Fibrin(ogen)olytic character of FIIa isolated from Agkistrodon acutus venom1
Introduction
Fibrin-specific clot lysis was initially the major goal to offer the opportunity for effective lysis of pathological thrombus without the risks of a systemic haemorrhagic diathesis[1]. But clinical studies show that even tissue plasminogen activator (t-PA), which exhibits strict selectivity toward fibrin-bound plasminogen, is associated with a significant although variable degree of fibrinogeno-lysis[2–4].
In our previous studies, fibrinolytic enzyme FIIa from Agkistrodon acutus venom was shown to dissolve both fibrin and fibrinogen in vitro. In vivo, FIIa was able to dissolve thrombus without hemorrhage at an effective dose for thrombolysis[5–7]. A few experiments were performed to investigate the fibrin(ogen)olytic character of FIIa such as the specificity to fibrin and fibrinogen and the specificity to different types of clots.
In this study, we compared fibrin(ogen)olytic character of FIIa with urokinase (UK) in vitro and in vivo.
Materials and methods
Snake venoms Agkistrodon acutus venom was collected in Yuanling, Hu-nan Province and lyophilized and stored in desiccator.
Reagents DEAE-Sephadex A-50 and Sephadex G-75 were from Pharmacia (Uppsala, Sweden); human fibrinogen (95% clottable) and bovine fibrinogen (70% clottable) was from Sigma (Saint Louis, USA); aprotinin was from Dadeli Biochemistry and Pharmaceutical Co Ltd (Lanzhou, China); thrombin was from Zhuhai Biochemical Pharmaceutical Factory (Zhuhai, China); UK was from Tianpu Biochemistry and Pharmaceutical Co Ltd (Guangzhou, China); 125I-labeled fibrinogen was from the Department of Experimental Nuclear Medicine of our college by the method of chloramines-T; fibrinogen concentration determination reagent pack (Clauss method) was from Sun Biotechnology Company (Shanghai, China); human plasma was from Guangzhou Blood Center was centrifugated at 3800×g for 8 min; whole blood was collected from healthy volunteers (n=5 male, age 24.0±4.2 a, weighing 61±2.4 kg). All materials were of analytical grade from commercial sources.
Animals Male New Zealand white rabbits (3–4 months old, weighing 2.2±0.1 kg, Grade II, certificate N
Purification of the enzyme FIIa, the fibrinolytic enzyme from Agkistrodon acutus venom was prepared according to the method described by Chen et al[7].
Lysis of 125I-labeled human plasma clots In vitro, 125I-labeled clots were prepared by the modified method of Gurewich et al[8]. Human plasma 5.5 mL were mixed with 125I-labeled fibrinogen (6.105×104 Bq) plus 55 µL CaCl2 (1 mol/L). This solution (0.5 mL) was added to 5-mm (ID) glass tube in the presence of 5 µL thrombin (100 kU/L). The clots were incubated at 37 ºC for 30 min and kept overnight at room tem-perature. All the clots were washed three times with 0.9% saline and the total radioactivity was measured before being transferred to 10-mm (ID) test tubes.
Plasma (4 mL) were incubated (37 ºC, 6 h) with radiolabeled clots in the presence of UK 20, 30, 40, 60, and 80 kU/L or FIIa 0.05, 0.1, 0.2, 0.4, and 0.8 g/L, respectively. The reaction was terminated by adding aprotinin (1×106 kU/L final concentration) into test tubes containing different concentrations of UK or edetic acid (5 µmol/L final concentration) into test tubes containing different concentrations of FIIa. Aliquots (1 mL) were removed for the measurement of radioactivity.
Clot lysis was calculated according to the formula: Clot lysis=Radioactivity in aliquot (modified volume)/total radio-activity×100%. The concentrations of UK or FIIa which could induce 20%, 40%, 50%, 60%, and 80% clot lysis were determined based on the concentration-response curve.
The same experiments were repeated with UK and FIIa at the concentrations that could induce 20%, 40%, 50%, 60%, and 80% clot lysis. Aliquots were removed for the measurement of fibrinogen concentration to get the fibrinogen degradation at the same percent of clot lysis.
Each experiment was performed three times.
