Blood pressure variability and baroreflex sensitivity are not different in spontaneously hypertensive rats and stroke-prone spontaneously hypertensive rats1
Introduction
Blood pressure (BP) is not constant. Spontaneous variations exist in BP. The extent of the variation in BP is defined as the blood pressure variability (BPV). This new parameter of cardiovascular function can be determined through continuous ambulatory BP monitoring using a computerized technique[1,2], which can provide beat-to-beat BP tracings. It is understood that BPV is independent of BP[2,3], and high BPV is a novel risk factor for cardiovascular damage, such as aortic hypertrophy and renal lesions[2,4,5]. In the post-genome era, attaching physiology and pharmacology to the genome has become more and more important, as the combined physiological, pharmacological and genomic information should facilitate the development of new therapeutics[6]. To obtain genomic information on BPV, one of the strategies is to use linkage analysis in segregating populations produced by crossbreeding of inbred strains[7]. The first step of this strategy is to select two parental strains for cross-breeding. It is generally realized that the greater the difference in phenotype between two parental strains, the better for the linkage study.
Spontaneously hypertensive rat (SHR), an inbred strain for genetic hypertension, has a higher BPV compared with normotensive controls, such as Wistar-Kyoto rats and Sprague-Dawley rats[1,5]. Stroke-prone spontaneously hypertensive rat (SHR-SP), a sub-strain of SHR, has a much higher BP level than SHR[8,9]. However, little is known about the BPV of SHR-SP. We hypothesized that SHR-SP had a higher BPV than SHR in parallel with their BP changes, and thus would be more appropriate as a parental strain crossbred with another parental strain of normal rats. To test this hypothesis, hemodynamics were compared between SHR and SHR-SP in the present study.
Materials and methods
Animals Male SHR and SHR-SP were provided by the Animal Center of the Second Military Medical University, Shanghai, China. Animals were housed at controlled temperature (23 °C–25 °C) and lighting (8:00–20:00) and with free access to tap water and rat chow. At the age of 15 weeks, hemodynamic parameters, including BP, BPV, and baroreflex sensitivity (BRS), were measured in conscious, freely moving rats. All procedures were in accordance with the guidelines for animal care of the Second Military Medical University.
Measurement of blood pressure and its variability Rats were anesthetized with an injection of ketamine (50 mg/kg, ip) and diazepam (5 mg/kg, ip). A catheter was placed into the lower abdominal aorta via the left femoral artery for measurement of BP and heart period (HP), and another catheter was inserted into the left femoral vein for drug administration. Both catheters were tunneled subcutaneously and exteriorized between the scapulae. After a 2-d recovery, animals were placed in individual cylindrical cages. The aortic catheter was connected to a BP transducer (PT14M2, Fudan University, Shanghai, China) via a rotating swivel that allowed the animal to move freely in the cage. After approximately 14 h habituation, the BP signals were digitized and processed by a microcomputer, which calculated systolic BP (SBP), diastolic BP (DBP), mean BP (MBP), and HP values on-line for 2 h–3 h. These values were sampled beat-to-beat at 1000 Hz. In off-line analysis, the mean values of SBP, DBP, MBP, and HP over a 1-h period (10:00–11:00) were calculated and served as SBP, DBP, MBP, and HP, respectively. The standard deviation values of SBP, DBP, and MBP over the 1-h period were calculated and defined as systolic BPV (SBPV), diastolic BPV (DBPV), and mean BPV (MBPV), respectively[1,3–5].
Measurement of baroreflex sensitivity After BP and BPV measurement, BRS was determined using a method described previously[10]. The principle of this method is to measure the HP prolongation in response to an elevation in blood pressure. A bolus intravenous injection of phenylephrine was used to raise SBP to between 30 mmHg and 40 mmHg. HP was plotted against SBP for linear regression analysis; the slope of the linear regression equation was expressed as BRS (ms/mmHg).
Statistical analysis Data are expressed as mean±SEM. The differences between 2 groups were evaluated using the two-tailed Student’s unpaired t-test. Statistical significance was judged at P<0.05.
