Differentiation of embryoid-body cells derived from embryonic stem cells into hepatocytes in alginate microbeads in vitro1
Introduction
Although liver transplantation has become a standard therapy for acute liver failure and end-stage liver disease, its application has been limited due to the shortage of donor organs. There has been extensive research in the field of hepatocyte transplantation and bioartificial livers for bridging and as an alternative to liver transplantation[1,2]. A necessity for the development of bioartificial livers and hepatocyte transplantation is the availability of sufficient high-quality hepatocytes.
Embryonic stem (ES) cells are pluripotent and can proliferate infinitely in an undifferentiated state in vitro. Therefore, ES cell-derived hepatocytes could serve as potential cell sources for bioartificial livers and hepatocyte transplantation. Since the first report on the differentiation of mouse ES cells into endodermal cells[3], many studies have demonstrated that ES cells can differentiate into hepatocytes in vitro and in vivo[4,5]. These differentiation protocols included the addition of growth factors, sodium butyrate, collagen scaffold, serum limitation, genetic manipulation, and coculture[6–11].
Although many methods have been applied in the differentiation of ES cells into hepatocytes, the research of scalable and controlled culture systems is needed before the clinical application of ES cells. The immobilization of cells within alginate microbeads allows high-density cell culture and free exchange of nutrients, oxygen, and bioactive pro-ducts, whereas the cells are protected from sheer strength[12]. Several investigators have described the induction of adult stem cell differentiation and the mass production of embryoid bodies following alginate encapsulation[13–15]. Alginate encapsulation has also been reported to increase the function of mature hepatocytes and maintain mature hepatocyte function within bioartificial livers[16]. However, studies describing the alginate encapsulation of embryoid-body cells derived from ES cells to generate differentiated hepatocytes in alginate microbeads have been limited.
In the present study, embryoid-body cells derived from ES cells were cultured in alginate microbeads with exogenous growth factors to promote hepatic histogenesis and characterized cells by evaluating the expression of hepatocyte-specific markers and function.
Materials and methods
Cell culture and formation of embryoid bodies The mouse ES cells line CCE was grown on mouse embryo fibroblasts treated with mitomycin C (Sigma, St Louis, MO, USA) in Dulbecco’s modified Eagle’s medium with high glucose (H-DMEM, GIBCO, Grand Island, NY, USA), supplemented with 15% fetal bovine serum, 100 mmol/L non-essential amino acids, 100 mmol/L 2-mercaptoethanol (GIBCO, USA), 2 mol/L L-glutamine (GIBCO, USA), 100 U/mL penicillin, and 100 U/mL streptomycin (all from GIBCO, USA). In order to maintain the ES cells in an undifferentiated state, we added 1000 U/mL mouse leukemia inhibitory factor (mL IF) (ESGRO, Chemicon, Temecula, CA, USA) to the culture medium. The media were changed every day. The cultures were split and passaged every 3 d. To induce the formation of embryoid bodies, undifferentiated cells were trypsinized and dissociated to single-cell suspension and then plated at 6×104 cells/mL in petri dishes in culture medium without mLIF. The media were changed every 48 h. All cell cultures were incubated in a humidified 37 °C, 5% CO2 environment. Only the ES cells between passages 10 and 20 were used for differentiation (Figure 1).
