with 5% FBS for 6 d, the phenotype of C2C12 cells displayed obvious changes. Elongated and multinucleated thin myotubes formed by cells fusing with each other were observed in uninfected C2C12 cells and cells infected with AAV-EGFP. In contrast, most cells infected with AAV-BMP7 revealed an unfused, mononuclear round-cell morphology, resembling that of osteoblastic cells (Figure 3).

Differentiation of C2C12 cells treated with AAV-BMP7 In order to further investigate the function of BMP7 expression, we examined the gene expression changes for osteoblast-specific genes, and also measured ALP activity and osteocalcin production, in C2C12 cells infected with AAV-BMP7. In AAV-BMP7-treated C2C12 cells the mRNA expression level of Cbfa1 (an osteoblast specific transcription regulatory factor[15]) was upregulated after 4 d of treatment, a significant increase was detected at d 6 and its level continued to increase until d 12 (Figure 4). In contrast, the expression level of muscle-specific regulatory factor MyoD was reduced dramatically after 8 d of treatment and became undetectable by d 12.

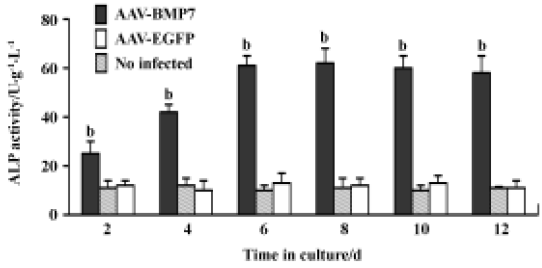
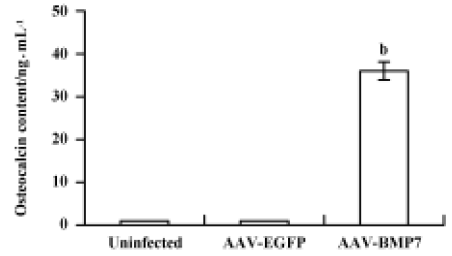
The uninfected and AAV-EGFP-infected C2C12 cells were found to have low levels of ALP activity with no significant change at all time points (Figure 5). A significant upregulation of ALP activity was observed in AAV-BMP7-treated cells, starting from d 2 and peaking at d 8. Osteocalcin production was detected at d 12 as 35±6 ng/mL (Figure 6); no detectable osteocalcin was found in non-treated and AAV-EGFP-treated cells.
Discussion
Our in vitro experiments demonstrated that C2C12 cells infected with AAV-BMP7 were able to generate BMP7 protein and showed a concomitant shift from myoblastic to osteoblastic differentiation. In addition, the highly efficient recombinant adeno-associated viral vector packaging system, rHSV-1/AAV hybrid helper viral system[10], provided us with high-titer purified AAV-BMP7 for further experiments. Our subsequent AAV-BMP7 transduction analysis suggested that recombinant AAV vector could mediate efficient transduction in C2C12 cells. At the same time, the amount of secreted BMP7 protein could reach the level of protein needed for cellular responses. The elevated ALP activity and osteocalcin production, together with osteoblast-like morphology, confirmed that the AAV-BMP7-infected C2C12 cells displayed differentiation to an osteoblastic phenotype.