Results
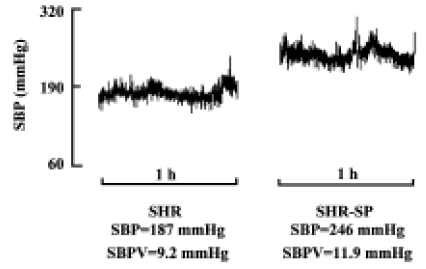
Blood pressure and its variability Body weight and heart period were not significantly different between SHR and SHR-SP at the age of 15 weeks (Table 1). BP was markedly higher in SHR-SP than SHR, whereas there was no significant difference in BPV. To show clearly that BPV was not changed in parallel with the BP changes in SHR and SHR-SP, BP and BPV were compared quantitatively using percentage difference (Table 1). Systolic, diastolic and mean BP were elevated significantly by 36.9%, 42.9%, and 39.5%, respectively, in SHR-SP compared with SHR (P<0.01). However, their variabilities were elevated only by 14.0%, 0.4%, and 10.1%, respectively, without statistical significance (P>0.05). Figure 1 shows the representative values of systolic blood pressure tracings during a 1-h period in SHR and SHR-SP.
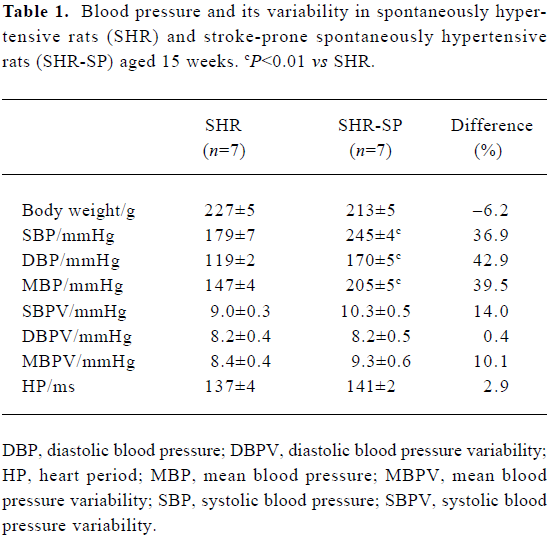
Full table
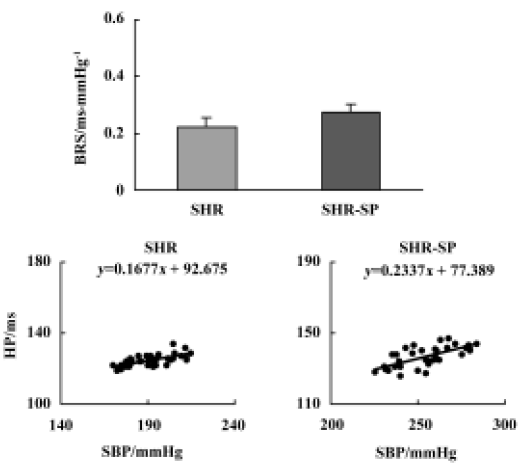
Baroreflex sensitivity There was no significant difference in BRS between SHR and SHR-SP (Figure 2, upper part, P>0.05). The representative values of BRS in SHR and SHR-SP are shown in the lower part of Figure 2.
Discussion
In the present study, 15-week-old rats were used for comparisons of hemodynamic phenotypes, including BP, BPV, and BRS. This is because BP levels reach a plateau at this adult age[9,11]. It is well known that BP level is markedly higher in SHR-SP than SHR in both conscious and anesthetized states[8,9,12]. Our present data are in accordance with previous results[8,9].
In contrast to our hypothesis, there was no significant difference in BPV between SHR-SP and SHR. In other words, BPV was not elevated in parallel with the remarkable elevations in BP in SHR-SP compared with SHR. The BPV data combined with the shorter life span of SHR-SP (about 45 weeks)[9] compared with SHR (about 75 weeks)[13] lead us to the unequivocal conclusion that SHR is more appropriate as a parental strain than SHR-SP for crossbreeding in BPV linkage studies.
Blood pressure variability is increased in almost all hypertensive patients and animals[1,14]. This often leads to the deduction that alterations in BP and BPV occur in parallel. The present study showed that SHR-SP, a sub-strain of SHR, had a markedly higher BP level without a significant increase in BPV, when compared with SHR. These provide a good example to delineate that BPV is not necessarily dependent on BP level. The facts were also demonstrated in other previous studies. Under physiological conditions, rabbits are known for their unstable BP, and when compared with rats, rabbits exhibit a higher BPV and a lower BP level[15]. In pathophysiological states of baroreflex interruption, BPV is increased markedly with no change in the average BP level after sinoaortic denervation or nucleus tractus solitarii lesions[2,4,5,16]. Frequent ventricular premature beats in myocardial infarction rats cause high BPV with unchanged BP level[3]. Finally, in pharmacological studies, BPV is reduced significantly by simultaneous infusion of phenylephrine and adenosine when the BP level maintains unchanged[17]. All of the above results confirm that BPV may not be associated with BP level.
