Cardioprotective effects of diltiazem reevaluated by a novel myocardial ischemic model in Chinese miniature swine1
Introduction
Coronary artery obstruction results in ischemic heart disease and even myocardial infarction. Under uncontrolled conditions, the attacked heart develops ischemic cardiomyo-pathy, which describes patients with coronary artery disease, enlarged hearts and clinical manifestations of congestive heart failure[1 ]. Although the underlying mechanisms of left ventricular dysfunction are complex and poorly understood, scientists have made efforts to explore new strategies to block the mentioned dysfunction process. Diltiazem, one of the first-generation classical calcium antagonists, has been used widely to treat coronary artery diseases. The mechanisms, by which diltiazem attenuates ischemic myocardial damages, includes dilating coronary artery, improving the cardiac output, and lowering myocardial oxygen consumption[2,3]. However, it is not generally recommended as first-choice therapeutic agents in infracted hearts on the basis of their detrimental effects in patients with heart failure, and is believed to result from the exacerbation of their negative inotropic properties[4,5]. Weighing the advantages and disadvantages of diltiazem in myocardial ischemia remains a challenge for cardiologists. It is necessary for the preclinical researchers to reevaluate the cardioprotective effects of diltiazem by a novel animal model.
To date, there are various myocardial ischemic animal models, such as ligating the coronary artery[6], placing constrictors on the coronary artery[7], putting microembolus into the coronary artery[8], or injecting ferric chloride via the vein[9]. However, operations in these methods lead to much damage in animals, and channelization can not be restored in the obstructed coronary artery. More consider-ably, the mimic pathophysiological alterations deviate from clinical data, especially autopsy. The ischemic model we used was developed in Chinese miniature swine, where myocardial ischemia was performed by injecting self-embolus into the middle segment of the left anterior descending (LAD) coronary artery without thoracotomy. To facilitate the surgical operation, we introduced the intervention technique and selective coronary angiography into present study. With the help of this model, we observed the heart functional parameters. Furthermore, we predicted that diltiazem was effective in inhibiting myocardial ischemia and restoring heart function in this novel model.
Materials and methods
Drugs and reagents Diltiazem (N
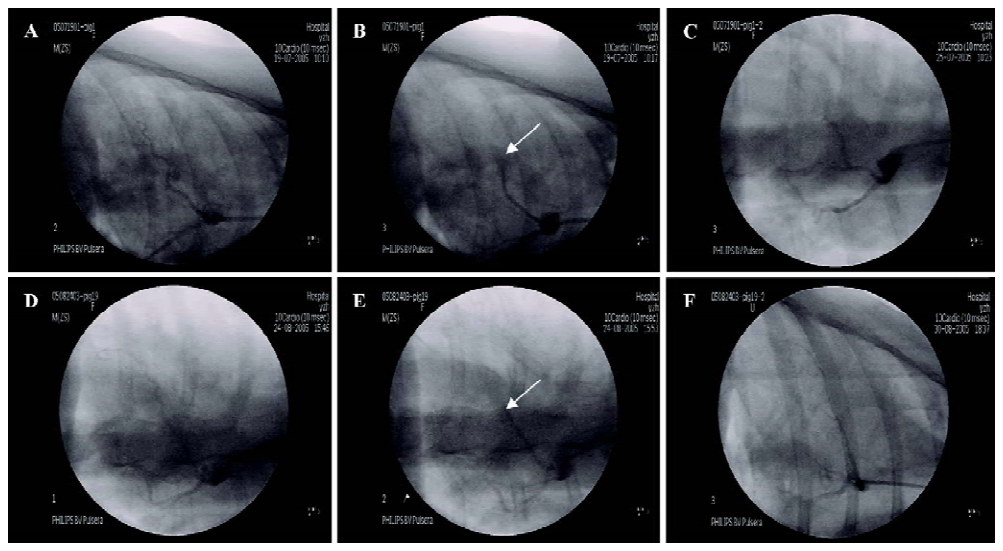
Myocardial ischemic model preparation Chinese miniature swine (38.9±4.7 kg) of either sex, purchased from the College of Animal Sciences, China Agriculture University (Beijing, China), were individually housed in metal cages and fed a standard pig diet. Before the surgery, the animals were anaesthetized with pentobarbital (30 mg/kg, iv). Through the cervical incision, a 6F sheath was inserted into the right common carotid artery. And heparin (200 U/kg) was injected to prevent the blood from coagulation. A 6F guiding catheter (Cordis, Miami, FL, USA) was positioned in the left coronary arterial ostium; a left coronary angiography was performed with C-arm, BV Pulsera (Philips, Veenpluis, 5684 PC Best, the Netherlands). After the left coronary angiogram, a 5F guiding catheter (Cordis, Miami, FL, USA), directed by the exchange wire, was introduced into the middle segment of the LAD. Then the prepared strip-shaped clot (induced by thrombin 100 U per 0.5 mL blood) was injected into the LAD to block the blood flow. Another coronary angiography was performed to confirm the complete occlusion of the LAD. After that, all the catheters were withdrawn, and the wound was sutured in layers under the sterile condition. The animals were individually housed in metal cages, with postoperative care (including antibiotics) thereafter. Electrocardiogram was continuously monitored during the procedure with an electrocardio-monitor (TEC-7621C, Nihon Kohden, Nishiochiai Shinjuku-ku, Tokyo, Japan).
