CD151 gene delivery increases eNOS activity and induces ECV304 migration, proliferation and tube formation1
Introduction
As a member of the tetraspanin family, CD151 possesses 4 transmembrane domains: cytoplasmic amino and carboxyl termini, and 2 extracellular loops, the larger of which contains the distinctive pattern of cysteine residues that help to define the family[1]. In vitro, CD151 antibody inhibition of cord-like structures on matrigel results seen in NIH3T3 cells, suggests that CD151 may contribute to angiogenesis in particular, and to branching morphogenesis in general[2,3]. Our research has revealed that CD151 promotes neova-scularization and angiogenesis, which suggests that CD151 is a potential therapeutic reagent for treating ischemia by inducing angiogenesis[4]. However, the precise mechanism by which CD151 induces angiogenesis response is not known.
Angiogenesis formation of new capillary blood vessels from existent microvessels occurs in physiological and pathological states. Angiogenesis involves endothelial cell (EC) movement, realignment to form sprouts, and subsequent fusion and extension to form mature capillary tubes. eNOS has been shown to regulate endothelial cell migration, proliferation and angiogenesis[5–8]. Whether eNOS is involved in CD151-induced angiogenesis remains to be determined.
We hypothesized that CD151 might increase eNOS activity and might affect cell migration, proliferation and tube formation. Here, we observed the effects of CD151 on eNOS activity, endothelial cell migration, proliferation and tube formation. Therefore, we aim to determine the mechanisms by which CD151 induces cell migration, proliferation and tube formation.
Materials and methods
Materials PzeoSV-CD151 plasmid was given as a gift by Prof Xin ZHANG, Department of Molecular Science, University of Tennessee Health Science Center (Memphis,Tennessee,USA). The restriction enzymes were from TaKaRa (Dalian, China). DNA marker was from Jingmei Company (Shenzhen, China). Dulbecco’s modified Eagle’s medium (DMEM) culture medium was purchased from Gibco (Losaneles, California, USA). Lipofectamine 2000 was from Invitrogen Co (Carlsbad, California, USA). Enhanced chemiluminescent (ECL) substrate was from Pierce (Rockford, Illinois, USA). Polyvinylidene difluoride (PVDF) membrane was from Schleicher and Schuell Co (Hahnestrasse, Dassel, Germany). Mouse anti-human CD151 was from Serotec (Kidlington, Oxford, UK). Rabbit anti-human β-actin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA). NG-nitro-L-arginine methyl ester (L-NAME) was purchased from Sigma Company (St Louis, MO, USA).
pAAV-CD151 and pAAV-anti-CD151 plasmid construction Two pairs of primers were designed: P1 For: 5'-GAGAT-CTATGGGTGAGTTCAACGAG-3', with a restriction site of Bgl II; P1 Rev: 5'-GGAATTCCTCAGTAGTGCTCCAGCTT-GAG-3',with a restriction site of Not I; P2 For: 5'-GGAATT-CCATGGGTGAGTTCAA CGAG-3',with a restriction site of Not I; P1 Rev: 5'-GAGATCTTCAGTAGTGCTC CAGCTTG-AG-3', with a restriction site of Bgl II. The entire coding region of human CD151 cDNA was obtained from the pZeoSV-CD151 plasmid by PCR. Then the pAAV-CD151 plasmid and the pAAV-anti-CD151 plasmid were constructed by inserting it into the pAAV vector in the sense and antisense directions next to the human cytomegalovirus promoter. They were then amplified by transferring into activated Escheria coli DH5a. They were confirmed by restriction endonuclease analysis (MBI, Hanover, MD, USA) and nucleotide sequencing (TaKaRa Biotechnology, China).