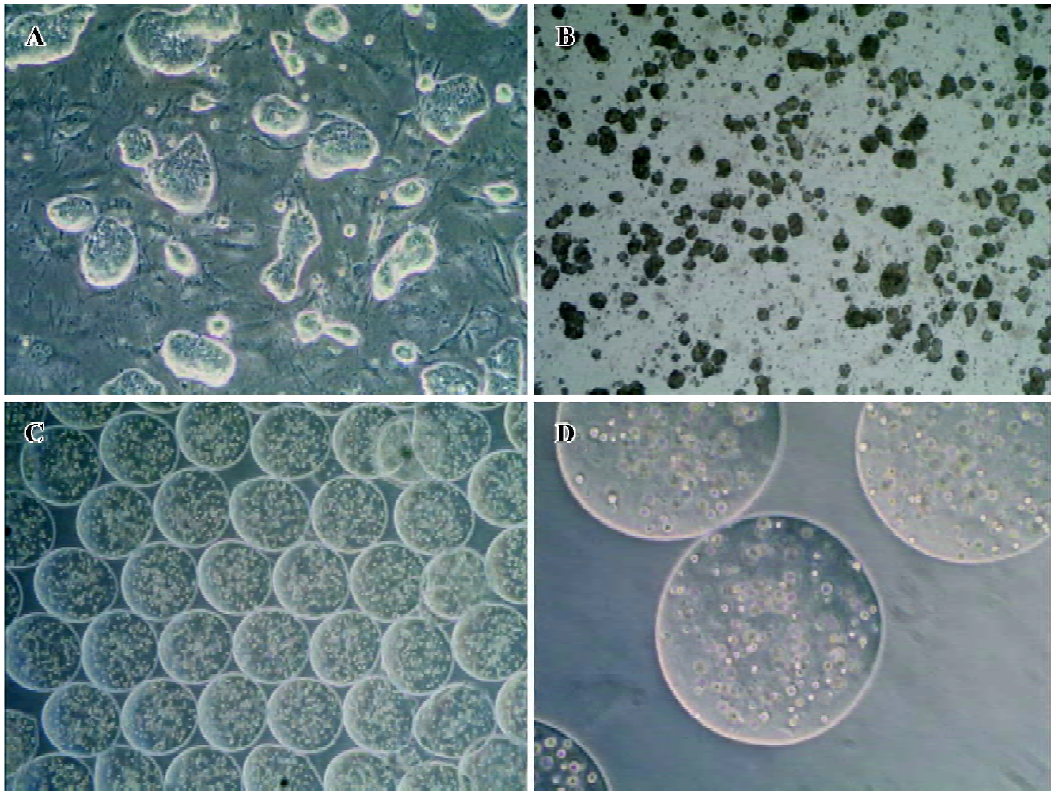
Encapsulation of embryoid-body cells and the induction of hepatic differentiation For the encapsulation in alginate microbeads, 5 d-old embryoid bodies were trypsinized with 0.25% trypsin-EDTA (GIBCO, USA), and 5×106 cells were mixed in 1 mL sterile filtered 2% alginate (Sigma, USA) dissolved in Ca2+ free DMEM (GIBCO, USA). The cell suspension was extruded through a droplet generator NISCO encapsulator (NISCO, Zurich, Switzerland). The beads formed were allowed to gel in a hardening bath (100 mmol/L CaCl2 [Sigma, USA], 10 mmol/L morpholinoethanesulphonic acid [MOPS; Sigma, USA], pH 7.4). After 10 min of hardening, the beads were washed 3 times with buffered saline (0.85% NaCl [Sigma, USA], 10 mmol/L 4-(2-hydroxyethyl)-1-pipera-zineethanesulfonic acid (HEPES) [Sigma, USA], pH 7.4). The beads were ultimately resuspended into H-DMEM medium containing 20% fetal bovine serum (without mLIF). The media were changed at every 3 days. The first day for embryoid-body cells embedded in alginate microbeads was designated as d 0. According to the method previously described with modification[8], several growth factors were added into the culture medium at varying days to induce the differentiation of hepatocytes, including acidic fibroblast growth factors (aFGF; R&D Systems, Minneapolis, MN, USA; 100 ng/mL) added between d 0 and 7, hepatocyte growth factor (HGF; R&D Systems, USA; 20 ng/mL) was then added between d 7 and 14, and oncostatin M (OSM; R&D Systems, USA; 10 ng/mL), dexamethasone (Sigma, USA; 10-7 mol/L), 5 mg/mL insulin, 5 mg/mL transferrin, and 5 µg/mL selenium (ITS, Sigma, USA) were added between d 10 and 14. The cells were cultured until d 14. As a control treatment for spontaneous differentiation, embryoid-body cells in alginate microbeads were cultured in vitro for 2 weeks in the absence of growth factors.
