Effects of huperzine A on secretion of nerve growth factor in cultured rat cortical astrocytes and neurite outgrowth in rat PC12 cells1
Introduction
Nerve growth factor (NGF) is a member of the neurotrophin family that promotes the survival and outgrowth of central cholinergic neurons[1]. The decrease in trophic support for the neurons in the aging brain is associated with neuronal death and appearance of neurodegenerative disorders such as Alzheimer disease (AD)[2]. A case study showed that topical application of NGF into the brain of one AD patient relieved symptoms of dementia[3]. However, the clinical utility of NGF is limited by an inability to cross the blood-brain barrier, necessitating invasive neurosurgical procedures for administration. In contrast, pharmacological stimulation of endogenous NGF synthesis or mimicking of NGF activity by compounds that penetrate membrane barriers may provide an alternative means to provide equivalent trophic actions in the central nervous system.
Accumulated data suggest that cholinergic mechanisms are involved in the regulation of NGF synthesis and release. It has been suggested that cholinergic activity in basal forebrain neurons may stimulate NGF synthesis in appropriate target areas during early postnatal development[4]. Addi-tionally, substances that augment cholinergic function, such as nicotine, muscarinic receptor agonists, and acetylcholine-releasing agents, can increase NGF expression and synthesis[5,6]. Although there have been no reports that acetylcholinesterase (AChE) inhibitors elevate NGF production, some AChE inhibitors are known to exert NGF-like activity by potentiating the neuritogenic effect of NGF[7] or increasing choline acetyltransferase (ChAT) activity[8]. These actions appear to be independent of AChE inhibition. Such findings led us to suspect that the neurotrophic effects of huperzine A (HupA), a novel acetylcholinesterase inhibitor isolated from the Chinese herb Huperzia serrata, involve NGF-like activity, directly or indirectly.
HupA has been proved to be one of the most promising new agents for AD therapy[9]. Our previous studies showed that, in addition to its potent inhibition of AChE, HupA exhibited neuroprotective effects both in vivo and in vitro[10,11]. For example, just as NGF can ameliorate neuronal degeneration in rat cerebral cortex and hippocampus after ischemic insult[12], HupA will attenuate ischemic damage from transient global ischemia in gerbils and hypoxic-ischemia (HI) in neonatal rats[13,14]. A multicenter, randomized, double-blind, placebo-controlled clinical trial in China proved that HupA markedly improved the cognitive function of vascular dementia (VD)[15–17]. In view of these findings it is natural to ask if HupA might influence intrinsic neurotrophic factors. The aim of this study was to examine the effects of HupA on neuritogenesis in rat pheochromocytoma cells and on the expression and secretion of NGF in primary cultures of rat cortical astrocytes.
Materials and methods
Materials HupA, provided by the Department of Phytochemistry at Shanghai Institute of Materia Medica is a colorless powder with mp 230 °C, and purity >99%. It was dissolved and diluted in phosphate-buffered saline (PBS). RPMI-1640 medium, DMEM/F12 medium, fetal bovine serum, horse serum, and N-2 supplement were purchased from Gibco (CA, USA). Cell cytotoxicity Kit, NGF Emax® Immunoassay System, Reverse Transcription System were purchased from Promega (Madison, WI, USA). Mouse monoclonal anti-AChE antibody was purchased from BD Biosciences (CA, USA). TRIzol reagent was purchased from Invitrogen (CA, USA). ECL kit was purchased from Pierce (Rockford, USA).
PC12 cell culture and neurite outgrowth assay Undifferentiated PC12 rat pheochromocytoma cells were obtained from ATCC. The cells were maintained in RPMI-1640 media containing 10% FCS, 5% HS, 100 kU/L penicillin, and 100 kU/L streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. For each experiment, cells (3.5×104 cells/well) were seeded into a 12-well collagen-coated plate and cultured overnight. Subsequently the cells were washed once with PBS and transferred to fresh serum-free DMEM containing an N-2 supplement. After a 2-h incubation with serum-free DMEM, NGF or HupA was added to the cultures, which were incubated for a further 48 h. Neurite formation was examined under a phase-contrast microscope, and processed longer than one cell diameter were scored as neurites. The percentage of neurite-bearing cells in relation to the total number of cells was examined in four fields from each of the eight culture wells per treatment condition.
