Urotensin II accelerates cardiac fibrosis and hypertrophy of rats induced by isoproterenol1
Introduction
Urotensin II (UII) is a vasoactive cyclic peptide which was originally isolated from fish urophysis, and has been cloned from humans since 1998[1]. UII has been identified as the endogenous ligand for the orphan G protein-coupled receptor, GPR14 (urotensin II receptor, UT)[2,3]. UII mRNA is predominately expressed in the spinal cord and certain brain areas, while UT mRNA is widely expressed in cardiovascula-ture such as the myocardium, vascular smooth muscle cells (VSMC) and endothelial cells[4,5]. Human UII effectively constricts isolated arteries from non-human primates. The potency of vasoconstriction is of a greater magnitude than that of endothelin 1, making UII the most potent mammalian vasoconstrictor[2]. UII also exhibits many other physiological actions, for example, UII induces proliferation of cultured VSMC and human endothelial cells[6,7], and accelerates foam cell formation in human monocyte-derived macrophages[8]. UII-induced hypertrophic responses in cultured neonatal rat cardiomyocytes have also been observed[4]. In isolated human atrial trabecular tissues, UII exhibits potent positive inotropic activity[9]. The increase of UII and UII mRNA, as well as greater density of UII binding sites were found in the myocardium of patients with congestive heart failure[3]. Gruson et al also found that in patients with congestive heart failure, UII content was related to the functional class of the disease and correlated negatively with left ventricular ejection fraction. Furthermore, UII correlated significantly with big-engdothelin-1 and brain natriuretic peptide, suggesting that UII could play a role in worsening the course of congestive heart failure[10]. In patients with coronary artery disease, the rise of plasma UII was significantly proportional to the parameters of cardiac dysfunction[11,12]. We previously showed that the density of binding sites for UII in sarcolemma of the myocardium increased in rats exposed to chronic hypoxia[13]. In addition, the expression of UII and UT protein increased in both infarcted and non-infarcted regions of the left ventricle in a rat model of heart failure after myocardial infarction[14]. Tzanidis et al reported that UII stimulates collagen synthesis of cardiac fibroblasts, suggesting that UII may be involved in myocardial fibrogene-sis[14]. Interestingly, Watanabe et al reported that UII acts synergistically with mildly oxidized low density lipoprotein (LDL) and serotonin in inducing VSMC proliferation and accelerates foam cell formation in human monocyte-derived macrophages[15,16]. Taken together, these data suggest that UII might contribute to cardiovascular diseases through synergistic interaction with other vasoactive substances. Accordingly, in this study, we determined UII contents in the plasma and myocardium, and UT mRNA expression in the myocardium with fibrogenesis of rats induced by isoproterenol (ISO). We also determined whether UII is involved in the development of cardiac hypertrophy and fibrogenesis.
Materials and methods
Materials Male Wistar rats weighing 180–200 g were supplied by the Animal Center, Health Science Center, Peking University (Beijing, China). Animal care and experimental protocols were in compliance with the Animal Management Rules of China (Documentation N
Rat UII (pEHGTAPECFWKYCI) and the radioimmunoassay (RIA) kit of rat UII were purchased from Phoenix Pharmaceuticals Inc (Belmont, CA, USA). The RIA kit of Ang II and ALD were purchased from Furui Pharmaceutical Inc (Beijing, China). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Sigma (St Louis, MO, USA). Fetal bovine serum (FBS) was from Hyclone (Logan, UT, USA). [3H]Thymidine and [3H]proline were from Amersham Pharmacia Biotech (Freiburg, Germany). Trizol and dNTP were from Clontech Laboratories (Palo Alto, CA, USA). Moloney murine leukemia virus transcriptase (MMLV), Taq DNA polymerase and oligo (dT)15 primer were from Promega (Madison, WI, USA). Oligonucleotides were synthesized by Sai Baisheng Biotechnology (Beijing, China). The sequences of oligonucleotide primers were: rUT-S: 5'-GCATC-TTCACCCTGACCATAA-3'; rUT-A: 5'-CCCAGAAGAGAA-GGACGATACC-3'; β-actin S: 5'-ATCTGGCACCACACCTTC-3'; and β-actin A: 5'-AGCCAGGTCCAGACGCA-3'.