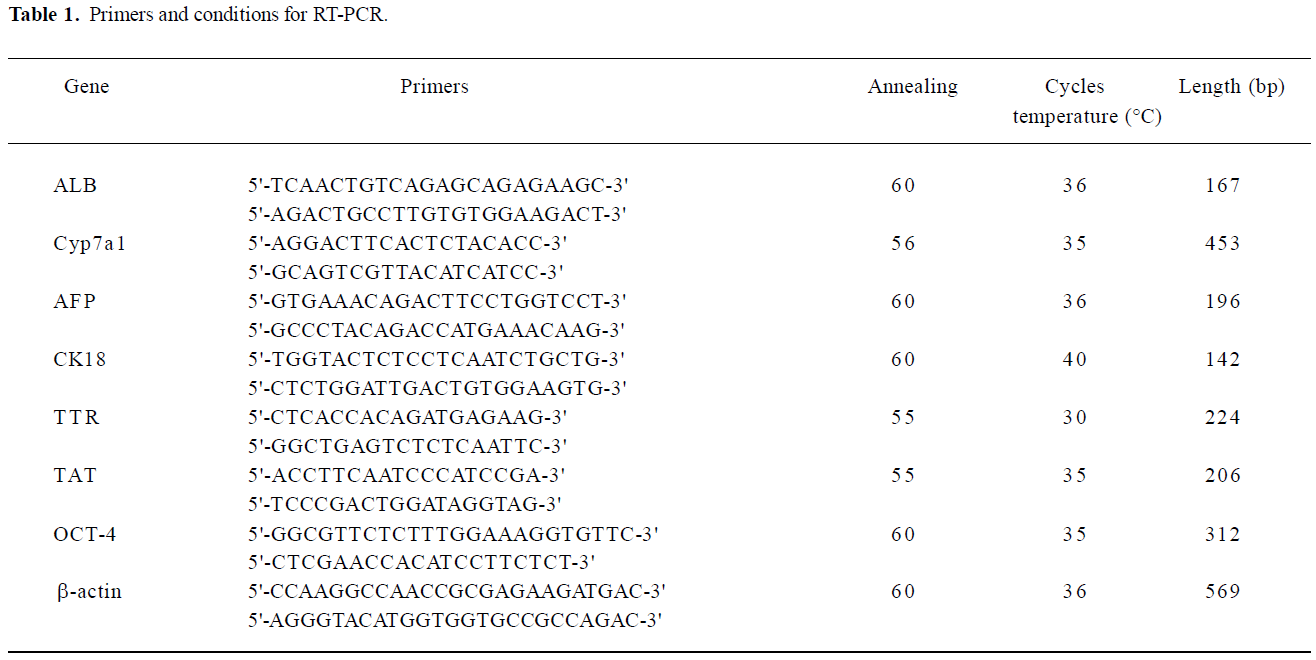
RT-PCR To extract RNA from encapsulated embryoid-body cells, the microbeads were washed with 0.1 mol/L phosphate buffered saline (PBS), and 55 mmol/L sodium citrate (Sigma, USA), containing 10 mmol/L MOPS (Sigma, USA), and 27 mmol/L NaCl (Sigma, USA) was added for 15 min at 37 °C to induce depolymerization. The released cells were then collected by centrifugation at 453×g (1500 r/min) for 5 min. Total RNA was extracted using Trizol reagent (GIBCO, USA) according to the manufacturer’s instructions. The concentration of RNA extracted was determined at wavelength of 260 nm using a biophotometer (Eppendorf, Netheler-Hinz, Hamburg, Germany). First-strand complementary DNA (cDNA) was synthesized by the reverse transcription system (Promega, Madison, WI, USA). Total RNA (1 µg) was reverse transcribed to first-strand cDNA in a 20 µL mixture containing 25 mmol/L MgCl2 (4 µL), reverse transcription 10×buffer (2 µL), 10 mmol/L dNTP mixture (2 µL), recombinant RNase inhibitor (0.5 µL), avian myeloblastosis virus (AMV) reverse transcriptase (15 U), and oligo (dT) 15 primers (0.5 µg). The reactions were incubated at 42 °C for 60 min, and then the samples were heated at 95 °C for 5 min. The PCR was performed by using a housekeeping gene β-actin as an internal standard. The total reaction volume was 50 µL for the PCR reaction, which was performed in a DNA thermal cycler (Supermix, GIBCO, USA). All primers were synthesized by Shanghai Sangon Biological Engineering Technology and Service (Shanghai, China). The primer sequences and PCR reaction conditions are shown in Table 1. The PCR samples, together with a 2000 bp DNA ladder, were analyzed by 2% agarose gel and visualized by ethidium bromide staining (Figure 2).
