Triptolide inhibits NF-κB activation and reduces injury of donor lung induced by ischemia/reperfusion
Introduction
The ischemia/reperfusion-induced injury of donor lungs is an important pathological process which influences the survival rate of donor lungs after transplantation. It is believed that the injury is mainly caused by inflammatory reactions[1]. The responses of ischemia/reperfusion-induced inflammation include the induction of adhesion molecules, infiltration of immune blood cells, and the release of cytokines. NF-κB is a key regulator of many inflammatory genes[2,3]. It plays an important role in modulating the expression of varieties of inflammatory cytokines. Our previous studies have reported that inhibiting NF-κB reduces the expression of inflammatory and apoptotic genes and protects endothelial and neuronal cells from apoptosis[4,5]. The Chinese herb Tripterygium, which is produced in the south of China, has the ability to control organ rejection and is highly considered an ideal drug. Triptolide (TRI) has been reported as a strong inhibitor of NF-κB[6,7] and protects donor hearts against ischemia/reperfusion-induced injury in heart transplantation[8]. NF-κB inhibitors were used during lung preservation and reperfusion periods to observe its influence on lung functions. This study sought to investigate the protective effect of TRI on ischemia/reperfusion-induced injury of transplanted rabbit lungs. As the results showed, TRI inhibited NF-κB-mediated immunoresponses and lung injury induced by ischemia/reperfusion.
Materials and methods
Reagents TRI and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (St Louis, MO, USA); the ELISA kit of intercellular adhesion molecule-1 (ICAM-1) was purchased from Xitang Biotechnology (Shanghai, China), and the NF-κB activity detecting kit was purchased from Boster Biotechnology (Wuhan, China).
Animals and grouping In total, 36 healthy adult rabbits of both sexes, weighing 2.5±0.5 kg were randomly divided into 3 groups. In each group, 6 rabbits were prepared as donor lungs, and the other 6 were the recipients. In group I (control, Con), the lungs were perfused and preserved in improved low-potassium dextran (LPD) solution and were used as the negative control. In group II (LPS), the lungs were perfused and preserved in LPD solution containing LPS (50 µg/L) and were used as the positive control. In group III (TRI), the lungs were perfused and preserved in LPD solution containing TRI (0.5 mg/L).
Preparation of the donor lungs The rabbits were anesthetized with 3% pentobarbital (45 mg/kg, ip). After tracheotomy was performed, the rabbits received mechanical ventilation at a rate of 32/min, tidal volume (VT) = 15 mL/kg, FiO2 = 60%. After median sternotomy, the pericardium was opened, and the pulmonary artery and ascending aorta were separated and cannulized. Then they were heparinized by injecting heparin (3 mg/kg) into the left auricle. After that, the pulmonary conus were transversely cut and a catheter was inserted via the left auricle. The hilum of right lung was ligated, then the lung was infused with TRI or LPS with a perfusion pressure of 60 cm H2O at 4 ºC. The infusion ended when the effluent from the left auricle became clear. After infusion, the left lung was retrieved as the donor lung and soaked in the corresponding preservation solution at 4 ºC for 4 h.
Transplantation of the donor lungs The recipient rabbits were anesthetized and thoracotomy was also performed. The inferior vena cava was dissociated and cannulized. Heparin was injected into the left auricle at a dose of 3 mg/kg. The inferior vena cava was slit at the distal end, ligated at the proximal end, and then connected to the donor pulmonary artery via a pump. The recipients? left auricle was connected with that of the donor. The blood circulation was resumed by pumping blood from the inferior vena cava into the donors? pulmonary artery at a flow rate of 10 mL/min, and flowed back to recipients? heart through a left auricle adaptor. The reperfusion continued for 1 h.
Measuring the levels of PvO2 of the donor lungs During the circulated perfusion, the PvO2 of vein blood gas of the dissociated lungs after regurgitation was measured. Changes of the PvO2 levels were recorded every 15 min by a blood gas analyzing machine (Radiometer ABL5, Denmark), it lasted for 1 h.
Measuring the wet weight of the donor lungs After reperfusion for 1 h, the donor lungs were taken out and weighed. They were then put into an 80 ºC oven for 48 h and weighed again after drying. The percentage of the wet weight was calculated according to the data. The formula was: wet weight (%) = (total weight–dried weight)/total weight.
Measuring the activity of myeloperoxidase of the donor lungs Detection of myeloperoxidase (MPO) activity was performed using the Myeloperoxidase (MPO) Detection Kit (Nanjing Jiancheng Bioengineering Institute, China. Approximately 0.05 g of lung tissue was dissected and rinsed with 0.9% NaCl to remove blood contamination; 5% homogenate was made according to its mass ratio. The procedure was done according to the protocol provided by the kit. Absorbency was measured (A) on a spectrophotometer at 460 nm and calculated the activity of MPO.