Fibrinogen degradation Fibrinogen degradation was performed according to the reagent pack instruction by the Clauss method.
Carotid artery thrombosis The rabbit carotid artery thrombosis model was established according to the method of Wang et al[9]. Forty-eight rabbits were randomly divided into 8 groups (n=6): 0.9% saline 1 mL/kg, as the negative control group; UK 5, 10, 20, and 40 kU/kg group; FIIa 0.5, 1.0, and 2.0 mg/kg group.
Two hours after the initiation of the carotid thrombosis, UK and FIIa were administered via ear-edge vein of rabbit. One hour later, the residual thrombus within the carotid artery was excised and blotted. The wet weight was measured. Blood was drawn from the right femoral artery cannula before and 1 h after administration for the measurement of plasma fibrinogen concentration. The blood sample was drawn into a plastic syringe containing 3.8% sodium citrate as the anticoagulant (1: 9 v/v, citrate/blood) and was centrifuged at 1500×g 4 ºC for 15 min. The platelet-free plasma was stored at -70 ºC until assayed.
Lysis of platelet-rich plasma (PRP) clots, platelet-poor plasma (PPP) clots, and whole-blood clots Whole blood from healthy volunteers was mixed with 3.8% sodium citrate (1:9 v/v, citrate/blood) and immediately centrifuged at room temperature at 250×g for 10 min to obtain PRP. PPP was obtained by centrifuging the original blood sample at 1500×g for 10 min[10]. 125I-labeled clots made from PRP, PPP, and whole blood were prepared as described above.