Products amplified by UT and β-actin primers were 399 bp and 291 bp, respectively.
Preparation of animals Thirty male Wistar rats (weight 190±10 g) were randomly and equally divided into 3 groups: group 1, control; group 2, ISO-treated; and group 3, ISO and UII co-treated. In groups 2 and group 3, ISO (5 mg·kg-1·d-1) was subcutaneously injected into the rats once a day. In group 1, the rats were given sc injections of 0.9% saline instead of ISO[17]. The rats of group 3 were also given iv injections of UII [3 nmol/kg (5 µg/kg)] on the first day, followed by sc injections of UII (1.5 µg/kg), twice daily. These treatments lasted for 7 d. On d 8, no special treatment was given to these rats. Caudal artery pressure and heart rate were measured with a determinator (sphygmomanometer, Chinese-Japanese Friendship Hospital, Beijing, China) before and after treatment.
On d 9, the animals were weighed and anesthetized with 0.6% pentobarbital sodium (60 mg/kg, ip). After the blood was collected from the inferior vena cava in pre-chilled tubes containing ethylenediamine tetraacetic acid and leupeptin, the rats were subsequently killed by decapitation; the heart was carefully isolated, then blotted slightly and weighed. The degree of ventricular hypertrophy was assessed by measuring the ratio of the heart weight/body weight (HW/BW). Several slices of left ventricular tissue were stored in 10% formalin for pathological examination microscopically after hematoxylin-eosin stain and Masson stain. Other cardiac tissues were stored at -70 °C for the determination of UT mRNA, UII contents, hydroxyproline concentration, Ang II contents, ALD contents, and malondialdehyde (MDA). Plasma was separated from the blood immediately and stored at -70 °C until determination.
Myocardial expression of UT mRNA was determined by RT-PCR. The UII contents of plasma and cardiac tissues, as well as cardiac Ang II and ALD contents were determined by RIA. Plasma lactate dehydrogenase (LDH) activity was measured on an automatic analyzer. Myocardial MDA was determined using thiobarbituric acid test[18].
RT-PCR assay The expression of UT mRNA in the atrium and ventricles were assessed by RT-PCR as described[19], with medulla oblongata as a positive control tissue. The total RNA extracted from the tissue was quantified by use of an UV spectrophotometer (UV2100, Shimadzu, Japan). Reverse transcription to cDNA was accomplished by priming 2 µg total RNA with oligo (dT)15 primer using MMLV trans-criptase. Products were then used for the following PCR amplification: 2.5 mmol/L each dNTP 1 µL, 10×PCR buffer (100 mmol/L Tris-HCl, pH 8.3, 15 mmol/L MgCl2, 500 mmol/L KCl) 2.5 µL, cDNA 1 µL, 5 µmol/L each of rUII-S and rUII-A primers or UT-S and UT-A primers 1 µL and 1.25 unit of Taq DNA polymerase, in a total volume of 25 µL. After denaturing at 95 oC for 5 min, PCR cycles were run at 94 oC for 30 s, 57 oC for 30 s and 72 oC for 30 s for 35 cycles, then 72 oC for 5 min. As an internal control for each PCR reaction, β-actin cDNA was also amplified for each sample under the same conditions. PCR products were separated in a 1.5% agarose gel and visualized by ethidium bromide staining. The intensity of the PCR product bands under UV light was measured using a gel image analyzer. Results were expressed as the ratios of UT PCR product (399 bp) to β-actin PCR product (291 bp). Each sample was repeated 3 times.
Determination of myocardial hydroxyproline concentration Myocardial hydroxyproline concentration was determined by the method of Stegemann and Stalder after acid (HCl) hydrolysis, as previously described[20]. Samples of ventricular tissue from all the rats were weighed for tissue analysis. Approximately 100 mg of left ventricular tissues were scissored and used for the spectrophotometric determination of hydroxyproline (558 nm).