Cell culture and plasmid transfection The ECV304 cell line was bought from the China Center for Type Culture Collection and cultured in DMEM supplemented with 10% fetal serum bovine (FBS), penicillin and streptomycin. ECV304 cells were grown on 6-well plates (70%–90% confluent) and pre-incubated in OptiMem media for 15 min at 37 °C. The pAAV-CD151 plasmids (1 µg), pAAV-anti-CD151 plasmids (1 µg) or pAAV-GFP plasmids (1 µg) was mixed with Lipofectamine 2000 (5 µL/well) and incubated for 15 min at 37 °C. The lipid-coated DNA was then added to each well containing 2 mL of DMEM media and incubated for 6 h. At the end of this period, the media were removed and replaced with complete media, after which the ECV304 cells were lysed or used in migration assays.
Western blot analysis The cells were scraped off the plates and lysed in radioimmunoprecipitation assay (RIPA) buffer [50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 1% Nonidet-P40, 0.5% deoxycholic acid and 0.1% sodium dodecyl sulfate]. Cell lysates (50 µg protein) were electrophoresed on SDS-PAGE gel and transferred to a polyvinyl difluoride membrane. Membranes were incubated with antibodies against CD151 or β-actin. After incubation, the corresponding secondary antibody signals were detected by the enhanced chemiluminescence reagents. The intensities of the various protein bands were quantified by densitometry.
Migration assay Cell migration assay was performed using Boyden transwell chambers (8.5 mm in diameter). Briefly, 200 µL DMEM containing 10% FBS was added to the bottom well. Cells were resuspended in the appropriate buffer at a concentration of 1×106 cells/mL, and 800 µL cell suspension was added to the top well of the Transwell chambers. Between the bottom well and the top well, there was the filter (pore size 8 µm; Costar, Cambridge, MA, USA). After incubation at 37 °C in 5% CO2 conditions, the cells that had not migrated were removed from the upper surface of the filters using cotton swabs, and those that migrated to the lower surface of the filters were fixed in methanol and stained with hematoxylin. Migration was determined by counting the cell number with a microscope at ×400. Five visual fields were chosen randomly for each assay. The average number of the migrating cells in the 5 fields was taken as the cell migration number of the group. The assays were repeated 3 times.
Proliferation assay The ECV304 cells were transfected with pAAV-CD151, pAAV-anti-CD151, or pAAV-GFP mediated by Lipofectamine 2000 in 6-well plates in triplicate, and 24 h later the cells were trypsinized and seeded in 96-well plates in triplicate (1×104 cells/well). After attachment, the cells were exposed to DMEM with 0.5% FBS for 48 h, and then the effects of CD151 on ECV304 proliferation were evaluated using the 3-[4,5-dimethylthiazol-2-yl]-2,5, diphenyltetra-zolium bromide (MTT) (Sigma, USA) colorimetric assay. Briefly, the medium was removed and replaced with medium containing 5 mg/mL MTT and incubated for 4 h. The medium was then aspirated, and the product was solubilized with DMSO. Absorbance was measured at 570 nm for each well using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA) according to the manufacturer’s protocol.
Tube formation analysis In vitro formation of capillary-like tube structures was examined on matrigel. Matrigel (0.5 mL) was polymerized on 24-well plates and 5×104 cells were then plated in full-growth media for 1 h. Once the cells were seeded, the media was replaced with media containing 0.5% serum. Tube formation was visualized using an inverted microscope (Nikon TE 2000, Tokyo, Japan) equipped with digital imaging. For each treatment, 10 high power field images were captured and the area of endothelial tubes and networks formed was quantified using the Scion Image Analysis System (Frederick, Maryland,USA) with background subtraction. To examine the effects of CD151 on tube formation, ECV304 cells were first transfected with different plasmids; 24 h later, they were plated in 24-well plates with matrigel followed by tube formation analysis as described earlier.