Mitogenic activity and cytotoxicity assay To analyze the effects of HupA on mitogenic activity, PC12 cells were seeded into 96-well plate at a density of 1×104 cell/well. After incubation of the cells with 10 µmol/L HupA for 48 h at 37 °C, [3H]thymidine (37 000 Bq) was added to the cultures and incubation was continued for another 8 h. The cells were harvested onto filters using a cell harvester and the retained radioactivity was determined in a scintillation counter. HupA cytotoxicity was evaluated by an assay kit, following the manufac-ture’s protocol to detect lactate dehydrogenase (LDH) release.
AChE activity assay AChE activity was measured by a standard spectrophotometric method[18].
Western blot analysis Changes in AChE levels were assessed by Western blot. For this purpose, cultured cells harvested 48 h after treatment with HupA were lysed in 1×SDS PAGE gel loading buffer [Tris-HCl 50 mmol/L pH 6.8, DTT 100 mmol/L, 2% (w/v) SDS, 10% (w/v) glycerol, 0.1% Bromophenol Blue] and boiled in a water bath for 10 min. Equal amounts of protein (40 µg) were loaded in each gel lane and, after electrophoresis, proteins were transferred to a nitrocellulose membrane. The membranes were blocked with TBST containing 5% non-fat milk and then were incubated at 4 °C overnight with primary antibody (a mouse monoclonal antibody raised against human AChE and crossreactive with rat AChE). Finally the blots were incubated with HRP-conjugated anti-mouse IgG at 37 °C for 2 h and target protein bands were detected by the ECL method according to the manufacture’s instruction.
Astrocytes cultures and experimental treatment For studies of NGF expression, primary cultures of astrocytes were prepared from 1-day-old neonatal Sprague-Dawley rats. Cerebral cortices were stripped of meninges and dissected in Ca2+-, Mg2+-free D-Hanks’ solution. Next the samples were trypsinized and passed through a monofilament mesh (pore size 80 μm). Cells were collected by centrifugation and resuspended in DMEM/F12 containing 10% FCS, 100 kU/L penicillin and 100 kU/L streptomycin for culture in a humidified atmosphere (95% air and 5% CO2) at 37 °C. Astrocytes were grown to confluence and oligodendrocyte and microglial cells were removed by shaking and washing with cold D-Hanks’ solution. The astrocytes were then trypsinized and plated onto 24-well plates at a density of 1×104 cell/cm2, washed with D-Hanks’ solution, and exposed for 24 h to serum-free medium. After this incubation, culture medium was collected and centrifuged to remove cell debris.
NGF content assay Released NGF was measured in the supernatant by a two-site enzyme-linked immunosorbent assay (ELISA) using the NGF Emax® Immunoassay system.
RT-PCR analysis RT-PCR was performed to determine the expression of mRNA for AChE in PC12 cells and for NGF and P75NTR in astrocytes. Total cellular RNA was isolated using TRIzol reagent following the manufacturer’s protocols and quantified by absorbance at 260 nm. RNA purity was determined using the A260/A280 ratio (average ratio >1.85). Total RNA of each sample was reverse-transcribed into cDNA using Reverse Transcription System. The cDNAs were amplified with the following specific primers. AChE: 5'-TCTTTGCTCAGCGACTTA-3' (upstream), 5'-GTCACAGG-TCTGAGCATCT-3' (downstream); NGF: 5'-CTTCAGCATT-CCCTTGACAC-3' (upstream), 5'-AGCCTTCCTGCTGAGCA-CACA-3' (downstream); P75NTR: 5'-AGCCAACCAGACCG-TGTGTG-3' (upstream), 5’-TTGCA GCTGTTCCACCTCTT-3' (downstream); β-actin: 5'-CCTGCGTCTGGACCTG GCTG-3' (upstream), 5'-CTCAGGAGGAGCAATGATCT-3' (down-stream). Amplifications were performed as follows: AChE: 30 cycles, 94 °C for 45 s, 56 °C for 45 s, 72 °C for 45 s; NGF: 24 cycles, 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 60 s; P75NTR: 25 cycles, 94 °C for 45 s, 55 °C for 30 s, and 72 °C for 90 s; β-actin: 30 cycles, 94 °C for 45 s, 57 °C for 45 s, and 72 °C for 60 s. The PCR products were normalized in relation to standards of β-actin mRNA.