Measuring the concentrations of the ICAM-1 of the donor lungs The ELISA method was adapted to measure the ICAM-1 by determining the absorbency at 492 nm. Approximately 10 mg of tissue of the donor lungs was processed following the protocol of the manufacturer. The concentration of the ICAM-1 was calculated according to the standard curve, which was made from a series of different known concentrations of standard bovine serum albumin.
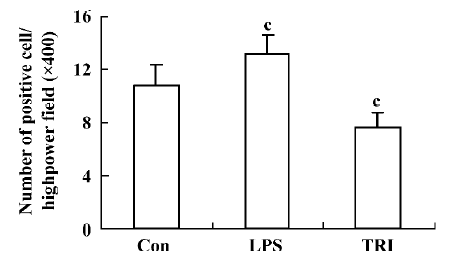
Measuring the expression of NF-κB of the donor lungs Immunohistochemistry was performed using the Strep-tavidin biotin complex kit to measure the expression of NF-κB. The first antibody was the anti-NF-κB (P65) mono-clonal antibody with a dilution of 1:100. Positive cells were identified as those whose nucleus had yellow-brown grains. Eight sections from each group were chosen, and 5 fields were observed randomly in each section. Then the number of positive cells was counted under high-power fields (?400). The average count of 5 fields was taken as the result.
Observing the ultrastructure of the donor lungs After reperfusion, 1 cm3 of tissue from the left lung was dissected and fixed in 4% glutaraldehyde to make electron microscopic samples. The changes in the ultrastructure were observed by using the H600 transmission electron microscope (Hitachi, Japan).
Statistical analysis The experimental data were shown as mean±SD. An analysis of these data was performed with ANOVA using the SPSS 12.0 statistical package (SPSS, Chicago, IL, USA). P-values of less than 0.05 were accepted as statistically significant.
Results
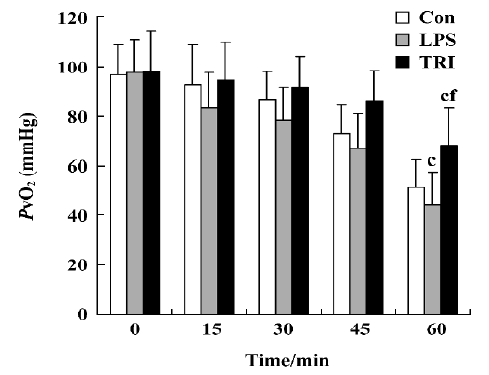
Effect of TRI on the levels of PvO2 in the donor lungs As the time of reperfusion was prolonged, the levels of PvO2 in the donor lungs of all groups decreased. In group I, the levels PvO2 decreased starting at 30 min after reperfusion and decreased continuously thereafter. In group II, significant decreases of the levels PvO2 were observed 15 min after reperfusion. On the other hand, although the levels of PvO2 were also reduced during reperfusion in group III, these changes were less intense than those in groups I and II which had statistical significance at the end of reperfusion (P<0.01; Figure 1).
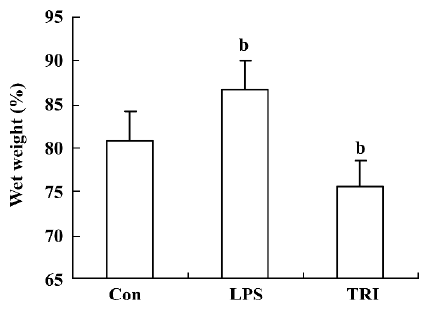
Effect of TRI on pulmonary edema of the donor lungs Pulmonary edema of the donor lungs in group II was higher than that in group I (P<0.05). In contrast, in group III, pulmonary edema was significantly lower compared to group I (P<0.05; Figure 2).
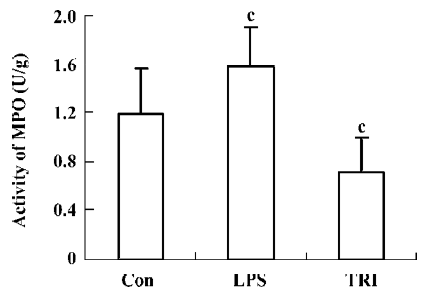
Effect of TRI on MPO activity in the donor lungs The MPO activity in group II was significantly higher than that in group I (P<0.01), while in group III, it was lower than that in group I (P<0.01; Figure 3).