Measurement of eNOS activity The activity of eNOS was assayed by L-[3H] citrulline production from L-[3H] arginine. In brief, cell lysates (100 µL) were incubated in 50 mmol/L Tris-HCl buffer (pH 7.5) containing the cofactors 100 nmol/L calmodulin, 2.5 mmol/L CaCl2, 1 mmol/L nicotinamide adenine dinucleotide phosphate (NADPH),10 µmol/L tetrahydro-biopterin, 1 mmol/L dithiotreitol, and the substrate, 1 µCi L-[3H]arginine, for 15 min at 37 °C. After the incubation period, the reaction was quenched by the addition of 1 mL of stop buffer [20 mmol/L N-2-hydroxyethylpiperazine-N'-2-ethane sulfonic acid (HEPES), 2 mmol/L EDTA, and 0.2 mmol/L ethyleneglycol-bis-(β-aminoethyl ether)-N,N,N',N'-tetra-acetic acid (EGTA), pH 3]. The reaction mixture was applied to a 1 mL column containing Dowex AG 50WX-8 resin (Na+ form, Bio-Rad, Hercules, CA, USA) that had been pre-equilibrated with the stop buffer. L-[3H]citrulline was eluted twice with 0.5 mL of stop buffer and radioactivity was determined by liquid scintillation counting.
Statistical analysis Values presented are the mean±SEM. Comparisons between 2 groups were performed using Student’s t test; 3 or more groups were compared by ANOVA, followed by the Newman-Keuls test. P values less than 0.05 were considered significant.
Results
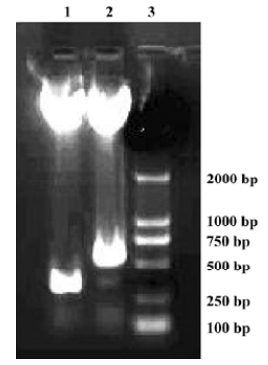
Construction and confirmation of pAAV-CD151 plasmid and pAAV-anti-CD151 plasmid To construct the recombinant pAAV-CD151 vector and pAAV-anti-CD151 vector, 799 bp CD151 cDNA with 2 enzyme sites (Bgl II/Not I) was linked with the pAAV plasmid in the sense and antisense directions, respectively. The enzymes of Bgl II and BamH I were isoschizomers. The BamH I enzyme restriction site was approximately 500 bp away from the translation initiation of the CD151 gene. pAAV-CD151 and pAAV-anti-CD151 were identified by restriction endonuclease digestion with BamH I and Not I, and electrophoresis generating a 300 bp fragment and 500 bp fragment, respectively, which was compared with the desired result (Figure 1). The DNA sequence was identical to the human CD151 gene sequence of GenBank (MIM: 602243).
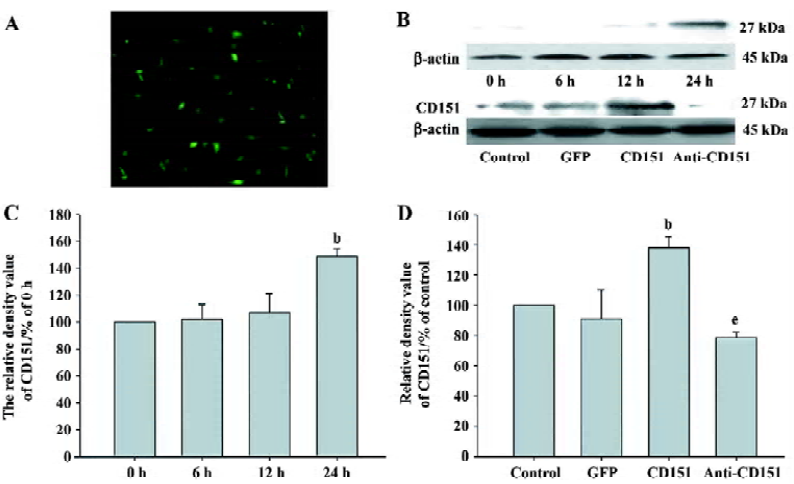
Expression of CD151 after transfection Using pAAV-GFP to control transfection efficiency, the transfection efficiency was at its highest at 24 h, which was approximately 60% (Figure 2A). To observe the time course of the expression of CD151 after transfection with pAAV-CD151, we found that CD151 levels did not increase at 0, 6, or 12 h, but significantly increased at 24 h (P<0.05). We also observed that the expression of CD151 in the cells transfected with pAAV-CD151 significantly increased (P<0.05) as compared with the control and pAAV-GFP groups 24 h after transfection. In addition, pAAV-anti-CD151 transfection dramatically decreased (P<0.05) the expression of CD151 24 h after transfection (Figure 2B–D).