Groups and administration After the surgery, 12 qualified miniature swine were randomly divided into 2 groups with 6 pigs in each group: model group, which was fed with normal pig diet, and the diltiazem group, fed a pig diet mixed with diltiazem 5 mg·kg-1·d-1. During the experiment, a special technician was appointed to observe the pigs eating so as to ensure the absorbed drug dose. After 6 successive days of medication, the animals were sacrificed.
Coronary angiography To determine the embolism of LAD pre-operation, instantly after the operation and 6 d after medication, a selective coronary angiography was performed.
Body surface electrocardiogram (BS-ECG) The 30 point electrode was placed on the chest surface in the cardiac projective area and a physiological polygraph (MP-100, Biopac, Santa Barbara, CA, USA) was connected to record BS-ECG before the operation or 6 d after the operation while the animals were anaesthetized. ST segment elevated to more than 0.8 mV was regarded as criterion to calculate the degree of myocardial ischemia (total mV of ST segment elevating, Σ-ST) and myocardial ischemic scope (total point number of ST segment elevating, N-ST).
Hemodynamics Before medication or 6 d after medication, a catheter was connected to the sheath, which was then inserted into the right common carotid artery to monitor blood pressure [systolic blood pressure (SBP) and diastolic blood pressure (DBP)] via a pressure transducer (MPU-0.5A, Nihon Khoden, Japan), and the mean blood pres-sure (MBP) was calculated with the function, MBP=DBP+(SBP-DBP)/3. The other hemodynamic indexes of the cardiac output (CO), cardiac index (CI), stroke volume (SV), stroke index (SI), systemic vascular resistance (SVR), systemic vascular resistance index (SVRI), left cardiac work (LCW), left cardiac work index (LCWI) and heart rate (HR) were acquired by needle electrodes connected with BioZ Impedance Cardiograph (CardioDynamics, San Diego, CA, USA) while the animals were anaesthetized.
Biochemistry After medication for 6 d, blood was taken from the sheath to segregate blood plasma; the activity of superoxide dismutase (SOD) and content of malondialdehyde (MDA) were determined with methods of thiobarbituric acid and xanthine oxidase.
Myocardial ischemic area At the end of the protocol, the animals sacrificed due to over blood loss[10]. The heart was taken out, washed with normal saline and weighed immediately. Under the coronary occlusion spot, the ventricle was transversely divided into 5 pieces of equal thickness. The pieces were then infiltrated with N-BT staining solution at 25 °C for 15 min. Both the ischemic area (N-BT non-stained area) and non-ischemic area (N-BT stained area) were determined by a multimedia color pathological image analytical system (MPIAS-500, Konghai company, Beijing, China), and the infarction percentage of the entire heart or ventricle was calculated[11].
Pathohistology The middle segment of the LAD and cardiac muscle of the third piece of ventricle around the LAD were cut, and the pathohistological changes of coronary embolus and the cardiac muscle were observed after 10% formalin fixation, paraffin imbedding and microtome section.
Statistical analysis Measurement data were expressed as mean±SD. Analysis of variance and the t-test were used to determine significant differences between groups. P values less than 0.05 were considered to be significant.
Results
Coronary embolism in myocardial ischemic miniature swine The LAD was embolized after self-embolus injection. Treatment with diltiazem (5 mg·kg-1·d-1, oral administration, 1–6 d after self-embolus injection) alleviated the LAD embolism of myocardial ischemic miniature swine (Figure 1).