Extraction and measurement of UII peptide from tissues and plasma Extraction and measurement of UII peptide from the tissues and plasma was operated according to the method reported previously by us[13]. In brief, the blood samples were immediately centrifuged for 15 min (2000×g at 4 °C), and the supernatant plasma was collected; UII was extracted from the plasma by passage through the C18 Sep-Pak cartridges (Waters, Milford, MA, USA) and stored at -70 °C until UII measurement was undertaken. The cardiac tissues were quickly excised, frozen in liquid nitrogen, and stored at -70 °C until the UII levels were measured. The frozen tissues were homogenized in 1 mol/L acetic acid and boiled for 10 min. The homogenate was then centrifuged for 15 min (17 000×g at 4 °C) and the supernatant containing UII peptide was stored at -70 °C. Rat UII levels in both plasma and tissues were measured using a specific RIA, which has a sensitivity of 1 pg/tube and 20% cross-reactivity with mouse UII, 1% cross-reactivity with human UII respectively, and has 0% cross-reactivity with UI, urocortin or Ang II. The within assay coefficient of variation was 6%.
Measurement of Ang II and ALD Ang II contents of myocardial tissues were determined using RIA as described earlier, which has a sensitivity of 10 pg/tube and no cross-reactivity with Ang I. ALD contents were also determined using RIA, which has a sensitivity of 3 pg/tube. The within assay coefficient of variation was 5%.
Culture of neonatal cardiac fibroblasts of rat Neonatal rat cardiac fibroblasts were prepared from the hearts of 1 d-old Wistar rats (Health Science Center, Peking University, Peking, China) as previously described[21]. The cells were purified by differential plating[22] and used at passages 2-4 for all experiments.
Determination of cell proliferation DNA synthesis was examined by measuring [3H]thymidine incorporation into the cellular DNA, as described previously[23]. Cultured cardiac fibroblasts were divided into the following groups: (1) control group: the cells were cultured in serum-free DMEM; and (2) UII groups: 5×10-9, 5×10-8, or 5×10-7 mol/L UII was added to serum-free medium. Each experiment was repeated 6 times.
Cardiac fibroblasts were first grown in DMEM with 10% FBS and 200 mg/L L-glutamine, and then seeded in 24-well plates at 1×105 cells/well in DMEM+10% FBS. After 24 h, cell growth was arrested in DMEM containing 0.5% FBS for 24 h. After synchronization of cardiac fibroblasts, the medium was changed to DMEM without serum. Cardiac fibroblasts were incubated with different concentrations of UII for 24 h and exposed to [3H]thymidine at the concentration of 1 µCi/well for the last 8 h of the 24 h incubation period. After the incubation, the cells were washed with ice cold PBS and 10% trichloroacetic acid. Acid-insoluble [3H]thymidine was collected on glass fiber filters (Whatman, Kent, UK) and determined by a liquid scintillation counter (LS 6500, Beckman, Fullerton, CA, USA).
Determination of collagen synthesis and secretion Collagen synthesis was examined by measuring [3H]proline incorporation into the cells. The groups were divided and treatment was the same as the experiment of cell proliferation, except that [3H]proline was used instead of [3H]thymidine. At the end of the experiment, the cells were harvested for the measurement of collagen synthesis, and supernatants were collected to measure the collagen secreted from the cells.
Collagen secretion was measured according to the method of Li[24]. Briefly, 100 µL of pepsin assay buffer (mixed with 25 μL of 5 mol/L acetic acid, pH 2−3, 25 µL of 1 g/L pepsin solution in 0.5 mol/L acetic acid, and 50 µL of 10 g/L proline) were added to 1 mL of the supernatant from a well and kept for 3 h at 4 °C. To precipitate protein fractions, 250 µL of 1.2 mol/L cold trichloroacetic acid was added to the samples and incubated on ice for 2 h. Precipitates were applied onto filter units (Whatman, UK), washed with trichloroacetic acid and ethanol, and counted in a scintillation counter.
Statistical analysis Results are shown as mean±SD. Comparisons were done with the use of the unpaired Student’s t test and one-way ANOVA, followed by the Student-Newman-Keuls test. A value of P<0.05 was considered statistically significant.