Effect of CD151 on ECV304 migration In the Boyden chamber assay (Table 1), at 0, 6, and 12 h after transfection with pAAV-CD151, there was no significance among the groups in the migrated cell number. At 24 h after transfection, increased expression of CD151 significantly increased cell migration (P<0.05), whereas decreased expression of CD151 significantly, inhibited cell migration (P<0.05). These results suggest that CD151 plays a critical role in ECV304 migration.
Full table
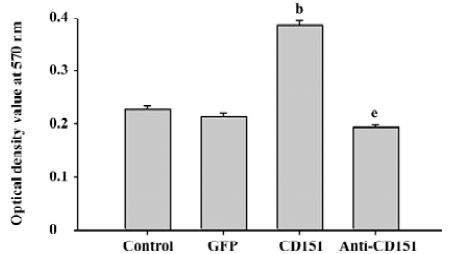
Role of CD151 in ECV304 proliferation To determine the effect of CD151 on ECV304 proliferation, a necessary process for angiogenesis, we determined the proliferative effects using MTT assay. ECV304 transfected with pAAV-CD151 significantly enhanced ECV304 proliferation (P<0.05) as compared with the groups transfected with pAAV-GFP and the control. pAAV-anti-CD151 transfection significantly inhibited the proliferative effects as compared with the groups transfected with pAAV-GFP and the control group (Figure 3), which suggested that CD151 promotes cell proliferation.
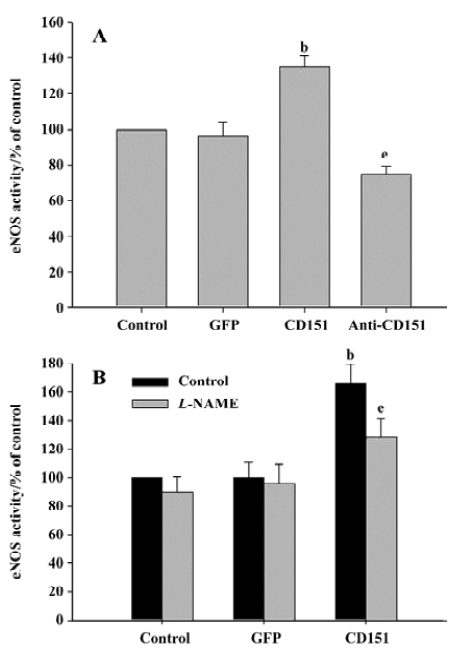
Effect of CD151 on eNOS activity To investigate whether CD151 was able to increase eNOS activity, ECV304 cells were transfected with pAAV-CD151 and pAAV-anti-CD151 and analyzed for NOS activity. As with cell migration, increased expression of CD151 increased NOS activity significantly (P<0.05), whereas decreased expression of CD151 significantly decreased NOS activity (P<0.05, Figure 4A). Our data show that CD151 increases eNOS activity, which is consistent with our hypothesis. In addition, increased eNOS activity induced by CD151 was inhibited by L-NAME (Figure 4B). Based on these results, we show that CD151 activates eNOS.