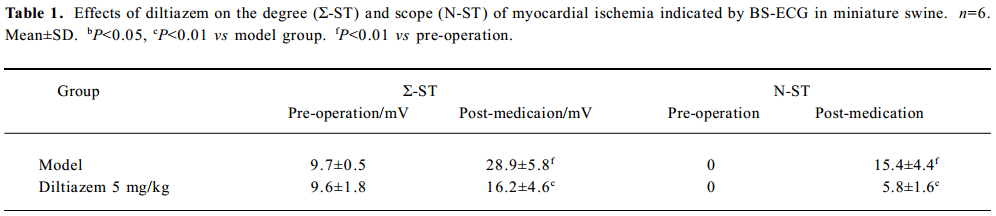
Degree (Σ-ST) and scope (N-ST) of the myocardial ischemia Six days after self-embolus injection, the Σ-ST increased obviously in the model group (P<0.01 vs pre-operation). Diltiazem (5 mg/kg) significantly reduced the Σ-ST by 44% in myocardial ischemic miniature swine (P<0.01). After feeding them a normal diet, there was no significant difference among the scope of myocardial ischemia (N-ST) in the models. Diltiazem (5 mg/kg) significantly decreased the N-ST by 62% as compared with that of the model group (P<0.01; Table 1).
Full table
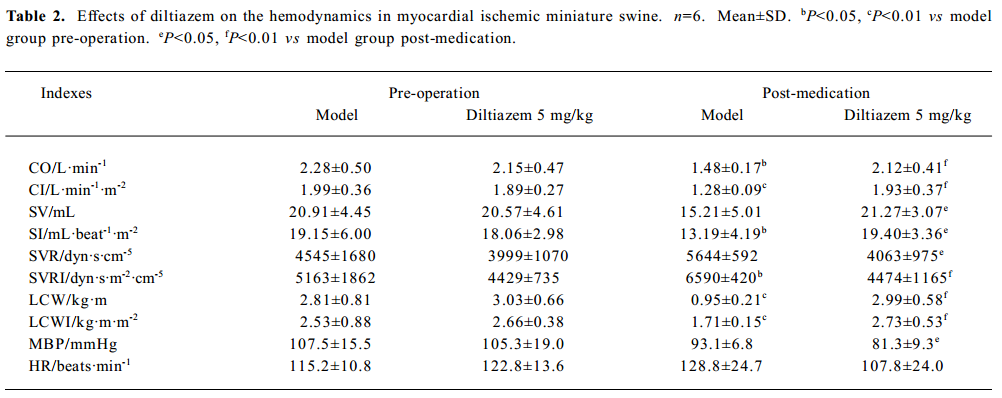
Hemodynamics in myocardial ischemic miniature swine Before the operation, there was no significant difference of hemodynamic indexes between the model and diltiazem groups. When ischemia lasted for 6 d, the CO, CI, LCW, and LCWI decreased in the model group. However, the SVRI of the model group increased significantly (P<0.05). The CO, CI, SV, SI, LCW, and LCWI of the diltiazem group were significantly higher than those of the model group; SVR, SVRI and MBP of diltiazem group decreased obviously compared with those of the model group (P<0.05). No obvious change in HR was determined between the treatment and non-treatment groups (Table 2).
Full table
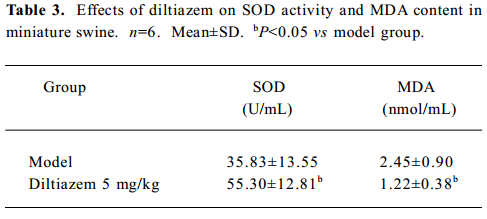
Biochemistry Six days after self-embolus injection, diltiazem (5 mg/kg) significantly increased the SOD activity and decreased MDA content in myocardial ischemic miniature swine (P<0.05, Table 3).
Full table
Myocardial ischemic area The ischemic area was indicated by NT-B staining (Figure 2). The ischemic area of the diltiazem group, and the percentage of heart and ventricle, were significantly lower than those of model group (P<0.05, Table 4, Figure 2).
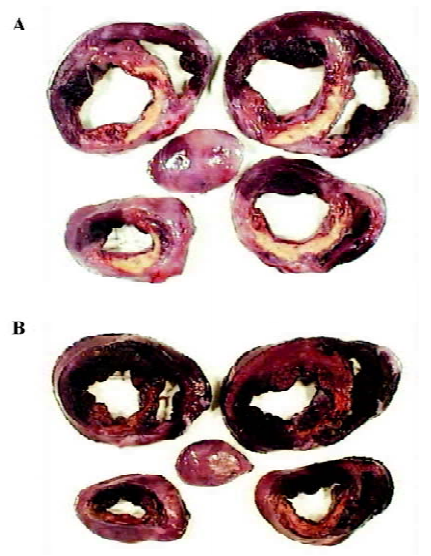
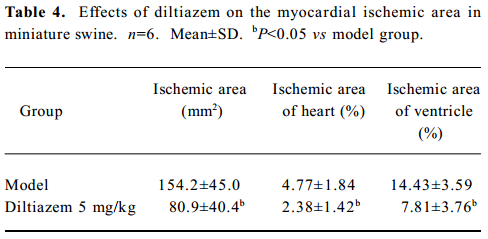
Full table
Pathohistology Myocardial ischemic pathohistology changes appeared in the miniature swine of the model group, but after 6 d medication, the myocardial ischemic changes were lessened in the miniature swine of the diltiazem group. Both groups showed large embolus in the LAD, but the embolus in the diltiazem group was relatively less than that of the model group (Figure 3).