Statistical analysis Data were expressed as mean±SEM. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test, with P<0.05 as the significant level.
Results
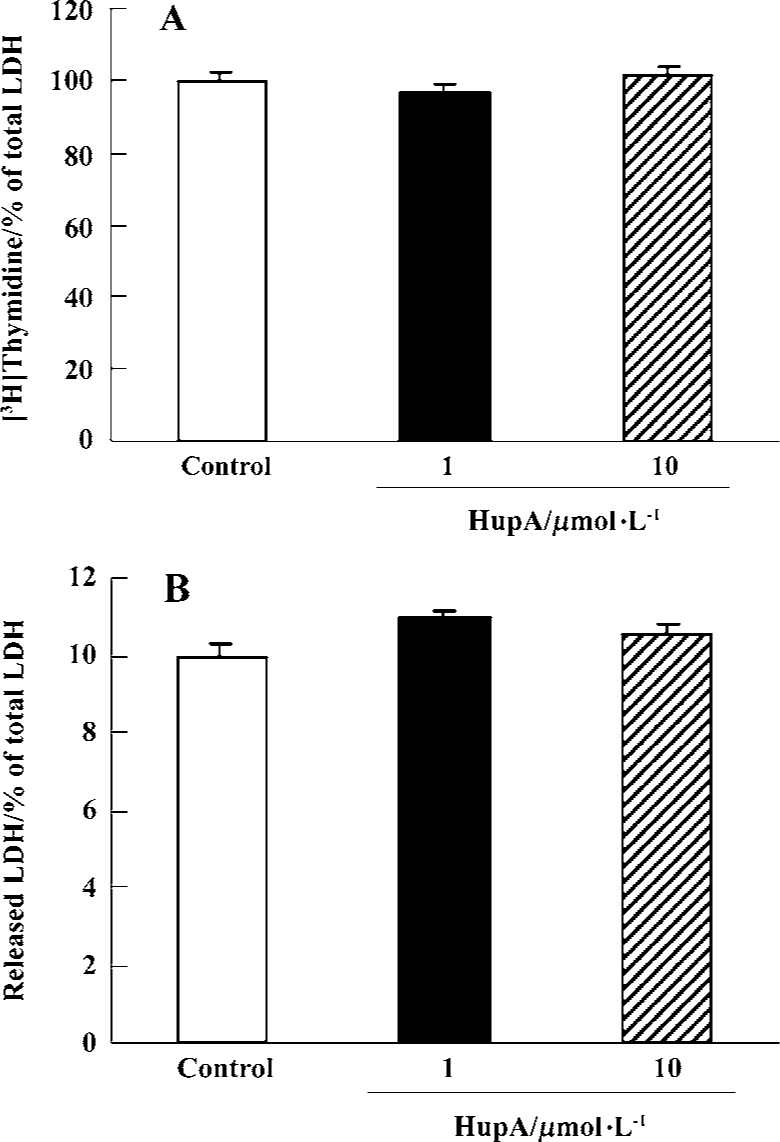
Mitogenic activity and cytotoxicity of HupA on PC12 cells There was no effect on cell proliferation during a 48-h exposure of PC12 cells to HupA 10 µmol/L. Likewise, measures of LDH release showed that HupA 10 µmol/L did not induce any cytotoxic effects (Figure 1).