Effect of TRI on NF-κB activity in the donor lungs The NF-κB immunoreactivity in group II was significantly higher than that in group I (P<0.01), while in group III, NF-κB immunoreactivity was lower than that in group I (P<0.01; Figure 4).
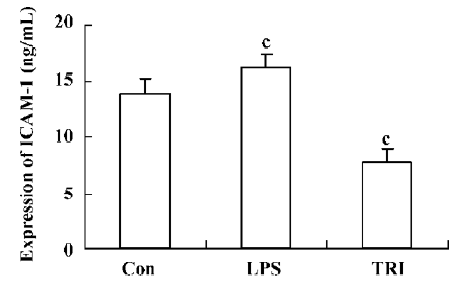
Effect of TRI on the expression of ICAM-1 in the donor lungs The levels of ICAM-1 in group II significantly increased compared with that in group I (P<0.01). However, compared with that in group I, the levels of ICAM-1 in group III decreased significantly (P<0.01; Figure 5).
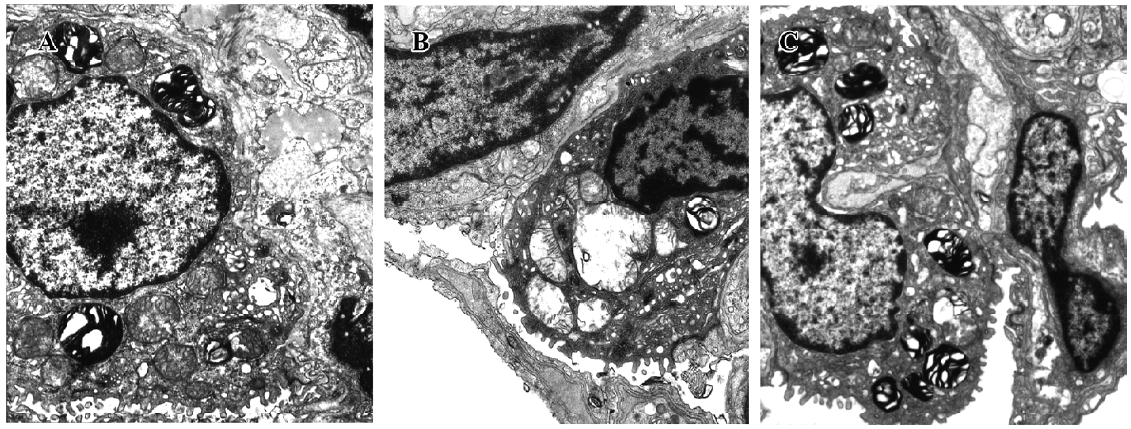
Effect of TRI on the ultrastructural changes in the donor lungs In group I, the microvilli of the type II pneumocyte were still dense, and the nucleus remained normal. Most of the mitochondria cristae in the cytoplasm were satiated and some mitochondria swelled occasionally. The endoplasmic reticulum did not enlarge. The osmiophilic multilamellar body was kept intact and the vessle structure integrity was maintained (Figure 6A). In group II, the microvilli of the type II pneumocyte were sparse. The nuclear chromatin was marginated and the mitochondria swelled significantly; their cristae were short and scarce. The morphology of some mitochondria changed and appeared vacuole-like. The osmium-philic multilamellar body developed poorly, and the vascular endothelial cells also swelled. The endoplasmic reticulum enlarged slightly (Figure 6B). In group III, the microvilli of the type II pneumocyte and the nuclear structure were normal. The mitochondria in the cytoplasm swelled slightly. The osmiumphilic multilamellar body was intact. The microvilli of the type I pneumocyte and the nuclear structure were also normal; the mitochondria and endoplasmic reticulum enlarged slightly (Figure 6C).
Discussion
Lung transplantation is becoming an effective means for the treatment of lung diseases in the terminal stage. The protection of the functions of donor lungs is one of the critical factors that determine the success of lung transplantation[9,10]. Most deaths occur within 30 d after transplan-tation. The main cause of death during the first postoperative month is non-specific graft failure secondary to ischemia/reperfusion-induced lung injury[11,12]. Many attempts have made to reduce injury, including improvement of preservation solutions, adequate setting of flushing solutions, reperfusion pressure and ventilation, inhaled nitric oxide, administration of prostaglandins, complementary inhibitors, the antagonists of the platelet-activating factor, and surfactant enhancers[13]. Generally, donor lungs can be safely preserved for 4?6 h. Among those that died after lung transplantation, 15%?20% died from lung injury induced by ischemia/reperfusion[14]. Lung injury induced by ischemia-reperfusion is characterized by increased pulmonary vascular resistance, decreased oxygenation capacity, worsened compliance, and edema formation[15]. Multiple mechanisms are involved in ischemia/reperfusion-induced lung injury. It is generally believed that the injury is a process of inflammatory reaction, which includes the activation of endothelial, the expression of adhesion molecules, and also the adherence, aggregation, and activation of leucocytes and platelets, as well as the release of free radicals. In these inflammatory reactions, the transcription factor NF-κB is the key regulator which controls the expression of many genes involved in inflammation, such as the activation of the endothelium and adherence of molecules. Therefore, NF-κB has become a new target for anti-inflammatory treat-ments[16].