Plasma (4 mL) were incubated with these three types of radiolabeled clots in the presence of FIIa 0.4 g/L or UK 125 kU/L (final concentration) at 37 ºC. Aliquots were removed for the measurement of radioactivity in plasma at subsequent times during a course of 12 h. The time-response relationship of FIIa and UK for these three types of clots were compared. Each experiment was perfomed three times[11].
Data analysis Values were expressed as mean±SD. Analysis of variance and t-test was used for comparisons of the mean values. P<0.05 was considered significant.
Results
Fibrin(ogen)olytic character of FIIa in vitro In vitro both UK and FIIa dose-dependently dissolved clots. Based on the dose-effect relationship of UK and FIIa, UK at 25, 35, 40, 45, 60 kU/L and FIIa at 0.08, 0.23, 0.4, 0.5, and 0.7 g/L caused an equivalent clot lysis (20%, 40%, 50%, 60%, and 80%, Figure 1, 2).
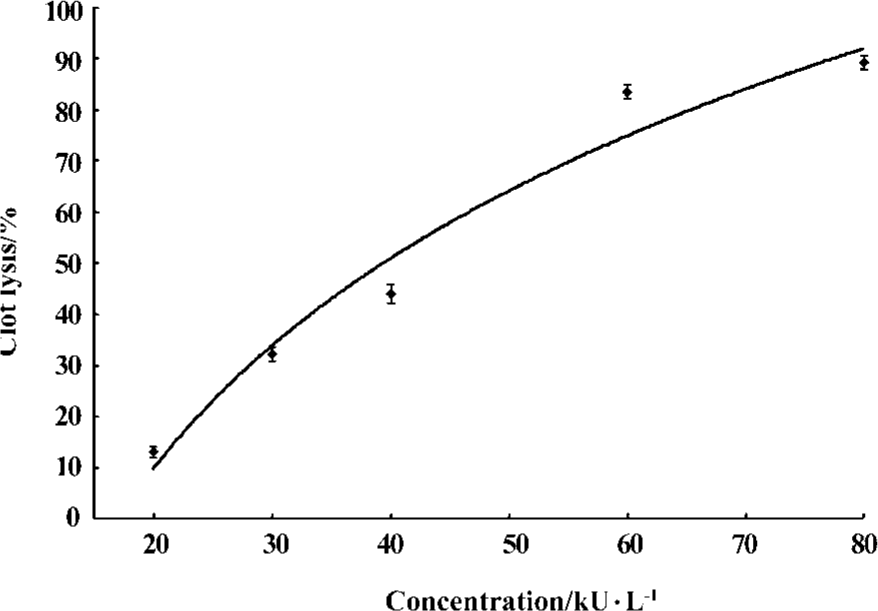
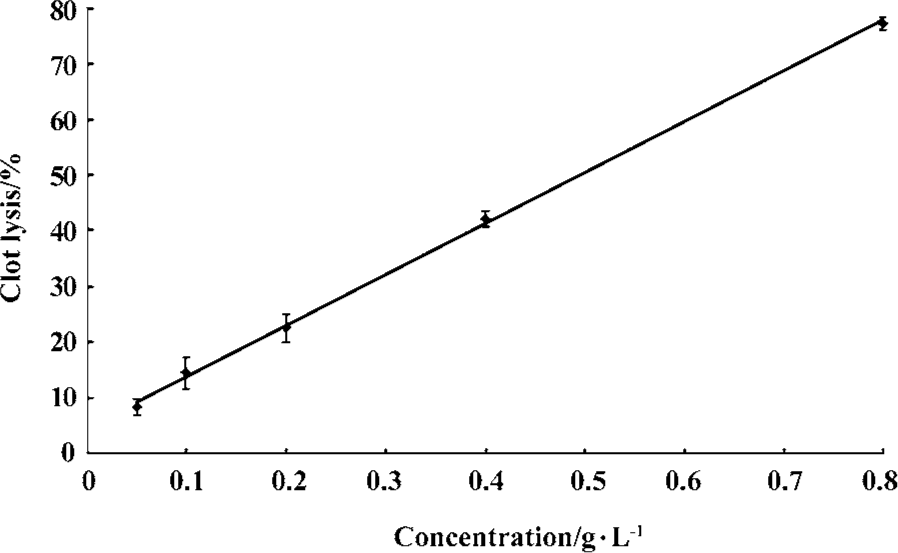
At the calculated concentration, which could induce the same clot lysis, the fibrinogen degradation induced by FIIa was much higher than that induced by UK. In the negative control group, the fibrinogen degradation was 0.01%±0.02% (0.02±0.05 g/L) (Table 1, 2).
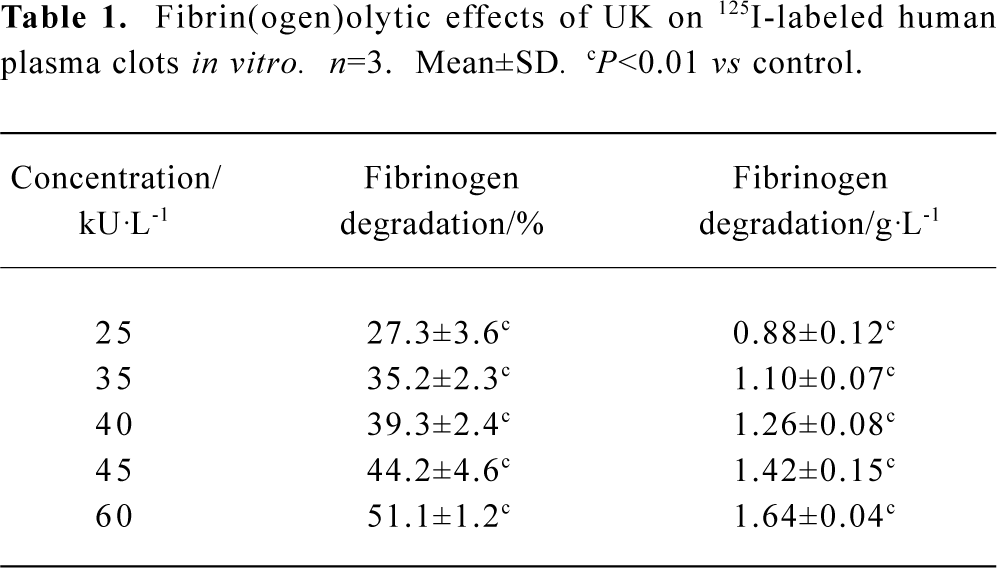
Full table
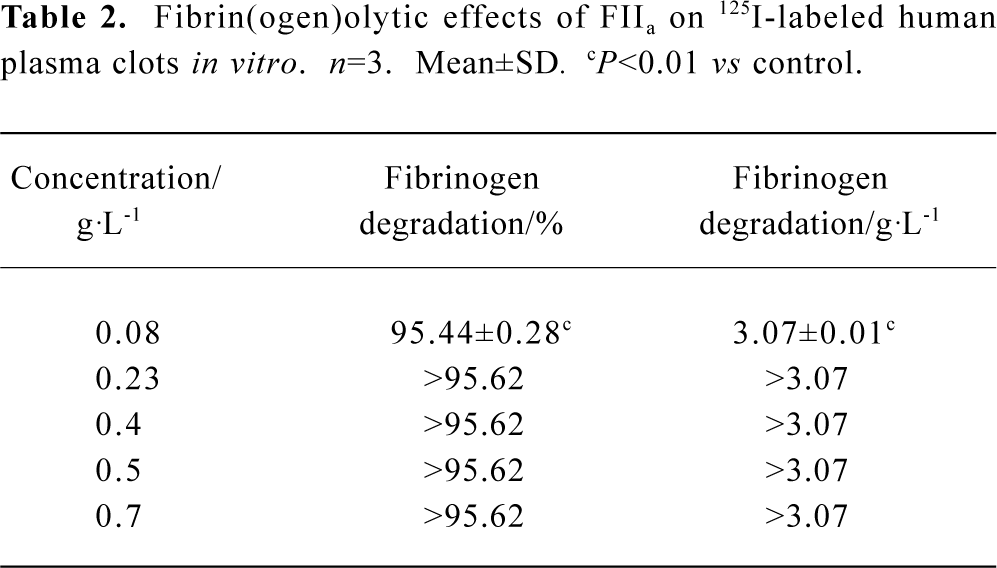
Full table