Results
Changes of HW/BW after ISO/ISO+UII administration During the experimental period, 1 rat from the ISO group and 2 rats from the ISO+UII group died. No death occurred among the control rats. In the ISO-treated group, the rats’ heart became enlarged markedly. Compared with the control rats, the HW/BW increased by 44.7% (P<0.01) in the ISO group, and 73.4% (P<0.01) in the ISO+UII group, respectively. Moreover, HW/BW increased by 19.8% (P<0.01) in the ISO+UII group compared with the ISO group (Table 1).
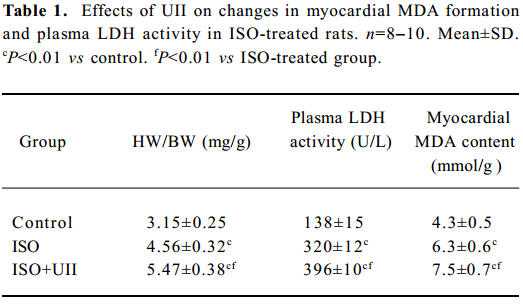
Full table
Myocardial injury Myocardial MDA formation and plasma LDH activity in the ISO group increased by 46.5% (P<0.01) and 132% (P<0.01), respectively, compared with the control group. Moreover, the ISO+UII-treated animals showed a further increase in myocardial MDA (P<0.01) and plasma LDH activity (P<0.01), which were higher than those of the ISO group (both P<0.01, Table 1).
Measurements of caudal artery pressure and heart rate of rats after ISO and ISO+UII administration ISO induced a marked increase in blood pressure. Compared with the control group or before ISO treatment, the blood pressure of the ISO rats elevated significantly (P<0.05), and it was even higher in the ISO+UII-treated rats (P<0.01). However, the heart rates in the ISO-treated rats did not change significantly. Although the heart rate in the UII+ISO rats was slightly lower than that before administration or the control group, there was no statistical difference (P>0.05, Table 2).
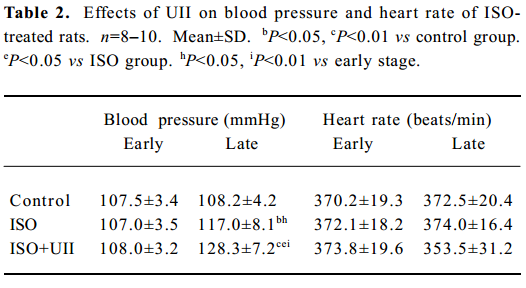
Full table
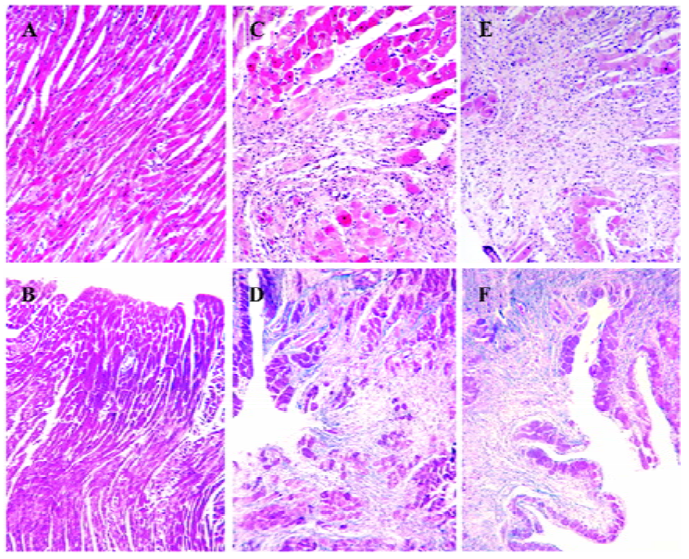
Myocardial necrosis and fibrosis Morphological studies showed that no fibrosis and necrosis occurred in the myocardium of normal rats, while marked fibrosis and necrosis in both the myocardium of the ISO-treated rats and the ISO+UII-treated rats, especially in the latter. Moreover, the greatest degree of fibrosis and necrosis were confined to the subendocardial areas (Figure 1).
Changes of myocardial hydroxyproline concentration after ISO and ISO+UII administration The change of myocardial collagen was measured by means of hydroxyproline quantification. The ISO group had an increased myocardial hydroxyproline concentration, compared with the control group (0.55±0.04 µg/mg vs 0.49±0.02 µg/mg, P<0.01), and the myocardial hydroxyproline concentration in the ISO+UII group (0.64±0.05 µg/mg) was further increased compared with the control and ISO groups (P<0.01).