Full table
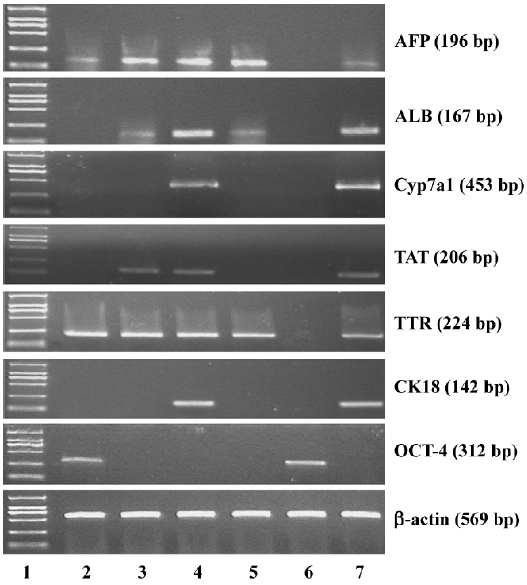
Immunofluorescence staining Albumin (ALB) and cytokeratin-18 (CK18) were used as markers of mature hepatocytes. Cells, including directed differentiated and spontaneous differentiated embryoid-body cells in alginate microbeads, were depolymerized and coated on the glass slides. The sections were fixed with acetone for 15 min at 4 °C and incubated in blocking buffer (0.1 mol/L PBS containing 0.5% normal goat serum and 0.2% Triton X-100) for 1 h and thereafter in primary antibodies overnight at 4 °C diluted in 0.1 mol/L PBS. The antibodies used in this study were rabbit anti-ALB (1:100 Abcam, Cambridge, UK) and mouse monoclonal anti-CK18 (1:100 Abcam, UK). The negative controls were processed using identical pro-cedures, except for incubation without the primary antibody. The secondary antibody used was immunoglobulin G fluorescein-isothiocyanate-conjugated goat antimouse (1:50, Sigma, USA) and tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat antirabbit (1:50, Sigma, USA). Incubation with the secondary antibody was performed at room temperature for 30 min.
ALB and urea production Conditioned media from embryoid-body cells in alginate microbeads (20 microbeads per well in a 24-well dish) cultured both with and without exogenous growth factors were collected on d 0, 4, 7, 10, and 14 and frozen at -20 °C until the assay. The conditioned media were assayed for ALB production using a quantitative ELISA kit (Bethel Laboratories, Montgomery, USA) according to the manufacturer’s recommendations. To analyze urea production, the embryoid-body cells in alginate microbeads (20 microbeads per well in a 24-well dish) were incubated with 1 mL medium containing 5 mmol/L NH4Cl (Sigma, USA) for 24 h in 5% CO2 at 37 °C on d 0, 4, 7, 10, and 14. Following this incubation, the supernatant was collected and the urea concentrations were measured through a colorimetric assay kit (Randox Laboratories, Antrim, UK). The culture medium was used as a negative control.
Results
Cell morphology and viability The cell morphology in the alginate microbeads was observed by the use of a contrast microscope (Olympus, Tokyo, Japan). Through our encapsulation system, the beads were round in shape, identical in size, with a mean diameter of 500–600 µm, and contained 600 cells in each bead. The cells were discretely scattered in the bead and there were no differences in shape and density throughout the 2-week experiment. Living cells appeared to be shiny in the alginate microbeads. Immediately following depolymerization, cell viability was determined by the trypan-blue exclusion test. In the alginate microbeads, the viability remained high (>90%) throughout the 2-week experiment. The duration of the in vitro culture had no significant effects on the viability of the cells.