Fibrin(ogen)olytic character of FIIa in rabbit carotid artery thrombosis UK or FIIa reduced the weight of residual thrombus and the level of fibrinogen in plasma in a dose-dependent manner. In the group treated with UK 40 kU/kg, the weight of residual thrombus was 9.0±2.5 mg. In the group treated with FIIa 1.0 mg/kg, the weight of residual thrombus was 7.8±3.5 mg. The fibrinolytic effects of these two groups were approximately the same, but the fibrinogen degradation of these two groups were 24.4%±6.2% and 4.1%±7.8%, respectively (P<0.05, n=6). In the negative control group, the weight of residual thrombus was 30.0±5.4 mg, and the fibrinogen degradation was 2.7%±2.7%. The difference between UK and control or FIIa and control was statistically significant (P<0.01, n=6, Table 3, 4).
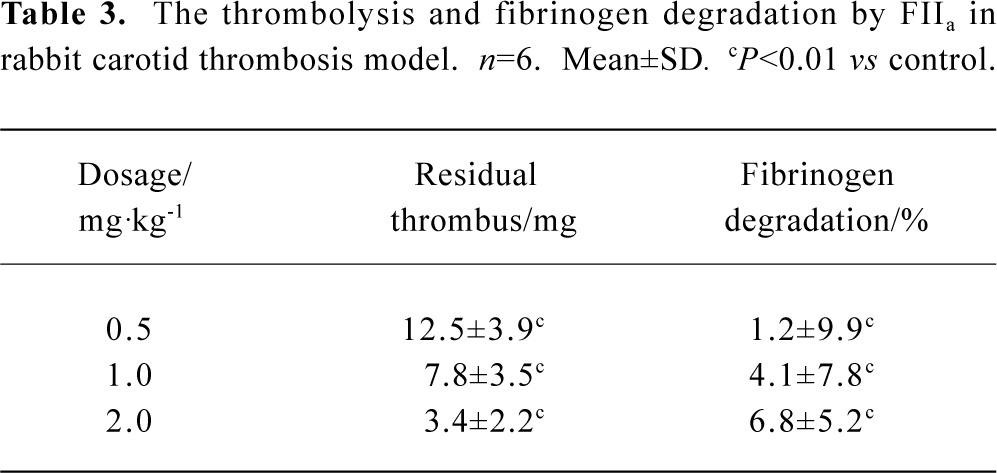
Full table
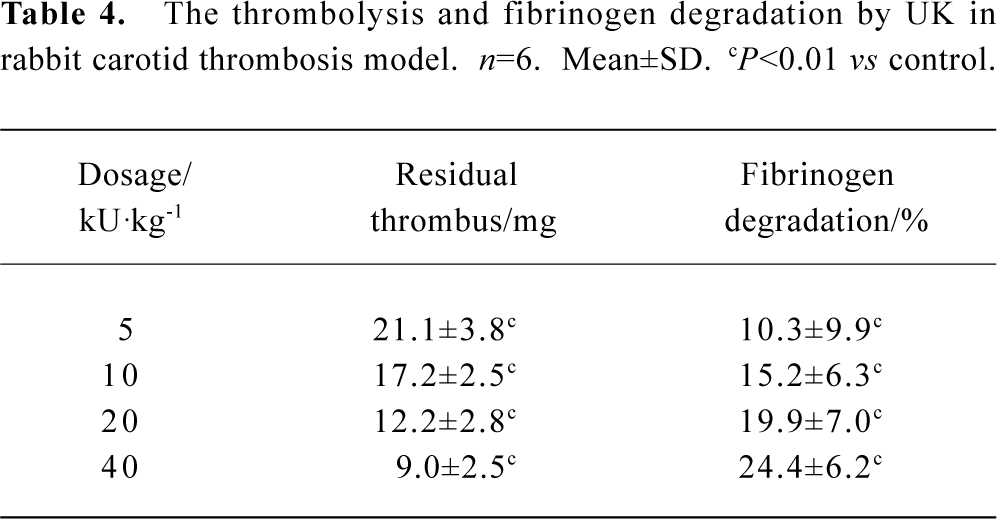
Full table
Fibrin(ogen)olytic character of FIIa in lysis of platelet-rich clots, platelet-poor clots and whole-blood clots The kinetics of FIIa-induced lysis were characterized by an initial lag phase followed by an accelerated lysis, whereas lysis by UK lacked the lag phase. The order of the lysis speed after UK 125 kU/L treatment PPP clots>the whole blood clots>PRP clots. The sequence for FIIa 0.4 g/L was PRP clots>PPP clots>whole blood clots. The clot lysis of these three types of clots with saline was below 10% during the course of 12 h (Figure 3 and 4).
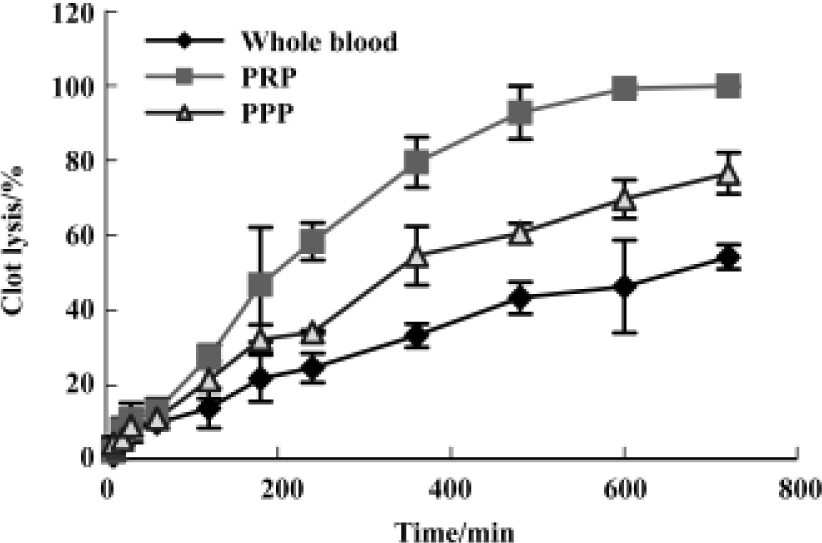
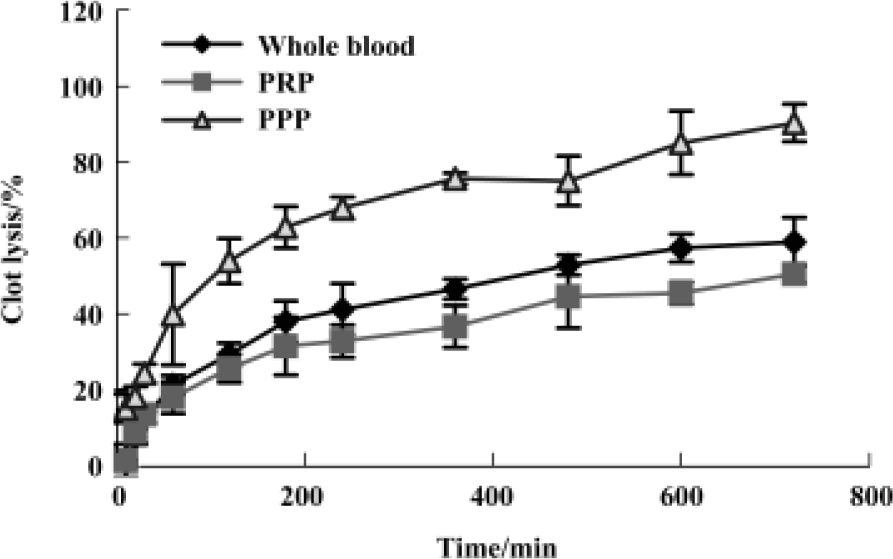
Discussion