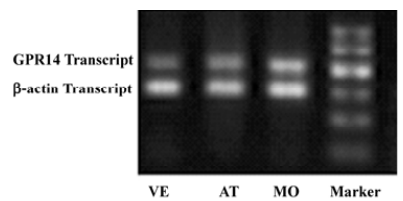
Changes of cardiac UT mRNA expression after ISO and ISO+UII administration Our results showed abundant expression of UT mRNA in rat atrium and ventricle (Figure 2). RT-PCR showed UT mRNA expression in ventricular tissue from both the ISO-treated rats and ISO+UII-treated rats were significant higher than that of the control (P<0.05, Figure 3). Although UT mRNA expression in the tissue of the ISO+UII rats had a tendency to increase than that of the ISO rats, there was no statistical significance between them (P>0.05, Figure 3). In the atrium, UT mRNA expression from both the ISO and ISO+UII groups were also significant higher than that of the control group (P<0.05), while there was no significant difference between the ISO and ISO+UII groups (P>0.05, Figure 4).
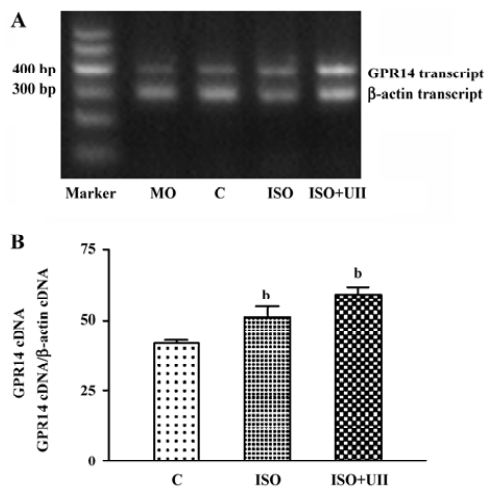
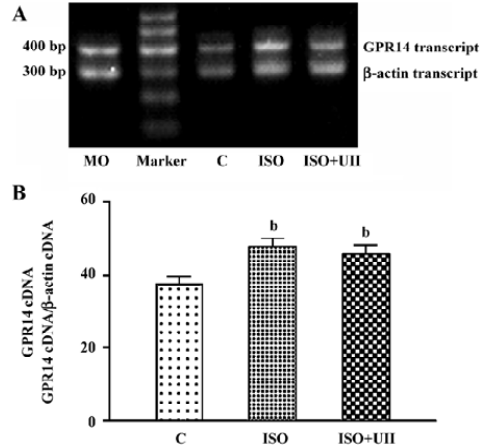
Changes of UII contents after ISO and ISO+UII administration Plasma UII levels increased by 17.3% in the ISO group and further increased by 19.8% in the ISO+UII group compared to the control rats (P<0.05). However, there was no significant difference in plasma UII levels between the ISO and ISO+UII groups (P>0.05, Table 3).
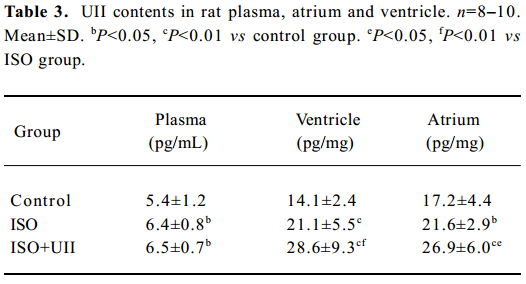
Full table
The ventricular UII contents significantly increased by 49.9% (P<0.01) in the ISO rats and by 103% in the ISO+UII rats compared to the control rats. Furthermore, the UII contents were elevated by 35.4% in the ISO+UII rats than the ISO rats (P<0.01, Table 3).
Atrial UII contents increased by 25.6% (P<0.05) in the ISO rats and by 56.0% (P<0.01) in the ISO+UII rats, respec-tively, compared with the control rats, and elevated by 24.2% (P<0.05) in the ISO+UII rats compared with the ISO rats (Table 3).