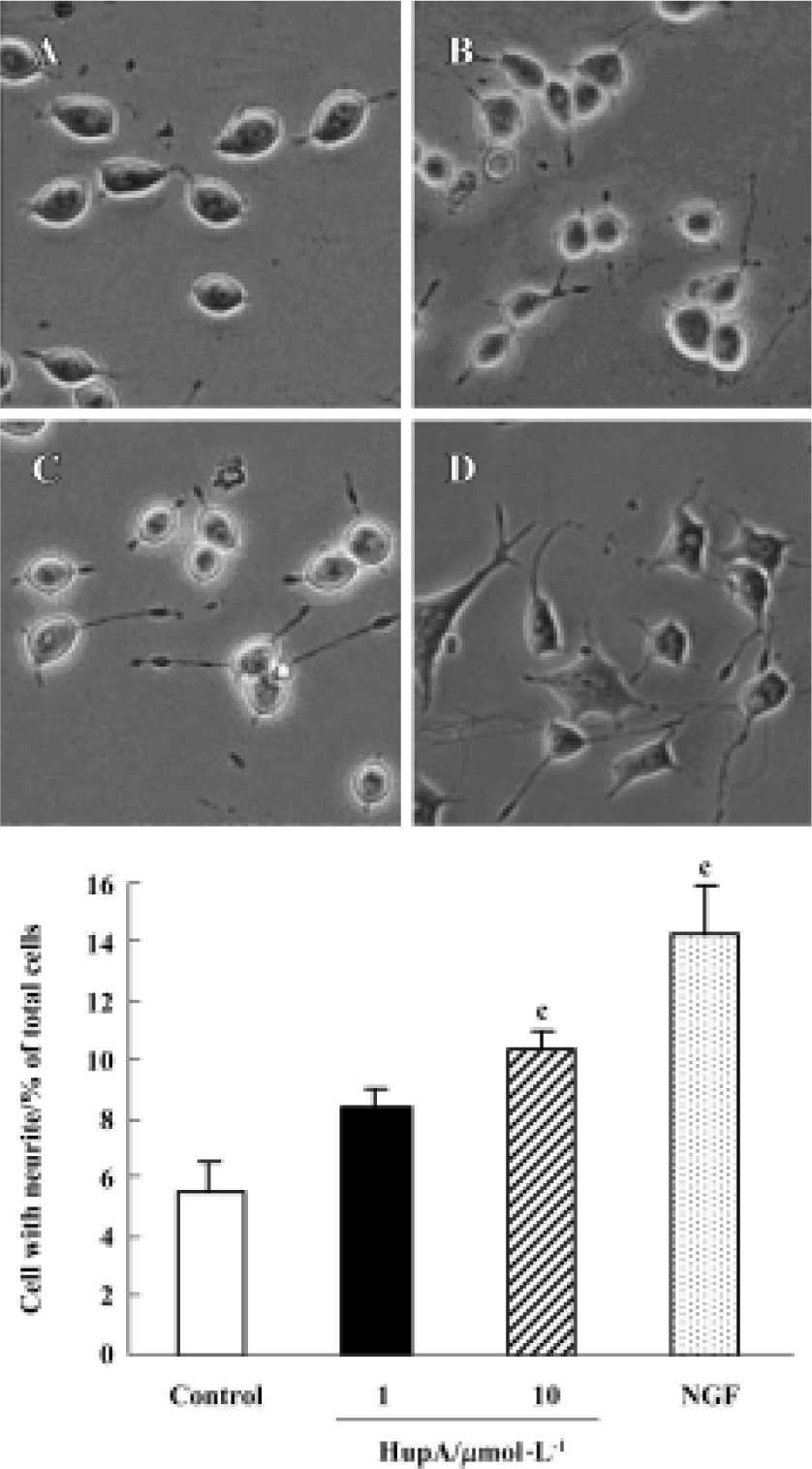
Effects of HupA on neurite outgrowth Most PC12 cells displayed a flat, polygonal, undifferentiated morphology after incubation under control conditions for 48 h, and only 5.5% of them exhibited neurites. However, after incubation with HupA 10 µmol/L for 48 h more cells differentiated and nearly twice as many (10.4%) were found to bear neuritis. Moreover, 14.2% of PC12 cells exhibited neuritis after incubation with 2 μg/L NGF (Figure 2).