In this paper, the fibrin(ogen)olytic character of FIIa was studied and compared with UK. The in vitro results showed that both FIIa and UK degraded clots and fibrinogen in a dose-dependent manner. FIIa degraded 95.4%±0.3% fibrinogen when the clot lysis was 20% and UK degraded 51.1%±1.2% fibrinogen when the clot lysis was 80%. In vitro, FIIa degraded more fibrinogen than UK did at the same percentage of clot lysis.
The in vivo results showed that both FIIa and UK could dose-dependently dissolve clots and fibrinogen. The fibrinolytic effects of UK 40 kU/kg or FIIa 1.0 mg/kg were approximately the same, but the fibrinogen degradation effects were 24.4%±6.2% and 4.1%±7.8%, respectively (P<0.05, n=6). In vivo, FIIa degraded less fibrinogen than UK did at the same percentage of clot lysis. The in vivo and in vitro results of the experiments are in accordance with the data of our previous studies[5,6,12].
UK and FIIa have different sensitivity and kinetics to different types of clots. UK dissolved most effectively the PPP clots, then the whole blood clots and PRP clots. The sequence for FIIa was PRP>PPP>whole blood clots.
Attention should be paid as to why the conclusion in vitro did not accord with the conclusion in vivo. It could be explained that the dosage of FIIa was much higher in vitro than that used in vivo, while the dosage of UK was opposite. The dosage was at least one of the causes of the inconsis-tency.
The main component of thrombus in rabbit carotid artery thrombosis model was platelet[9] and the main component in in vitro clots was fibrin. FIIa dissolves most effectively the platelet rich clots compared with UK that dissolves most effectively fibrin rich clots (Figure 3 and 4), which could explain why FIIa dissolved clot at less dosage in vivo than in vitro but the clot lysis effect of UK was just the opposite.