Changes of cardiac Ang II contents after ISO/ISO+UII administration The results showed that the ventricular Ang II content significantly increased in the ISO rats (367.1±20.6 pg/mg) and the ISO+UII rats (399.1±36.0 pg/mg), compared with the control rats (321.2±26.6 pg/mg; P<0.05, P<0.01 respectively). Although atrial Ang II contents of the ISO rats (552.8±89.5 pg/mg Prot) and the UII+ISO rats (617.8±83.36 pg/mg) were slightly elevated compared to the controls (533.1±74.71 pg/mg), there was no statistical significance (P>0.05).
Changes of cardiac ALD contents after ISO and ISO+UII administration The ventricular ALD content of the ISO rats (84.80±7.85 pg/mg) was slightly higher than that of the controls (77.93±6.88 pg/mg). However, there was no statistic significance between the 2 groups. The ventricular ALD contents of the ISO+UII rats was higher than that of the controls (93.71±10.94 pg/mg vs 77.93±6.88 pg/mg, P<0.05), but there was no significant difference between the ISO and the UII+ISO group (P>0.05).
UII promoted the proliferation of neonatal cardiac fibroblasts In cultured neonatal cardiac fibroblasts, UII stimulated the proliferation in a concentration-dependent manner, as assessed by [3H]thymidine incorporation experiment compared with the control; [3H]thymidine incorporations increased by 1.1, 1.9 and 2.5 times respectively in the 5×10-9, 5×10-8 and 5×10-7 mol/L UII groups (P<0.01).
UII promoted collagen synthesis and secretion of cardiac fibroblasts The results also showed that UII stimulated [3H]proline incorporation in a concentration-dependent manner. [3H]Proline incorporation increased by 30%, 45%, and 57%, respectively in the 5×10-9, 5×10-8, and 5×10-7 mol/L groups compared with the control (all P<0.01).
UII also stimulated collagen secretion from cultured cardiac fibroblasts in a dose-dependent manner, as shown in Table 4. UII at the concentrations of 5×10-9–5×10-7 mol/L increased collagen secretion by 38%–61% (P<0.01), compared with the control group.
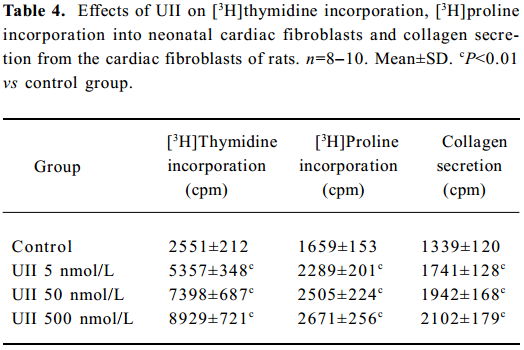
Full table
Discussion
UII displays strong vasoconstrictive effects in isolated arteries and some other cardiovascular effects, including stimulating proliferation of VSMC and inducing strong hypertrophic growth of cardiomyocytes[6,15,16]. Moreover, we previously reported an increase in the density of binding sites for UII in the myocardium of rats exposed to chronic hypoxia[13]. Douglas et al showed an up-regulation of UII content and mRNA expression, as well as greater density of UII binding sites in the myocardium of patients with congestive heart failure[3]. UII is also elevated in children with congenital heart disease[25]. Furthermore, Tzanidis et al observed that UII and UT proteins increased in both infarct and non-infarct regions of the left ventricle in the rat model of heart failure after myocardial infarction. In vitro, they also found that UII stimulated collagen synthesis of neonatal cardiac fibroblasts[9]. However, the significance of UII in the development of cardiac fibrosis has still not been clarified completely.
The present study shows that repeated injections of small doses of ISO results in subacute impairment of the heart, such as an increase of cytosolic enzyme LDH leaking into the extracellular space, promotion of lipid peroxidation, myocardial hypertrophy and necrosis, over expression of extracellular matrix proteins and massive fibrosis. Moreover, the addition of UII significantly aggravated myocardial damage. This study showed, for the first time, that plasma and myocardial UII contents, as well as UT mRNA expression, increased significantly in the process of ventricular hypertrophy and fibrosis induced by ISO. These indicated that UII was involved in myocardial hypertrophy and fibrogenesis by acting synergistically with ISO.