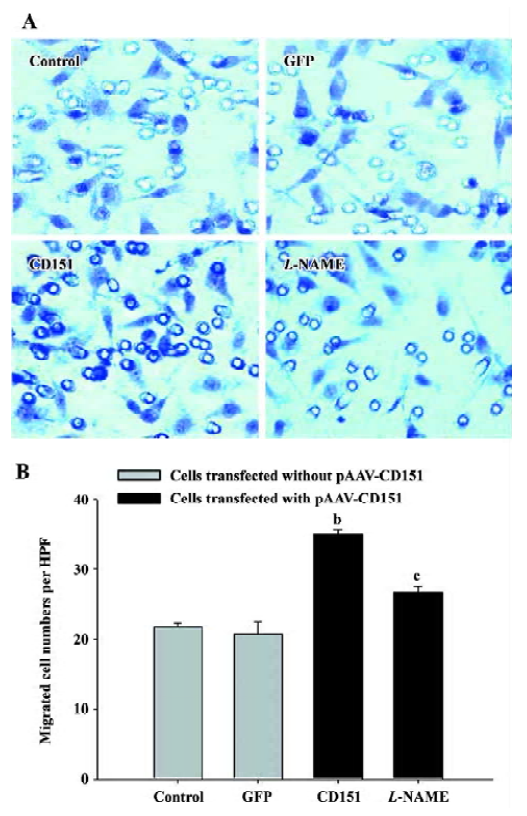
Role of eNOS in CD151-induced ECV304 migration We next investigated whether eNOS mediates CD151-induced cell migration. For this reason, an eNOS inhibitor (L-NAME) was used. The results showed that the cell number significantly increased (P<0.05) in the cells transfected with pAAV-CD151 as compared with the groups transfected with pAAV-GFP and the control group, which is consistent with Table 1. In addition, migrated cell number in cells transfected with p-AAV-CD151 decreased significantly (P<0.05) in the presence of L-NAME (0.5 mmol/L, Figure 5). Taken together, the data indicate that CD151-induced cell migration is partly dependent of the activation of eNOS.
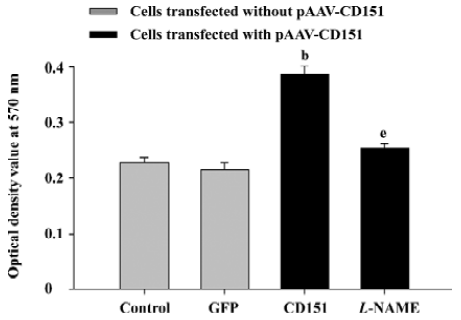
Role of eNOS in CD151-induced ECV304 proliferation In the MTT assay, we obtained similar results. The results showed that CD151 significantly increased cell proliferation (P<0.05) as compared with the groups transfected with pAAV-GFP and the control group. L-NAME (0.5 mmol/L) significantly inhibited cell proliferation induced by CD151 (P<0.05, Figure 6). Taken together, the data indicate that CD151-induced cell proliferation is partly dependent on the activation of eNOS.
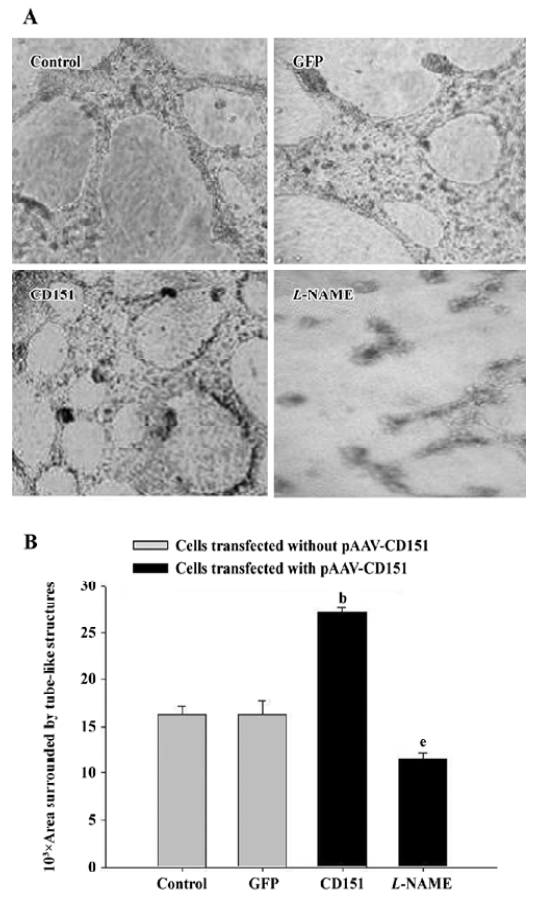
Role of eNOS on CD151-induced ECV304 tube formation We next examined whether CD151 promotes ECV304 tube formation. Matrigel tests demonstrated that transfection with pAAV-CD151 dramatically (P<0.05) increased tube formation; L-NAME dramatically (P<0.05) decreased the process (Figure 7). These data suggested that CD151 enhanced capillary tube formation, which was inhibited by L-NAME, indicating that eNOS activation played a critical role in CD151-induced tube formation.