Our previous study showed that FIIa influenced blood coagulation by inhibiting platelet aggregation induced by ADP in rat PRP and degrading factor X and prothrombin[12]. It is also one of the reasons in the dosage inconsistency.
There are two opinions about the mechanism of the fibrinolytic enzyme from snake venom inhibiting platelet aggregation. (1) Fibrinolytic enzymes inhibit platelet aggregation by hydrolyzing α-fibrinogen to prevent fibrinogen from combining with fibrinogen receptor (GPIIb-IIIa), such as α-fibrinolytic enzyme from Agkistrodon contortrix contor-trix[13], A rhodostoma[14], and T mucrosquamatus[15]. (2) The disintegrin-like domain may target the fibrinolytic enzyme to a particular site such as platelets where the metalloproteinase domain may cleave relevant substrates including integrins, coagulant proteins, matrix, or other latent proteins[16]: such as collagen receptor antagonist Jararhagin from Bothrops jararaca[17], Mutalysin I from Lachesis muta muta[16], Crovidisin from C viridis[18]; such as GPIIb-IIIa antagonist Barbourin[19] with KGD sequence. Our previous study showed FIIa degraded α and β chains of fibrinogen[5]. Is there any relationshiop between the inhibitory activity of FIIa on platelet aggregation and the proteolytic activity of FIIa on fibrinogen or membrane protein? This topic will be further explored in the near future.