The mechanism of UII facilitating myocardial hypertrophy and fibrogenesis is not clear. It was reported that UII might contribute to cardiac remodeling by the stimulation of cardiomyocyte hypertrophy via UT, and through the upregulation of inflammatory cytokines such as interleukin-6[4]. Tzanidis et al also found that UII stimulated cardiac hypertrophy significantly under conditions of UT upregula-tion[14]. In TE-671 cells, the functional UII high affinity binding sites could be specifically upregulated by interferon-gamma[26]. The present study showed that ISO treatment markedly induced increases of UT mRNA expression and UII contents. UII could potentiate ISO-induced cardiac impairment. In vitro, UII significantly promoted the proliferation and collagen synthesis and secretion, being consistent with the Tzanidis et al study[14]. We propose that the upregulation of UT might produce a necessary condition by which UII could sufficiently stimulate hypertrophy of cardiomyocytes and proliferation as well as collagen synthesis of cardiac fibroblasts, thereby accelerating ISO-induced myocardial injuries and contributing to the development of cardiac hypertrophy and fibrosis.
It is reported that neuroendocrine factors play an important role in the development of ISO-induced injures. Grim et al[27] reported that plasma renin activity and cardiac ACE activity increased significantly in ISO-treated rats. The present study found that with the addition of increased ventricular Ang II, the myocardial UII-UT system was upregulated in the ISO-treated rats, indicating that the UII-UT system is a new regulating system involved in cardiac fibrogenesis. UII could accelerate cardiac injuries induced by ISO. In addition, myocardial ALD content in the UII+ISO-treated rats also increased, being similar to a report that UII could stimulate the elevation of interrenal ALD secretion in axolotl, Ambystoma mexicanum[28], which suggests that there is a relationship between the 2 systems, however, it needs to be further investigated.
In summary, the present study showed that HW/BW, plasma LDH activity, myocardial MDA and hydroxyproline concentration and cardiac expression of UT mRNA markedly increased in the ISO-treated rats. Morphological studies showed marked myocardial fibrosis and necrosis in these rats. The ISO-treated rats were also characterized by hormonal activations including elevations of myocardial UII and ventricular Ang II contents. ISO plus UII treatment significantly increased the degree of myocardial impairment and exacerbated the degree of hypertrophy and fibrosis. In vitro, UII stimulated proliferation and collagen synthesis of neonatal cardiac fibroblasts in a concentration-dependent manner. These results indicate that UII is a factor in accelerating the progress of cardiac fibrogenesis and plays an important role in the cardiac remodeling by synergistic effects with catecholamine.
References
- Coulouarn Y, Lihrmann I, Jegou S, Anouar Y, Tostivint H, Beauvillain JC, et al. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc Natl Acad Sci USA 1998;95:15803-8.
- Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature 1999;401:282-6.
- Douglas SA, Tayara L, Ohlstein EH, Halawa N, Giaid A. Congestive heart failure and expression of myocardial urotensin II. Lancet 2002;359:1990-7.
- Johns DG, Ao Z, Naselsky D, Herold CL, Maniscalco K, Sarov-Blat L, et al. Urotensin-II-mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol 2004;370:238-50.
- Egginger JG, Camus A, Calas A. Urotensin-II expression in the mouse spinal cord. J Chem Neuroanat 2006;31:146-54.
- Zhang YG, Qi YF, Xia CF, Pang YZ, Yang J, Zhang ZK, et al. Stimulating proliferation of aorta smooth muscle cells of rat by urotensin II. Chin Pharmacol Bull 2001;17:155-7.
- Shi L, Ding W, Li D, Wang Z, Jiang H, Zhang J, et al. Proliferation and anti-apoptotic effects of human urotensin II on human endothelial cells. Atherosclerosis 2006;188:260-4.
- Watanabe T, Suguro T, Kanome T, Sakamoto Y, Kodate S, Hagiwara T, et al. Human urotensin II accelerates foam cell formation in human monocyte-derived macrophages. Hypertension 2005;46:738-44.