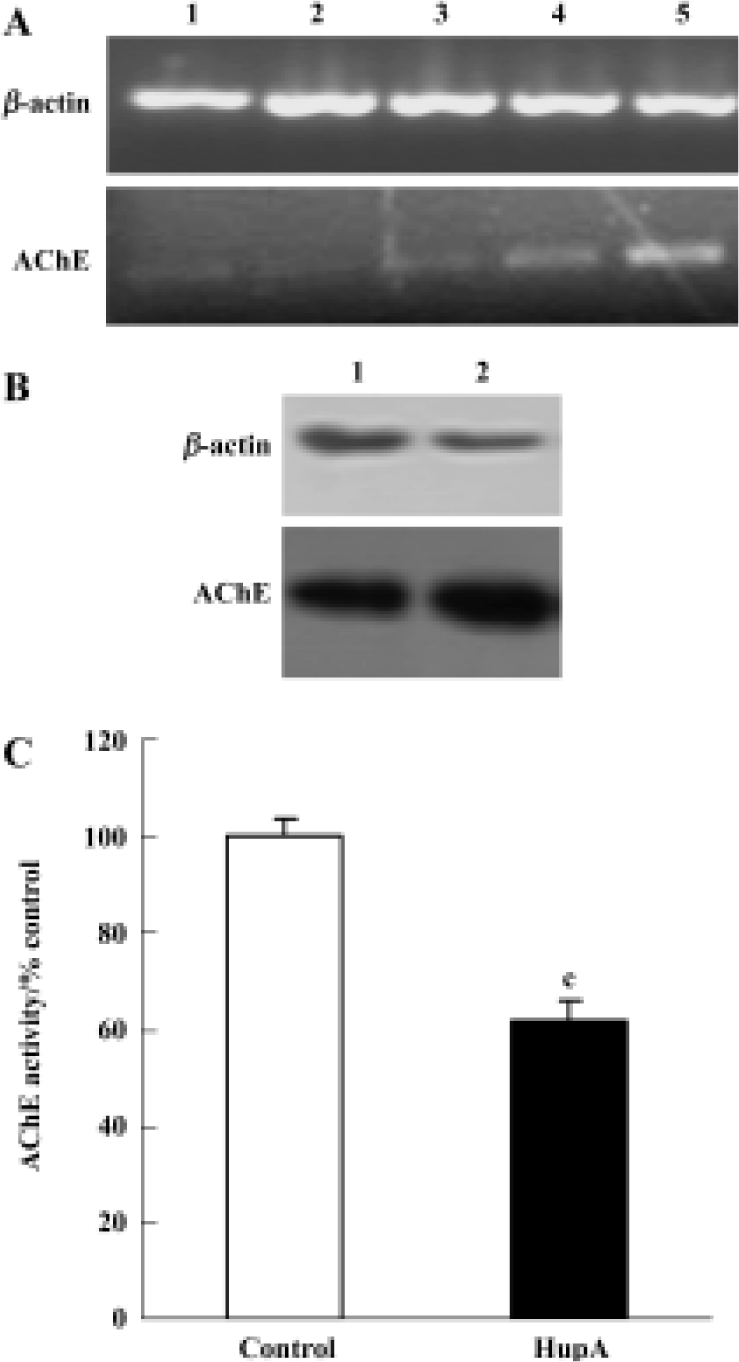
Effects of HupA on activity, expression, and protein levels of AChE In PC12 cells the activity of AChE was inhibited substantially by HupA treatment. The expression of AChE was also affected. AChE mRNA was increased at 6 h and 10 h. Consistent with the late rise in mRNA, AChE protein levels rose after a 48-h incubation with HupA (Figure 3).
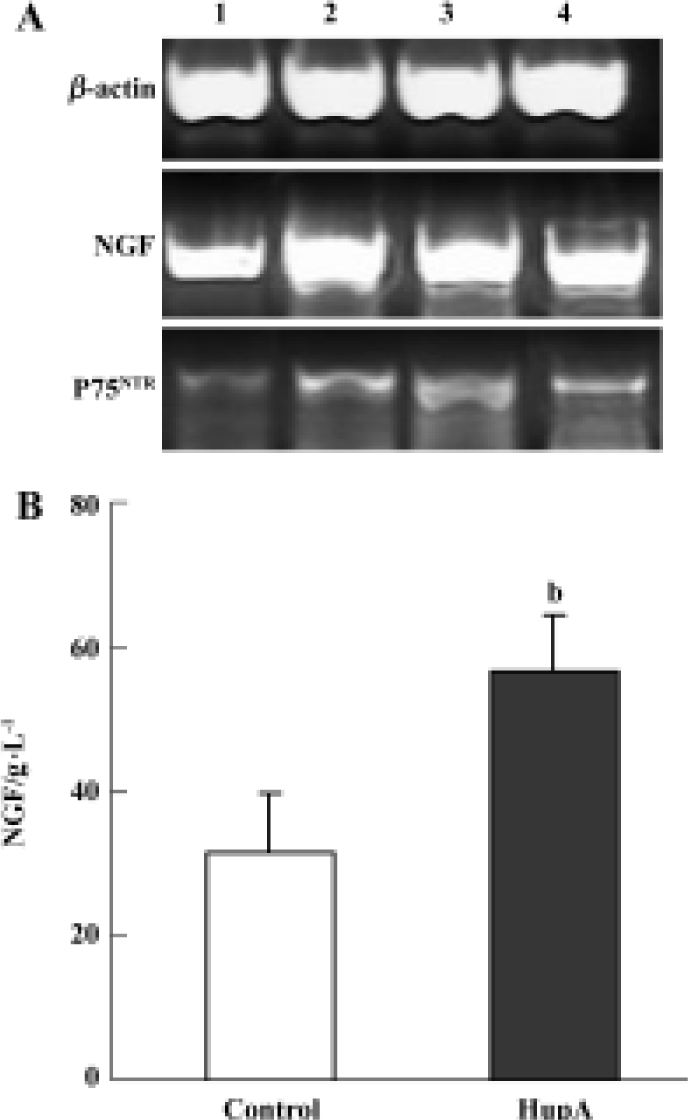
Effects of HupA on the expression and secretion of NGF Effects of HupA on NGF and P75NTR mRNA were determined by RT-PCR in primary rat cortical astrocytes, a cell type known to express the corresponding proteins. Figure 4A represents an example of RT-PCR products visualized after electrophoresis in 1.5% agarose gel containing ethidium bromide. The results suggested that NGF mRNA transcripts were slightly upregulated at 2, 4, and 6 h, while the levels of mRNA for P75NTR appeared greatly increased. Moreover, an ELISA for NGF protein showed a large and statistically significant increase in cultures incubated with HupA 10 µmol/L for 24 h when compared to controls incubated for the same length of time in normal medium (Figure 4B).
Discussion
PC12 cells cannot secret NGF, but they have NGF receptors and can respond to NGF. In the presence of NGF, PC12 cells cease cell division, differentiate into sympathetic neuron-like cells, and extend neuritis. So it is a putative model to determine the neuritogenic activity[19]. Different from PC12 cells, astrocytes can secret NGF. In the injured brain, for example, astrocytes play an important role in neurotrophic support[20]. So the two different cells were used to determine the direct or indirect neurotrophic activity of HupA. In the present study, HupA was demonstrated to increase neurite outgrowth from undifferentiated PC12 cells and to enhance the expression and secretion of NGF in primary astrocytes. These findings indicate