Gene characterization of the embryoid-body cells in the alginate microbeads To determine the level of differentiation that occurred in the encapsulated cells, RNA from the capsules was extracted and gene characterization for liver-specific genes, including α-fetoprotein (AFP), ALB, Cyp7a1, CK18, transthyretin (TTR), and tyrosine aminotransferase (TAT) was performed. AFP is a marker of endodermal differentiation, as well as an early fetal hepatic marker, and its expression decreases as the liver develops into an adult phenotype. The expression of ALB, the abundant protein synthesized by mature hepatocytes, starts in early fetal hepatocytes and reaches the maximal level in adult hepatocytes. Cyp7a1 is expressed in the liver, but not in the yolk sac tissue, and thus it can be a good marker for hepatocytes[17]. CK18 is a cytoskeletal protein and is expressed in mature hepatocytes. TAT represents an excellent enzymatic marker for perinatal or postnatal hepatocyte-specific differentiation. Since hormone-regulated TAT activity is strictly limited to the parenchymal cells of the adult liver, it has been used extensively for monitoring cellular differentiation in experimental models for liver development/maturation in vitro. TTR represents endodermal differentiation and is first expressed in early embryos and throughout liver maturation[18].
In our results, embryoid-body cells in alginate microbeads on d 0 expressed mRNA of TTR and AFP. In the differentiating embryoid-body cells with growth factors, mRNA of AFP, ALB, TAT, and TTR were expressed on d 7. Cyp7a1 and CK18 were expressed until d 14, but in the embryoid-body cells without growth factors in the alginate microbeads, Cyp7a1, CK18, and TAT mRNA could not be detected on d 14. Thus, growth factors, such as aFGF, HGF and OSM seem to be preferable for inducing hepatic differentiation in encapsulated embryoid-body cells.
As expected, the pluripotent marker Oct-4 was expressed in undifferentiated ES cells. The gene was also expressed in embryoid-body cells in alginate microbeads on d 0, but the Oct-4 gene expression diminished completely on d 7.
Immunofluorescence staining To further confirm the hepatic differentiation from embryoid-body cells in the alginate microbeads, the expression of ALB and CK18 were analyzed by immunohistochemistry. The directed differentiated cells were stained positively for both CK18 and ALB (Figure 3) on d 14. Some of the positive cells were binuclear. From Figure 3, we can see that ALB and CK18 were found in the cytoplasm. The percentages of ALB- and CK18-positive cells were approximately 49%±3.8% and 50%±3.3% in direct differentiated embryoid-body cells, respectively.
ALB and urea production Spontaneous differentiation groups did not secrete ALB, whereas after induction of hepatic differentiation, ALB secretion increased significantly on d 10 and reached maximal values on d 14 (Figure 4A).
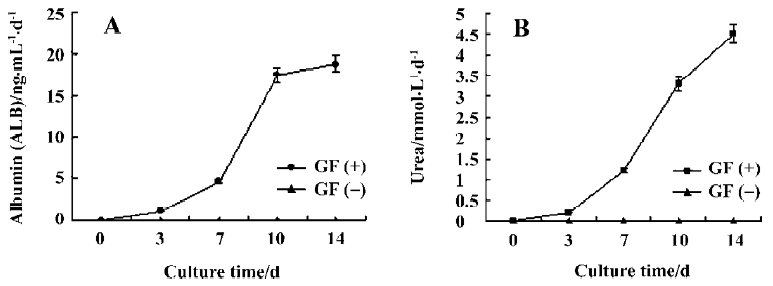
In addition, urea production was performed to confirm whether directed differentiated embryoid-body cells in alginate microbeads maintain hepatic metabolic functions. Urea production in spontaneous differentiated groups did not secrete urea. Directed differentiated embryoid-body cells produced urea on d 7. On d 14, the amount of urea produced by directed differentiated groups reached its maximum (Figure 4B).
Discussion
Previous studies of the role of alginate encapsulation in ES cell differentiation have provided conflicting results[15,19–21]. Recently, Dean et al reported whether ES cells or human ES cells, either in vivo or in vitro, differentiate spontaneously in alginate encapsulation[19]. Maguire et al reported similar results[20]. As we know, the efficiency of spontaneous ES cell differentiation into hepatocytes is very low. It is a good way to direct differentiated ES cells into hepatocytes by specific growth factor supplementation. Our study showed that the alginate microbeads maintained embryoid-body cell viability and gave rise to cells displaying gene expression patterns, morphological features, and metabolic activities characteristic of hepatocytes.
Previous studies have shown that adherent culture is needed to further hepatocyte differentiation after the formation of embryoid bodies, but adherent culture is limited by the absence of large-scale processing considerations. It has not been reported whether embryoid-body single cells keep viability in alginate encapsulation. Our results show that embryoid-body cells were cultured for 2 weeks with the medium changed every 2–3 d. During the 2 week period, the cell viability did not significantly decrease. The encapsulated embryoid-body cells did not form large cellular aggregates and remained in their current formation of single cells and/or in small clumps.