References
- Verstraete M, Lijnen HR, Collen D. Thrombolytic agents in development. Drugs 1995;50:29-42.
- Collen D, Verstraete M. Systematic thrombolytic therapy of acute myocardial infarction. Circulation 1983;68:462-5.
- Rao AK, Pratt C, Berke A, Jaffe A, Ockene I, Schreiber TL, et al. Thrombolysis in myocardial infarction (TIMI) trial-phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol 1988;11:1-11.
- Stump DC, Califf RM, Topol EJ, Sigmon K, Thornton D, Masek R, et al. Pharmacodynamics of thrombolysis with recombinant tissue-type plasminogen activator: correlation with characteristics of and clinical outcomes in patients with acute myocardial infarction. Circulation 1989;80:1222-30.
- Liang XX, Chen JS, Zhou YN, Qiu PX, Yan GM. Purification and biochemical characterization of FIIa, a fibrinolytic enzyme from Agkistrodon acutus venom. Toxicon 2001;39:1133-9.
- Chen JS, Liang XX, Qiu PX, Yan GM. Thrombolysis effect with FIIa from Agkistrodon acutus venom in different thrombosis model. Acta Pharmacol Sin 2001;22:420-2.
- Chen JS, Liang LG, Sun JJ. The pharmacology characterizations of fibrinolytic fraction II from five-pace snake venom. Chin Pharmacol Bull 1993;9:22-5.
- Gurewich V, Pannell R, Louis S, Kelley P, Robert L, Suddith RL, et al. Effective and fibrin-specific clot lysis by a zymogen precursor from a study in vitro and in tow animal species. J Clin Invest 1984;73:1731-9.
- Wang QC, Xu YL, Liu GF. A simple rabbit carotid thrombus model and the fibrinolytic effect of acutinase. Chin Pharmacol Bull 1993;9:228-9.
- Datta G, Dong A, Witt J, Tu AT. Biochemical characterization of basilase, a fibrinolytic enzyme from Crotalus basiliscus basiliscus. Arch Biochem Biophys 1995;317:365-73.
- Gurewich V, Pannell R. A comparative study of the efficacy and specificity of tissue plasminogen activator and pro-urokinase: demonstration of synergism and of different thresholds of non-selectivity. Throm Res 1986;44:217-28.
- Wang QQ, Chen JS, Liang XX, Qiu PX, Wang YW, Yan GM. Hemorrhagic activity and mechanism of FIIa, a fibrinolytic enzyme from Agkistrodon acutus venom. Acta Pharmacol Sin 2004;25:514-21.
- Manning MC. Sequence analysis of fibrolase, a fibrinolytic metalloproteinase from Agkistrodon contortrix contortrix. Toxin 1995;33:1189-200.
- Huang TF, Chang MC, Peng HC, Teng CM. A novel alphatype fibrinogenase from Agkistrodon rhodostoma snake venom. Biophim Biophys Acta 1992;1160:262-8.
- Sugihara H, Mori N, Nikai T, Kishida M, Akagi M. Comparative study of three proteinases from the venom of the Chinese hahu snake (Trimeresurus mucrosquamatus). Comp Biochem Physiol B 1985;82:29-35.
- Estêvão-costa MI, Diniz CR, Magalhães A, Markland FS, Sanchezâ EF. Thromb Res 2000;99:363-76.
- Kamiguti AS, Hay CRM, Zuzel M. Inhibition of collagen-induced platelet aggregation as the result of cleavage at α2β1-integrin by the snake venom metalloproteinase jararhagin. Biochem J 1996;320:635-C41.
- Liu CZ, Huang TF. Crovidisin, a collagen-binding protein isolated from snake venom of Crotalus viridid, prevents platelet-collagen interaction. Arch Biochem Biophys 1997;337:291-9.
- Perutelli P. Disintegrins: potent inhibitors of platelet aggregation. Recent Prog Med 1995;86:168-74.