that HupA is not only an effective AChE inhibitor, but it also possesses NGF-inducing activity and an ability to induce certain NGF-like effects. The question arises whether these effects are closely linked, and what kind of relation they have to AChE. It has been reported that AChE plays an important role in neuronal proliferation and differentiation during early development of the central and peripheral nervous system. Neural AChE typically appears while axons are growing and before synaptic connections form[21]. Accumulating evidence indicates that AChE may influence neurite outgrowth through a non-catalytic mechanism such as cell-cell or cell-substratum adhesion[22]. It has been observed that the trophic activity of AChE is blocked by inhibitors that interact with the peripheral anionic site (PAS) but not by inhibitors that interact only with the active site, deep within the catalytic gorge[23,24]. Such findings suggest strongly that surface features of AChE rather than catalytic activity are responsible for its trophic effect. Hence, it is not contradictory to find that HupA affected neurite outgrowth in a positive rather than negative manner despite causing marked AChE inhibition. In fact, the differentiation-promoting effect of HupA may well reflect increased amounts of AChE protein. In other cell lines and to some extent in vivo, increased neurite outgrowth has been seen to parallel the level of AChE expression[25–27]. Conversely, decreasing expression of AChE expression, using antisense techniques, reduced outgrowth[28]. Our present results showed that AChE mRNA expression and protein levels were significantly up-regulated after treatment with HupA. This finding is consistent with other reports of increased AChE gene expression after exposure to AChE inhibitors[29,30]. It remains to be determined whether feedback regulation of AChE synthesis is involved in these changes.