Previous studies have shown that BMP7 at 200 ng/mL can completely inhibit myogenic differentiation of C2C12 cells and successfully induce expression of both important early and late osteoblastic differentiation markers (ALP and osteocalcin, respectively)[6]. Although BMP7 protein could satisfactorily induce C2C12 cell differentiation into osteoblastic cells in vitro, it requires supra-physiological amounts of BMP7 to overcome rapid clearance due to its short life span, in order to accelerate fracture healing, bridge seg-mental bone defects and generate spine fusion in animal models[16]. Hence, protein therapies are hampered by high manufacturing costs, unpredictable side effects and the lack of an ideal matrix to deliver protein in a continuous manner over times[16]. Local BMP7 gene therapy provides an alternative method for the delivery of BMP7 protein to stimulate bone regeneration[17–20]. Recent studies have demonstrated that recombinant AAV vector is an ideal vector to deliver therapeutic factors. Recombinant AAV vector is non-pathogenic and elicits no inflammatory response. It is also an advantage that recombinant AAV vector often leads to efficient long-term expression of secreted proteins in vivo and in vitro[21]. However, whether AAV-BMP7 could induce bone formation in vitro and in vivo remains unclear. In this study, a recombinant AAV-BMP7 vector was constructed success-fully. The sustained secretion of BMP7 protein was detected in culture medium up to d 28 by C2C12 cells infected with AAV-BMP7. Mouse myoblast C2C12 cells displayed an osteoblastic phenotype. It has been reported that C2C12 cells are pluripotent mesenchymal precursor cells, capable of differentiating into myoblasts, adipocytes and osteoblasts under appropriate stimulation conditions[22,23]. Further studies demonstrated that BMP7 protein was a potent inducer of osteoblastic differentiation of C2C12 cells by upregulating Runx2/Cbfa1 gene transcription[24]. The core binding factor Runx2/Cbfa1 is a specific transcriptional activator and a molecular switch of osteoblast differentiation[25]. Thus, C2C12 cells infected with AAV-BMP7 could be finally restricted to give rise to one terminally differentiated cell type expressing the markers of osteoblasts (eg ALP and osteo-calcin). This osteogenic committed differentiation mediated by genetic modification is safe as it gives the desired cell type and avoids the possibility of giving rise to unlimited cell growth or unwanted cell types. The committed differentiation could also be controlled by using tissue-specific promoters or the “tet switch system”.
In this study, we found an interesting feature of the AAV vector, namely delayed transgene expression. The production of BMP7 protein reached a peak as late as 6 d after infection, compared with adenovirus-mediated gene delivery in C2C12 cells in which the desired BMP2 protein could be produced as early as 24 h after infection[7]. This difference may be due to the fact that AAV is a single-stranded DNA virus; there is, therefore, a rate-limiting step of second-strand DNA synthesis in the nucleus of infected cells[21]. However, the delayed BMP7 protein expression using the AAV vector did not affect the osteogenic biological function of BMP7 in our study. Some in vivo examinations have also demonstrated the same delayed transgene expression[26,27]. From a clinical standpoint, it seems that the short period of delayed osteoinductive protein production does not hamper the treatment of relative longer-term and non-emergency cases of fracture healing or spinal fusion. Furthermore, delayed transgene expression might protect secreted therapeutic proteins from immunologic attack induced by destruction of the vascular barrier at the time of virus injection[28].
We first report that AAV-based BMP7 gene transfer could represent a new and feasible way to induce a committed osteoblast differentiation in an in vitro culture system using C2C12 cells. This approach would be preferred in a great range of applications for some orthopedic disorders in which it is necessary to increase the formation of bone. Unlike the adenoviral-based vectors that are limited by their non-specific immune response[1], the AAV-BMP7 vectors could be injected directly into the segmental bone defect that does not heal spontaneously to promote bone formation by recruiting locally responsive cells to differentiate into osteoblasts. In addition, the AAV-BMP7 vectors could be added to autologous bone grafts to serve as an osteoinduc-tive agent to augment bone regeneration at the fusion site and decrease the need for extensive harvesting of autograft from the iliac crest. Furthermore, direct injection of these vectors into the spine could generate spine fusion in a less invasive way. For some systemic and metabolic bone diseases such as osteoporosis, the efficient long-term secretion of BMP7 proteins mediated by AAV is another outstanding advantage. The advantages of direct gene therapy strategy also include relatively simple technique requirements, minimized invasion and the potential for lower costs.
Although the detailed mechanisms of C2C12 cells differentiating into osteoblasts remains unclear, our current study suggests that AAV-BMP7 may be a valuable viral vector in the treatment of orthopedic disorders. Further in vivo studies are under consideration.
References
- Hannallah D, Peterson B, Lieberman JR, Fu FH, Huard J. Gene therapy in orthopaedic surgery. J Bone Joint Surg Am 2002;84A:1046-61.
- Monahan PE, Samulski RJ. AAV vectors: is clinical success on the horizon? Gene Ther 2000;7:24-30.
- Luk KD, Chen Y, Cheung KM, Kung HF, Lu WW, Leong JC. Adeno-associated virus-mediated bone morphogenetic protein-4 gene therapy for in vivo bone formation. Biochem Biophys Res Commun 2003;308:636-45.
- Chen Y, Luk KD, Cheung KM, Xu R, Lin MC, Lu WW, et al. Gene therapy for new bone formation using adeno-associated viral bone morphogenetic protein-2 vectors. Gene Ther 2003;10:1345-53.
- Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, et al. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther 2004;9:587-95.
- Yeh LC, Tsai AD, Lee JC. Osteogenic protein-1 (OP-1, BMP-7) induces osteoblastic cell differentiation of the pluripotent mesenchymal cell line C2C12. J Cell Biochem 2002;87:292-304.
- Okubo Y, Bessho K, Fujimura K, Iizuka T, Miyatake S. Expression of bone morphogenetic protein-2 via adenoviral vector in C2C12 myoblasts induces differentiation into the osteoblast lineage. Biochem Biophys Res Commun 1999;262:739-43.
- Kim YJ, Lee MH, Wozney JM, Cho JY, Ryoo HM. Bone morphogenetic protein-2-induced alkaline phosphatase expression is stimulated by Dlx5 and repressed by Msx2. J Biol Chem 2004;279:50773-80.
- Wu ZJ, Wu XB, Hou YD. Construction of a series of adeno-associated virus vectors and their expression of β-galactosidase gene. Chin J Virol 2000;16:1-6.
- Wu ZJ, Wu XB, Hou YD. Generation of a recombinant herpes simplex virus which can provide packaging function for recombinant adeno-associated virus. Chin Sci Bull 1999;44:715-9.
- Wu XB, Dong XY, Wu ZJ, Cao H, Niu DB, Qu JG, et al. A novel method for purification of recombinant adeno-associated virus vectors on a large scale. Chin Sci Bull 2000;45:2071-5.
- Ponnazhagan S, Yoder MC, Srivastava A. Adeno-associated virus type 2-mediated transduction of murine hematopoietic cells with long-term repopulating ability and sustained expression of a human globin gene in vivo. J Virol 1997;71:3098-104.
- Bonnelye E, Merdad L, Kung V, Aubin JE. The orphan nuclear estrogen receptor-related receptor α (ERRα) is expressed throughout osteoblast differentiation and regulates bone formation in vitro. J Cell Biol 2001;153:971-84.
- Song CL, Guo ZQ, Ma QJ, Chen ZQ, Liu ZJ, Jia HT, et al. Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem Biophys Res Commun 2003;308:458-62.
- Xiao ZS, Liu SG, Hinson TK, Quarles LD. Characterization of the upstream mouse Cbfa1/Runx2 promoter. J Cell Biochem 2001;82:647-59.
- Li R, Wozney J. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol 2001;19:255-65.
- Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther 2000;11:1201-10.
- Franceschi RT, Wang D, Krebsbach PH, Rutherford RB. Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem 2000;78:476-86.
- Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg 1999;42:488-95.
- Mason JM, Grande DA, Barcia M, Grant R, Pergolizzi RG, Breitbart AS. Expression of human bone morphogenic protein 7 in primary rabbit periosteal cells: potential utility in gene therapy for osteochondrial repair. Gene Ther 1998;5:1098-104.
- Schwarz EM. The adeno-associated virus vector for orthopaedic gene therapy. Clin Orthop 2000.Suppl:S31-9.
- Fux C, Langer D, Fussenegger M. Dual-regulated myoD- and msx1-based interventions in C2C12-derived cells enable precise myogenic/osteogenic/adipogenic lineage control. J Gene Med 2004;6:1159-69.
- Fux C, Mitta B, Kramer BP, Fussenegger M. Dual-regulated expression of C/EBP-α and BMP-2 enables differential differentiation of C2C12 cells into adipocytes and osteoblasts. Nucleic Acids Res 2004;32:e1.
- Tou L, Quibria N, Alexander JM. Transcriptional regulation of the human Runx2/Cbfa1 gene promoter by bone morphogenetic protein-7. Mol Cell Endocrinol 2003;205:121-9.
- Zheng H, Guo Z, Ma Q, Jia H, Dang G. Cbfa1/osf2 transduced bone marrow stromal cells facilitate bone formation in vitro and in vivo. Calcif Tissue Int 2004;74:194-203.
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet 2000;24:257-61.
- Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, et al. Sustained secretion of human α-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci USA 1998;95:14384-8.
- Aubert D, Pichard V, Durand S, Moullier P, Ferry N. Cytotoxic immune response after retroviral-mediated hepatic gene transfer in rat does not preclude expression from adeno-associated virus 1 transduced muscles. Hum Gene Ther 2003;14:473-81.