Discussion
The present study shows that CD151 increases migration, proliferation and tube formation of ECV304 cells in vitro. The antisense nuclei acid technology is an important method for investigating the gene function and retarding the adverse proteins, and has been extensively used in molecular biology for its specificity, ease of use, and stability. So we constructed p-AAV-anti-CD151 which was used to decrease the expression of CD151. Cell migration and proliferation were suppressed when the expression of CD151 decreased after transfection with rAAV-anti-CD151. Results revealed that CD151 plays a critical role in cell proliferation and migra-tion. In addition, we showed that CD151 stimulates tube-like structures after CD151 gene delivery. These data indicate that a critical role of CD151 is in cell migration, proliferation and angiogenesis. However, the precise mechanisms are still unclear.
It has been shown that the tetraspanin CD151 is involved in the regulation of the migration of neutrophils, endothelial cells and various tumor cell lines[5,9,10]. However, no biochemical signaling events have been ascribed to the activity of CD151. Recent research has identified CD151 as a potential target for therapeutic angiogenesis[4]. The mechanisms that CD151 control angiogenesis and signaling pathways are not completely understood at present. The present study also demonstrated that CD151 increases eNOS activity 24 h after transfection with pAAV-CD151. Conversely, inhibiting the expression of CD151 by transfection with p-AAV-anti-CD151 leads to decreased migrated cell numbers and proliferation of ECV304, accompanied by a decrease of eNOS activity. The promotion or inhibition of cell migration and proliferation were linked to the activity of eNOS, which indicates that eNOS plays a critical role in CD151-induced cell migration and proliferation of ECV304 cells. Considering CD151-induced cell migration and proliferation mediated by eNOS, we next tried to determine whether the effects of CD151 observed in the present study were related to increased eNOS activity. We then examined influences of an eNOS inhibitor (L-NAME) and found the addition of L-NAME to ECV304 cells transfected with pAAV-CD151 partly attenuated CD151-induced cell migration, proliferation and tube formation, suggesting that eNOS is involved in part to the antagonistic effect of L-NAME. It seems likely that CD151-induced cell migration, proliferation and angiogenesis through the activation of eNOS.
Mechanisms that CD151 regulate cell migration, proliferation and tube formation take into account that eNOS activated by CD151 plays a critical role in cell migration, proliferation and tube formation. eNOS is an important regulator in angiogenesis and had been implicated in all steps in the process of angiogenesis, including endothelial cell migration, proliferation and organization into a network structure[11-13]. In addition, inhibitors of eNOS could reduce CD151-induced cell proliferation, migration and tube formation. This result indicates that eNOS is an important mediator in CD151-induced cell migration, cell proliferation and angiogenesis.
From the currently available data, we have no explanation for the ability of CD151 to activate eNOS; future studies might help to fully understand the role of eNOS in signaling by CD151. Based on our study, the most likely mechanism is that CD151 can function as a transmembrane link interacting with proteins regulating eNOS activity. Indeed, earlier studies have shown that tetraspanins could influence downstream signaling events, including tyrosine phosphorylation of cellular proteins and activation of the serine-threonine kinase PKB/c-Akt. In addition, transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta (1) integrins[14]. The formation of PKC-TM4SF-integrin complexes is important for the “outside-in” signal transduction[15]. A published report has shown that PKCalpha could activate eNOS and increase arterial blood flow in vivo, which suggests that eNOS might be involved in the PKC-TM4SF-integrin pathway[16]. A detailed analysis of signaling events in the present study indicates that CD151 affects cell migration, proliferation and tube formation via a novel mechanism. In the present study, we showed that CD151 targets eNOS signaling and these events are connected with CD151-induced cell migration, proliferation and tube formation.