After demonstrating that embryoid-body cells can survive within the alginate microbeads, we evaluated the effect of alginate microbeads on hepatocyte differentiation from embryoid-body cells. It has been reported that ES cells differentiate to form embryoid bodies in sodium alginate at low concentrations, and sodium alginate at high concentrations inhibits the differentiation of ES cells[15]. Wang et al[21] also reported that the expression of ES cells markers remains high over 2 weeks of culture in alginate encapsulation in vitro. Our result showed that embryoid-body cells in alginate microbeads could not differentiate into functional hepatocytes during spontaneous differentiation, although hepatocyte genes, such as AFP and ALB were expressed. The hepatocyte-related genes are expressed not only in the liver, but also in the yolk sac of early embryos[22], but Cyp7A1 could not be detected, which was thought to be a liver-specific gene. This was similar to the results obtained by Asahina et al, who found that embryoid bodies in suspension culture could not differentiate into hepatocytes, and hepatocyte genes could be detected only in adherent culture[17].
Our next goal is to confirm whether embryoid-body cells in alginate microbeads can differentiate into hepatocytes by additional exogenous growth factors. Although the mechanism of ES cell differentiation remains unknown, many investigators have incorporated ES cell differentiation strategies into the generation of a renewable hepatocyte cell source using a variety of differentiation techniques[8,9,23–28]. The most abundant factors that were used were aFGF, HGF, and OSM, aFGF was added to imitate in vivo development, since it is secreted by the mesoderm and is the first factor to commit the foregut endoderm to form the liver primordium. HGF supports fetal hepatocytes during mid-stage hepatogenesis. OSM is produced by hematopoietic cells and induces the maturation of fetal hepatocytes[8,9,23,24]. Most of the differentiation strategies required embryoid body intermediates. Our results showed that embryoid-body cells in alginate microbeads could differentiate into functional hepatocytes by adding exogenous growth factors. This finding is noteworthy because it indicates the absence of transport limitations for exogenous growth factors in the alginate micro-beads, and embryoid-body cells in alginate microbeads can differentiate into hepatocytes by additional exogenous growth factors.
The benefits of the alginate encapsulation system in in vitro culture are numerous. Alginate microbeads can be depolymerized through the use of divalent cation chelators, facilitating rapid cell recovery for downstream analysis and application. Alginate microbeads, through variations in the encapsulation process (concentration, bead diameter, and cell seeding density) can discretely control key culture parameters. When alginate beads are applied for cell transplantation in vivo, they can easily be coated in a polylysine membrane for immune isolation.
In conclusion, our findings illustrate that the differentiation of embryoid-body cells into hepatocytes in alginate microbeads gives rise to functional hepatocytes containing exogenous growth factors. ES cells may provide an alternative source of hepatocytes for the development of the bioartificial liver system and hepatocyte transplantation.
References
- Stockmann HB, Jzermans JN. Prospects for the temporary treatment of acute liver failure. Eur J Gastroenterol Hepatol 2002;14:195-203.
- Strain AJ, Neuberger JM. A bioartificial liver — state of the art. Science 2002;295:1005-9.
- Abe K, Niwa H, Iwase K, Takiguchi M, Mori M, Abe SI, et al. Endoderm-specific gene expression in embryonic stem cells differentiated to embryoid bodies. Exp Cell Res 1996;229:27-34.
- Choi D, Lee HJ, Jee S, Jin S, Koo SK, Paik SS, et al. In vitro differentiation of mouse embryonic stem cells: enrichment of endodermal cells in the embryoid body. Stem Cells 2005;23:817-27.
- Yin Y, Lim YK, Salto-Tellez M, Ng SC, Lin CS, Lim SK. AFP(+), ESC-derived cells engraft and differentiate into hepatocytes in vivo. Stem Cells 2002;20:338-46.
- Fair JH, Cairns BA, Lapaglia M, Wang J, Meyer AA, Kim H, et al. Induction of hepatic differentiation in embryonic stem cells by co-culture with embryonic cardiac mesoderm. Surgery 2003;134:189-96.