Two reports have analyzed the BRS data of SHR and SHR-SP within the same study. In these previous studies, BRS was estimated either in an anesthetized state using intravenous infusion of phenylephrine for 30 min[12] or in a conscious state using cross-spectral analysis[9]. In the present study, BRS was determined in conscious, freely moving rats using a bolus intravenous injection of phenylephrine. These three studies provide similar evidence showing that the impairment of baroreflex function, expressed as decreased BRS, is not significantly different between SHR and SHR-SP.
In summary, the current study demonstrated and compared the hemodynamic phenotypes of BP, BPV and BRS in SHR and SHR-SP. For the first time, we have provided evidence that in addition to BRS, BPV is not changed in parallel with BP changes in SHR-SP and SHR. Together with the short life span of SHR-SP, these findings indicate that SHR is more appropriate than SHR-SP for crossbreeding in future linkage studies on BPV and BRS. The data are meaningful in attaching the physiological variables of BPV and BRS to the genome, especially as the rat has become researchers’ favorite laboratory animal due to the recent availability of its genome sequence[6,18,19].
References
- Miao CY, Shen FM, Su DF. Blood pressure variability is increased in genetic hypertension and L-NAME-induced hypertension. Acta Pharmacol Sin 2001;22:137-40.
- Su DF, Miao CY. Blood pressure variability and organ damage. Clin Exp Pharmacol Physiol 2001;28:709-15.
- Miao CY, Xu LP, Liu JG, Xie HH, Yuan WJ, Su DF. Frequent ventricular premature beats increase blood pressure variability in rats. Acta Pharmacol Sin 2004;25:545-53.
- Miao CY, Su DF. The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens 2002;20:1865-72.
- Miao CY, Yuan WJ, Su DF. Comparative study of sinoaortic-denervated rats and spontaneously hypertensive rats. Am J Hypertens 2003;16:585-91.
- Jacob HJ, Kwitek AE. Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet 2002;3:33-42.
- Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol Rev 2000;80:135-72.
- Griffin KA, Churchill PC, Picken M, Webb RC, Kurtz TW, Bidani AK. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am J Hypertens 2001;14:311-20.
- Nagai R, Nagata S, Fukuya F, Higaki J, Rakugi H, Ogihara T. Changes in autonomic activity and baroreflex sensitivity with the hypertension process and age in rats. Clin Exp Pharmacol Physiol 2003;30:419-25.
- Xie HH, Miao CY, Liu JG, Su DF. Effects of long-term treatment with candesartan on organ damages in sinoaortic denervated rats. J Cardiovasc Pharmacol 2003;41:325-31.
- Miao CY, Liu KL, Benzoni D, Sassard J. Acute pressure-natriuresis function curve shows early impairment in Lyon hypertensive rats. J Hypertens 2005;23:1225-31.
- Chan JY, Chen WC, Lee HY, Chan SH. Elevated Fos expression in the nucleus tractus solitarii is associated with reduced baroreflex response in spontaneously hypertensive rats. Hypertension 1998;32:939-44.
- Wang X, Ajikobi DO, Salevsky FC, Cupples WA. Impaired myogenic autoregulation in kidneys of Brown Norway rats. Am J Physiol Renal Physiol 2000;278:F962-9.
- Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target organ damage in hypertension. J Hypertens 1987;5:93-8.
- Sato K, Chatani F, Sato S. Circadian and short-term variabilities in blood pressure and heart rate measured by telemetry in rabbits and rats. J Auton Nerv Syst 1995;54:235-46.
- Buchholtz RA, Nathan NA. Chronic lability of the arterial pressure produced by electrolytic lesions of the nucleus tractus solitarii in the rat. Circ Res 1984;54:227-38.
- Jacob HJ, Alper RH, Brody MJ. Lability of arterial pressure after baroreceptor denervation is not pressure dependent. Hypertension 1989;14:501-10.
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 2004;428:493-521.
- Abbott A. Laboratory animals: the renaissance rat. Nature 2004;428:464-6.