In summary, we investigated in detail and found that CD151 promotes cell migration, proliferation and tube formation. Furthermore, we established that this effect arises from the marked effect of CD151 on the activation of eNOS. To our knowledge, there have been no previous studies regarding the effects of CD151 on eNOS activity. Further studies will provide clues for better understanding the function of CD151, and may help uncover novel therapeutic strategies for regulating the angiogenic process.
Acknowledgement
We are grateful to Prof Xin ZHANG for providing PzeoSV-CD151 plasmid (Department of Molecular Science, University of Tennessee Health Science Center, Memphis, Tennessee, USA).
Footnote
Project supported by a grant from the National Natural Science Foundation of China (N
References
- Stipp CS, Kolesnikova TV, Hemler ME. Functional domains in tetraspanin proteins. Trends Biochem Sci 2003;28:106-12.
- Chometon G, Zhang ZG, Rubinstein E, Boucheix C, Mauch C, Aumailley M. Dissociation of the complex between CD151 and laminin-binding integrins permits migration of epithelial cells. Exp Cell Res 2006;312:983-95.
- Zhang XA, Kazarov AR, Yang X, Bontrager AL, Stipp CS, Hemler ME. Function of the tetraspanin CD151-alpha6beta1 integrin complex during cellular morphogenesis. Mol Biol Cell 2002;13:1-11.
- Lan RF, Liu ZX, Liu XC, Song YE, Wang DW. CD151 promotes neovascularization and improves blood perfusion in a rat hind-limb ischemia model. J Endovasc Ther 2005;12:469-78.
- Lan RF, Liu ZX, Liu XC, Song YE, Wang DW. Effects of pAAV-CD151 and pAAV-antiCD151 on the migration of human tongue squamous carcinoma cell line Tca8113. J Huazhong Univ Sci Tech Med Sci 2004;24:556-9.
- Zhao X, Lu X, Feng Q. Deficiency in endothelial nitric oxide synthase impairs myocardial angiogenesis. Am J Physiol Heart Circ Physiol 2002;283:H2371-78.
- Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in tumor angiogenesis. Cancer Treat Res 2004;117:155-67.
- Isenberg JS. Nitric oxide modulation of early angiogenesis. Microsurgery 2004;24:385-91.
- Yauch RL, Berditchevski F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable, and specific association of integrin α3β1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell 1998;9:2751-65.
- Yanez-Mo M, Alfranca A, Cabañas C, Marazuela M, Tejedor R, Ursa MA, et al. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with α3β1 integrin localized at endothelial lateral junctions. J Cell Biol 1998;141:791-804.
- Dimmeler S, Dernbach E, Zeiher AM. Phosphorylation of the endothelial nitric oxide synthase at Ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett 2000;477:258-62.
- Gooch KJ, Dangler CA, Frangos JA. Exogenous, basal, and flow-induced nitric oxide production and endothelial cell proliferation. J Cell Physiol 1997;171:252-8.
- Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Investig 1998;101:2567-78.
- Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta (1) integrins. J Biol Chem 2001;276:25005-13.
- Berditchevski F, Gilbert E, Griffiths MR, Fitter S, Ashman L, Jenner SL. Analysis of the CD151- alpha3beta1 integrin and CD151-tetraspanin interactions by mutagenesis. J Biol Chem 2001;276:41165-74.
- Partovian C, Zhuang Z, Moodie K, Lin M, Ouchi N, Sessa WC, et al. PKCalpha activates eNOS and increases arterial blood flow in vivo. Circ Res 2005;97:482-7.