- Gerecht-Nir S, Cohen S, Ziskind A, Itskovitz-Eldor J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol Bioeng 2004;88:313-20.
- Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, et al. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett 2001;497:15-9.
- Imamura T, Cui L, Teng R, Johkura K, Okouchi Y, Asanuma K, et al. Embryonic stem cell-derived embryoid bodies in three-dimensional culture system form hepatocyte-like cells in vitro and in vivo. Tissue Eng 2004;10:1716-24.
- Kanda S, Shiroi A, Ouji Y, Birumachi J, Ueda S, Fukui H, et al. In vitro differentiation of hepatocyte-like cells from embryonic stem cells promoted by gene transfer of hepatocyte nuclear factor 3 beta. Hepatol Res 2003;26:225-31.
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, et al. Development of definitive endoderm from embryonic stem cells in culture. Development 2004;131:1651-62.
- Goosen MF. Physico-chemical and mass transfer considerations in microencapsulation. Ann N Y Acad Sci 1999;875:84-104.
- Dang SM, Zandstra PW. Scalable production of embryonic stem cell-derived cells. Methods Mol Biol 2005;290:353-64.
- Ma HL, Hung SC, Lin SY, Chen YL, Lo WH. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res A 2003;64:273-81.
- Magyar JP, Nemir M, Ehler E, Suter N, Perriard JC, Eppenberger HM. Mass production of embryoid bodies in microbeads. Ann N Y Acad Sci 2001;944:135-43.
- Dvir-Ginzberg M, Gamlieli-Bonshtein I, Agbaria R, Cohen S. Liver tissue engineering within alginate scaffolds: effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng 2003;9:757-66.
- Asahina K, Fujimori H, Shimizu-Saito K, Kumashiro Y, Okamura K, Tanaka Y, et al. Expression of the liver-specific gene Cyp7a1 reveals hepatic differentiation in embryoid bodies derived from mouse embryonic stem cells. Genes Cells 2004;9:1297-308.
- Lavon N, Benvenisty N. Study of hepatocyte differentiation using embryonic stem cells. J Cell Biochem 2005;96:1193-202.
- Dean SK, Yulyana Y, Williams G, Sidhu KS, Tuch BE. Differentiation of encapsulated embryonic stem cells after transplantation. Transplantation 2006;82:1175-84.
- Maguire T, Novik E, Schloss R, Yarmush M. Alginate-PLL microencapsulation: Effect on the differentiation of embryonic stem cells into hepatocytes. Biotechnol Bioeng 2006;93:581-91.
- Wang X, Wang W, Ma J, Guo X, Yu X, Ma X. Proliferation and differentiation of mouse embryonic stem cells in APA micro-capsule: A model for studying the interaction between stem cells and their niche. Biotechnol Prog 2006;22:791-800.
- Sellem CH, Frain M, Erdos T, Sala-Trepat JM. Differential expression of albumin and alpha-fetoprotein genes in fetal tissues of mouse and rat. Dev Biol 1984;102:51-60.
- Baharvand H, Hashemi SM, Kazemi Ashtiani S, Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol 2006;50:645-52.
- Hu AB, Cai JY, Zheng QC, He XQ, Shan Y, Pan YL, et al. High-ratio differentiation of embryonic stem cells into hepatocytes in vitro. Liver Int 2004;24:237-45.
- Kania G, Blyszczuk P, Jochheim A, Ott M, Wobus AM. Generation of glycogen- and albumin-producing hepatocyte-like cells from embryonic stem cells. Biol Chem 2004;385:943-53.
- Kuai XL, Cong XQ, Li XL, Xiao SD. Generation of hepatocytes from cultured mouse embryonic stem cells. Liver Transpl 2003;9:1094-9.
- Teratani T, Yamamoto H, Aoyagi K, Sasaki H, Asari A, Quinn G, et al. Direct hepatic fate specification from mouse embryonic stem cells. Hepatology 2005;41:836-46.
- Jochheim A, Hillemann T, Kania G, Scharf J, Attaran M, Manns MP, et al. Quantitative gene expression profiling reveals a fetal hepatic phenotype of murine ES-derived hepatocytes. Int J Dev Biol 2004;48:23-9.