- Russell FD, Molenaar P. Investigation of signaling pathways that mediate the inotropic effect of urotensin-II in human heart. Cardiovasc Res 2004;63:673-81.
- Gruson D, Rousseau MF, Ahn SA, van Linden F, Ketelslegers JM. Circulating urotensin II levels in moderate to severe congestive heart failure: Its relations with myocardial function and well established neurohormonal markers. Peptides 2006;27:1527-31.
- Heringlake M, Kox T, Uzun O, Will B, Bahlmann L, Klaus S, et al. The relationship between urotensin II plasma immunoreactivity and left ventricular filling pressures in coronary artery disease. Regul Pept 2004;121:129-36.
- Russell FD, Meyers D, Galbraith AJ, Bett N, Toth I, Kearns P, et al. Elevated plasma levels of human urotensin-II immunoreactivity in congestive heart failure. Am J Physiol Heart Circ Physiol 2003;285:H1576-81.
- Zhang YG, Li JX, Cao J, Chen JJ, Yang J, Zhang ZK, et al. Effect of chronic hypoxia on contents of urotensin II and its functional receptors in rat myocardium. Heart Vessels 2002;16:64-8.
- Tzanidis A, Hannan RD, Thomas WG, Onan D, Autelitano DJ, See F, et al. Direct actions of urotensin II on the heart: implications for cardiac fibrosis and hypertrophy. Circ Res 2003;93:246-53.
- Watanabe T, Suguro T, Kanome T, Sakamoto Y, Kodate S, Hagiwara T, et al. Human urotensin II accelerates foam cell formation in human monocyte-derived macrophages. Hypertension 2005;46:738-44.
- Watanabe T, Pakala R, Katagiri T, Benedict CR. Synergistic effect of urotensin II with mildly oxidized LDL on DNA synthesis in vascular smooth muscle cells. Circulation 2001;104:16-8.
- Gallego M, Espina L, Vegas L, Echevarria E, Iriarte MM, Casis O. Spironolactone and captopril attenuates isoproterenol-induced cardiac remodelling in rats. Pharmacol Res 2001;44:311-5.
- Asakawa T, Matsushita S. Thiobarbituric acid test for detecting lipid peroxides. Lipids 1979;14:401-6.
- Jiang W, Jiang HF, Cai DY, Pan CS, Qi YF, Pang YZ, et al. Relationship between contents of adrenomedullin and distributions of neutral endopeptidase in blood and tissues of rats in septic shock. Regul Pept 2004;118:199-208.
- Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta 1967;18:267-73.
- Gurantz D, Cowling RT, Villarreal FJ, Greenberg BH. Tumor necrosis factor-alpha upregulates angiotensin II type 1 receptors on cardiac fibroblasts. Circ Res 1999;85:272-9.
- Toraason M, Luken ME, Breitenstein M, Krueger JA, Biagini RE. Comparative toxicity of allylamine and acrolein in cultured myocytes and fibroblasts from neonatal rat heart. Toxicology 1989;56:107-17.
- Yu SM, Tsai SY, Guh JH, Ko FN, Teng CM, Ou JT. Mechanism of catecholamine-induced proliferation of vascular smooth muscle cells. Circulation 1996;94:547-54.
- Li YR. Biochemistry of extracellular mesenchyma and study methods, 1st ed. Beijing: People’s Medical Publishing House; 1988.
- Simpson CM, Penny DJ, Stocker CF, Shekerdemian LS. Urotensin-II is elevated in children with congenital heart disease. Heart 2006;92:983-4.
- Birker-Robaczewska M, Boukhadra C, Studer R, Mueller C, Binkert C, Nayler O. The expression of urotensin II receptor (U2R) is up-regulated by interferon-gamma. J Recept Signal Transduct Res 2003;23:289-305.
- Grimm D, Elsner D, Schunkert H, Pfeifer M, Griese D, Brucks-chlegel G, et al. Development of heart failure following isoproterenol administration in the rat: role of the renin-angiotensin system. Cardiovasc Res 1998;37:91-100.
- Gupta OP, Hanke W. Regulation of interrenal secretion in the axolotl, Ambystoma mexicanum. Exp Clin Endocrinol 1994;102:299-306.