Previous observations suggest that NGF regulates the phenotype and survival of basal forebrain cholinergic neurons[31] and protects hippocampal and cortical neurons against excitotoxic and ischemic damage[32]. Neurons surviving from transient ischemia highly expressed P75NTR, suggesting that this low affinity neurotrophin receptor could contribute to the cytoprotective effect of NGF[33]. Our results showed that HupA enhanced the expression and secretion of NGF as well as increasing P75NTR mRNA level in astrocytes. We conclude that these two responses may be key to the neuroprotective effects that have been observed in vitro and in vivo after treatment with HupA.
The exact mechanism by which HupA increases NGF secretion remains to be determined. There is evidence that cholinergic and adrenergic mechanisms as well as PKC activation all affect NGF gene expression in astrocytes. Our previous studies have demonstrated that HupA has enhancing effects on PKC[34], and on cholinergic and adrenergic systems[35]. Such effects may participate in the modulation of NGF synthesis. This provides another possible mechanism for HupA to promote survival of damaged neurons, which may interact synergistically with other pathways to exert the neuroprotective effect of this drug.
In the present study, HupA induced the NGF synthesis of cultured astrocytes and enhanced the neurite outgrowth of undifferentiated PC12 cells in vitro. These effects provide the possibility that HupA increase NGF-induced enhancement of neurons survival and their function improvement that was helpful in the rescue of injured neurons.
In summary, our study has demonstrated for the first time that HupA induces neurite outgrowth in PC12 cells and stimulates expression of NGF, P75 mRNA, and secretion of NGF in cultured rat cortical astrocytes. These effects might be helpful to restore and maintain neural cells in neuro-degenerative disease.
Acknowledgement
The authors wish to thank Prof Stephen BRIMIJOIN (Mayo Clinic, USA) for helpful discussion and English revision to the manuscript.
References
- Hefti F, Hartikka J, Knusel B. Function of neurotrophic factors in the adult and aging brain and their possible use in the treatment of neurodegenerative disease. Neurobiol Aging 1989;10:515-33.
- Mufson EJ, Bothwell M, Kordower JH. Loss of nerve growth factor receptor-containing neurons in Alzheimer’s disease: a quantitative analysis across subregions of the basal forebrain. Exp Neurol 1989;105:221-32.
- Olson L, Nordberg A, von Holst H. Nerve growth factor affects [C]nicotine binding, blood flow, EEG, and verbal episodic memory in an Alzheimer patient (case report). J Neural Transm (P-D Sect) 1992; 4: 79–95.
- da Penha Berzaghi M, Cooper J, Castren E, Zafra F, Sofroniew M, Thoenen H, et al. Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J Neurosci 1993;13:3818-26.
- French SJ, Humby T, Horner CH, Sofroniew MV, Rattray M. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Brain Res Mol Brain Res 1999;67:124-36.
- Ghelardini C, Galeotti N, Bartolini A, Furukawa S, Nitta A, Manetti D, et al. Memory facilitation and stimulation of endogenous nerve growth factor synthesis by the acetylcholine releaser PG-9. Jpn J Pharmacol 1998;78:245-51.
- Shigeta K, Ootaki K, Tatemoto H, Nakanishi T, Inada A, Muto N. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by a Coptidis Rhizoma extract and protober-berine alkaloids. Biosci Biotech Biochem 2002;66:2491-4.
- Kato K, Hayako H, Ishihara Y, Marui S, Iwane M, Miyamoto M. TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons. Neurosci Lett 1999;260:5-8.
- Tang XC, He XC, Bai DL. Huperzine A: a novel acetylcholinesterase inhibitor. Drugs Fut 1999;24:647-63.
- Xiao XQ, Wang R, Tang XC. Huperzine A and tacrine attenuate beta-amyloid peptide-induced oxidative injury. J Neurosci Res 2000;61:564-9.
- Wang R, Zhang HY, Tang XC. Huperzine A attenuates cognitive dysfunction and neuronal degeneration caused by beta-amyloid protein (1-40) in rat. Eur J Pharmacol 2001;421:149-56.
- Buchan AM, Williams L, Bruederlin B. Nerve growth factor: pretreatment ameliorates ischemic hippocampal neuronal injury. Stroke 1990;21:177.
- Zhou J, Zhang HY, Tang XC. HupA attenuates cognitive deficits and hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci Lett 2001;313:137-40.
- Wang LS, Zhou J, Shao XM, Tang XC. Huperzine A attenuates cognitive deficits and brain injury in neonatal rats after hypoxia-ischemia. Brain Res 2002;949:162-70.