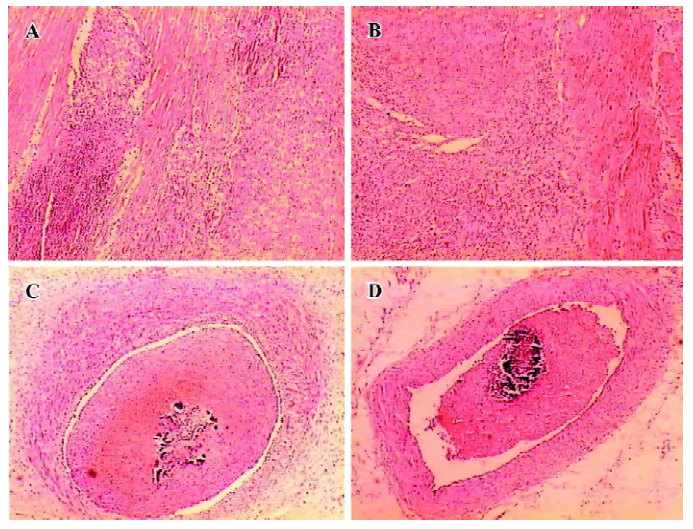
Discussion
The miniature swine was used as research subject because of its anatomic similarity in coronary circulation to human beings[12]. In general, thoracotomy was performed before ischemic approaches, such as ligating, constricting and blocking. However, the intervention technique we introduced in the present study, avoided thoracotomy and disturbance to the environment of thoracic cavity. In addition, this model did not require balloon inflation or intracoronary injection of chemical agents. Moreover, cardiovascular variations could be chronically, continually and systematically observed in this model.
Self-embolus injection was widely used in the study of the pulmonary embolism model[13] and the cerebral infarction model[14]. For the first time, we injected embolus made from its own blood into the LAD to establish a myocardial ischemic model in a Chinese miniature swine. Auto-implanted embolus, homologous with experimental animals, rendered little rejection and inflammation. Application of this model facilitated researchers to observe the pharmacological action and mechanisms, both anti-ischemic drugs and thrombolytic drugs. In our present investigation, the LAD was embolized after self-embolus injection; 6 d after medication, the LAD embolism of the model group was also serious, with the ST segment raised, and the hemodynamic indexes, CO, CI, LCW, and LCWI degraded. The cardiac muscle slices showed a large area of infarction or ischemia; the cardiac muscle appeared to show a larger area of degeneration, necrosis, fibroplasias, inflammatory cell infiltrate and granulation tissue hyperblastosis. The results of the present study indicate that we have established the myocardial ischemic model in miniature swine successfully.
Diltiazem is a calcium channel blocker, effective in the treatment of angina pectoris, and a first-line treatment for hypertension. Since ischemia in angina is essentially preceded by an increase in HR, calcium channel blockers with negative chronotropic properties may be able to perform better for this purpose than nonchronotropic compounds[15]. Diltiazem is useful in improving left ventricular systolic function following acute myocardial infarction in patients[16].
From this novel animal model, we reevaluated the cardioprotective effects of diltiazem. The results indicated that diltiazem remarkably attenuated the LAD embolism, degree (Σ-ST) and scope (N-ST) of myocardial ischemia in miniature swine. Additionally, the ischemic area of the diltiazem group, and the percentage of heart and ventricle, were significantly lower than those of the model group, which is due to coronary dilation[17]. SOD activity significantly increased and the MDA content of diltiazem group decreased obviously compared with those of the model group. Diltiazem improved the hemodynamics of ischemic miniature swine, the CO, CI, SV, SI, LCW, and LCWI significantly increased, and the SVR, SVRI and MBP decreased obviously. At the same time, the cardiac muscle also appeared to have pathological damage, but the area and the degree of damage were lessened. Thus, the drug was effective in inhibiting myocardial ischemia and restoring heart function in this novel model.
In conclusion, the closed-chest myocardial ischemic model in Chinese miniature swine may be prepared by self-embolus injection with cardiac catheter via LAD, effects of diltiazem on improving cardiac function, lowering-lipid peroxidation; decreasing myocardial ischemic area can be reevaluated by the model. There are extensive applications on the study of coronary heart disease and new drug exploitation by using the novel myocardial ischemic model in the future.
Acknowledgement
We greatly appreciate the helpful and constructive suggestion from Dr Yong-qiu ZHENG (Department of Pharma-cology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing 100091, China).
References
- Pantely GA, Bristow JD. Ischemic cardiomyopathy. Prog Cardiovasc Dis 1984;27:95-114.
- Klassen GA, Yeung PK, Barclay KD, Pollak PT, Hung OR, Buckley SJ. Effect of diltiazem on intraarterial blood pressure and heart rate during stress testing in patients with angina: a gender comparison study. J Clin Pharmacol 1997;37:297-303.
- Boden WE, van Gilst WH, Scheldewaert RG, Starkey IR, Carlier MF, Julian DG, et al. Diltiazem in acute myocardial infarction treated with thrombolytic agents: a randomised placebo-controlled trial. Incomplete infarction trial of European research collaborators evaluating prognosis post-thrombolysis (INTERCEPT). Lancet 2000;355:1751-6.
- Parameshwar J, Poole-Wilson PA. The role of calcium antagonists in the treatment of chronic heart failure. Eur Heart J 1993;14 Suppl A:38-44.
- Reicher-Reiss H, Barasch E. Calcium antagonists in patients with heart failure. Drugs 1990;42:343-64.
- Takahashi M, Tanonaka K, Yoshida H, Koshimizu M, Oikawa R, Daicho T, et al. Effects of angiotensin I-converting enzyme inhibitor and angiotensin II type 1 receptor blocker on the right ventricular sarcoglycans and dystrophin after left coronary artery ligation. Eur J Pharmacol 2005;522:4-93.
- Laham RJ, Rezaee M, Post M, Novicki D, Sellke FW, Pearlman JD, et al. Intrapericardial delivery of fibroblast growth factor-2 induces neovascularization in a porcine model of chronic myocardial ischemia. J Pharmacol Exp Ther 2000;292:795-802.
- Huang Y, Hunyor SN, Jiang L, Kawaguchi O, Shirota K, Ikeda Y, et al. Remodeling of the chronic severely failing ischemic sheep heart after coronary microembolization: functional, energetic, structural, and cellular responses. Am J Physiol Heart Circ Physiol 2004;286:H2141-50.
- Dogne JM, Rolin S, Petein M, Tchana-Sato V, Ghuysen A, Lambermont B, et al. Characterization of an original model of myocardial infarction provoked by coronary artery thrombosis induced by ferric chloride in pig. Thromb Res 2005;116:431-42.
- Kang RT, Li XS, Cao RQ, Wu WH, Dong ZM. Effects of propofol on lung injury following ischemia-reperfusion of hind limbs in rats. Chin J Anesthesiol 2005;25:346-50.
- Liu JX, Shang XH, Fu JH, Li XZ, Wang G. Effects of recombinant staphylokinase on coronary thrombosis in Chinese experimental miniature swine. Acta Pharmacol Sin 2002;23:509-15.
- Hughes GC, Post MJ, Simons M, Annex BH. Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. J Appl Physiol 2003;94:1689-701.
- Lacoursiere L, Millward S, Veinot JP, Labinaz M. Percutaneous removal of pulmonary artery emboli with hydrolyser catheter in pigs. Can Assoc Radiol J 2001;52:118-25.
- Busch E, Kruger K, Hossmann KA. Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain Res 1997;778:16-24.
- van der Vring JA, Daniels MC, Holwerda NJ, Withagen PJ, Schelling A, Cleophas TJ, et al. Combination of calcium channel blockers and beta blockers for patients with exercise-induced angina pectoris: a double-blind parallel-group comparison of different classes of calcium channel blockers. The Netherlands working group on cardiovascular research (WCN). Angiology 1999;50:447-54.
- Nicolau JC, Ramires JA, Maggioni AP, Garzon SA, Pinto MA, Silva DG, et al. Diltiazem improves left ventricular systolic function following acute myocardial infarction treated with streptokinase. The Calcium Antagonist in Reperfusion Study (CARES) Group. Am J Cardiol 1996;78:1052-79.
- Tadokoro H, Miyazaki A, Satomura K, Ryden L, Kaul S, Kar S, et al. Infarct size reduction with coronary venous retroinfusion of diltiazem in the acute occlusion/reperfusion porcine heart model. J Cardiovasc Pharmacol 1996;28:134-41.