- Li HN, Min QY. Huperzine A improved the cognition of vascular dementia: a report of 30 patients in therapeutics. Chin J Clin Rehabil 2001;5:59.
- Ma YX, Zhu Y, Gu YD, Yu ZY. Y SM, Ye YZ. Double-blind trial of huperzine-A (HUP) on cognitive deterioration in 314 cased of benign senescent forgetfulness, vascular dementia, and Alzheimer’s disease. Ann NY Acad Sci 1998;854:506-7.
- Pi X, Liu Y, Jiang ZY, Hu XQ, Zhu GZ. Clinical observation on treatment of light and moderate vascular dementia with meclofenoxate plus huperzine A. Shanghai Med Pharm J 2004;255:409-11.
- Wang YE, Yue DX, Tang XC. Anti-cholinesterase activity of huperzine A. Acta Pharmacol Sin 1986;7:110-3.
- Greene LA, Tischler AS. PC12 pheochromocytoma cultures in neurobiological research. Adv Cell Neurobiol 1982;3:373-414.
- Lu B, Yokoyama M, Dreyfus CF, Black IB. NGF gene expression in actively growing brain glia. J Neurosci 1991;11:318-26.
- Drews U. Cholinersterase in embryonic development. Prog Histochem Cytochem 1975;7:1-53.
- Brimijoin S, Koenigsberger C. Cholinesterases in neural develop-ment: new findings and toxicologic implications. Environ Health Perspect 1999;107 Suppl 1:59-64.
- Munoz FJ, Aldunate R, Inestrosa NC. Peripheral binding site is involved in the neurotrophic activity of acetylcholinesterase. Neuroreport 1999;10:3621-5.
- Sharma KV, Koenigsberger C, Brimijoin S, Bigbee JW. Direct evidence for an adhesive function in the noncholinergic role of acetylcholinesterase in neurite outgrowth. J Neurosci Res 2001;63:165-75.
- Karpel R, Sternfeld M, Ginzberg D, Guhl E, Graessmann A, Soreq H. Overexpression of alternative human acetylcholinesterase forms modulates process extensions in cultured glioma cells. J Neurochem 1996;66:114-23.
- Koenigsberger C, Chiappa S, Brimijoin S. Neurite differentiation is modulated in neuroblastoma cells engineered for altered acetylcholinesterase expression. J Neurochem 1997;69:1389-97.
- Layer PG, Willbold E. Novel functions of cholinesterases in development, physiology and disease. Prog Histochem Cytochem 1995;29:1-94.
- Grifman M, Soreq H. Differentiation intensifies the susceptibility of pheochromocytoma cells to antisense oligodeoxynucleo-tide-dependent suppression of acetylcholinesterase activity. Antisense Nucleic Acid Drug Dev 1997;7:351-9.
- Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 1998;393:373-7.
- Chiappa S, Padilla S, Koenigsberger C, Moser V, Brimijoin S. Slow accumulation of acetylcholinesterase in rat brain during enzyme inhibition by repeated dosing with chlorpyrifos. Biochem Pharmacol 1995;49:955-63.
- Whittemore SR, Seiger A. The expression, localization and functional significance of beta-nerve growth factor in the central nervous system. Brain Res 1987;434:439-64.
- Semkova I, Wolz P, Schilling M, Krieglstein J. Selegiline enhances NGF synthesis and protects central nervous system neurons from excitotoxic and ischemic damage. Eur J Pharmacol 1996;315:19-30.
- Lee TH, Abe K, Kogure K, Itoyama Y. Expressions of nerve growth factor and p75 low affinity receptor after transient forebrain ischemia in gerbil hippocampal CA1 neurons. J Neurosci Res 1995;41:684-95.
- Zhang HY, Yan H, Tang XC. HupA enhances the level of secretory amyloid precursor protein and protein kinase C-alpha in intracerebroventricular beta-amyloid-(1-40) infused rats and human embryonic kidney 293 Swedish mutant cells. Neurosci Lett 2004;360:21-4.
- Ou LY, Tang XC, Cai JX. Effect of HupA on working memory in reserpine- or yohimbine-treated monkeys. Eur J Pharmacol 2001;433:151-6.