Tripterygium extracts have been used widely to treat inflammatory and autoimmune diseases such as rheumatoid arthritis and immune complex nephritis in China[17,18]. In organ transplantation, both clinical and experimental studies have demonstrated that Tripterygium extracts effectively prolong allograft survival[19]. TRI is an active ingredient extracted from Tripterygium wilfordii multiglycoside[20]. It is a colorless, needle-like crystal, with a relative molecular weight of 360 g/mol; its molecular formula is C2OH24O6. It has complex chemical compositions and diverse pharmacological actions. It is TRI that makes Tripterygium wilfordii multiglycoside an effective immunosuppressant. The immunosuppressive effects of TRI can be partially attributed to its potent inhibition of T cell activation and interleukin-2 production[21]. Remarkably, the inhibitory effect of TRI on T cell activation has been shown to be more potent than that exhibited by cyclosporine[22] and Tacrolimus (FK-506)[23].
MPO is a specific enzyme in the cytoplasm of neutrophils, which induces the activation and aggregation of neutrophils. The changes in MPO in the lung can reflect the levels of neutrophil aggregation in the lung after reperfusion. This study also found that the levels of PaO2 decreased after reperfusion, the activities of MPO increased, and the structure was damaged in the donor lungs. These pathological changes in the donor lungs were exacerbated by LPS treat-ment. In contrast, these pathological changes in the TRI-treated group were improved, suggesting that TRI can protect donor lungs against ischemia/reperfusion-induced injury. The present study further demonstrated that the ischemia/reperfusion-induced elevation in NF-κB and the NF-κB target gene ICAM-1 significantly decreased by TRI. These results suggest that TRI may reduce injury of the donor lungs induced by ischemia/reperfusion through inhibiting the activity of NF-κB and reducing the expression of ICAM-1. Along with other studies[24,25], TRI may have a wide application in organ transplantation as an anti-inflammatory and immunosuppressant. However, TRI also has toxicity, just as many Chinese medicines do. It affects the digestive system, genital system, hematopoietic system, and so on. Thus, it is better to extract its effective monomer component to study its metabolic process so as to raise its pharmacodynamic action and reduce its toxicity.
References
- Peralta C, Fernández L, Panés J, Prats N, Sans M, Piqué JM, et al. Preconditioning protects against ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selection up-regulation in the rat. Hepatology 2001;33:100-13.
- Azzolina A, Bongiovanni A, Lampiasi N. Substance P induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells. Biochem Biophys Acta 2003;1643:75-83.
- Jiang B, Xu S, Brecher P, Cohen RA. Growth factors enhance interleukin-1 beta-induced persistent activation of nuclear factor-kappa B in rat vascular smooth muscle cells. Aterioscler Thromb Vasc Biol 2002;22:1811-6.
- Qin ZH, Wang YM, Nakai M, Chase TN. Nuclear factor-κB contributes to excitotoxin-induced apoptosis. Mol Pharmacol 1998;53:33-42.
- Qin ZH, Chen RW, Wang YM, Nakai M, Chuang DM, Chase TN. NF-κB nuclear translocation up-regulates c-Myc and p53 during N-methyl-D-aspartate receptor-mediated apoptosis. J Neurosci 1999;19:4023-33.
- Hu K, Liu Z, Liu D. Triptolide inhibits vascular endothelial growth factor expression and production by endothelial cells. Zhonghua Yixue Zazhi 2001;81:1106-9. Chinese..
- Liu H, Liu ZH, Chen ZH, Yang JW, Li LS. Triptolide: a potent inhibitor of NF-kappa B in T-lymphocytes. Acta Pharmacol Sin 2000;21:782-6.
- He JK, Gu ZL, Yu SD, Shen ZY. Triptolide on inhibition of reperfusion injury and prolongation of allograft survival in rat cardiac transplants. Chin J New Drugs Clin 2001;20:423-6. Chinese..
- Luh SP, Tsai CC, Shau WY, Shiau SY, Jan JS, Kuo SH, et al. Protective effects of inhaled nitric oxide and gabexate mesilate in lung reperfusion injury after transplantation from non-heart-beat donors. J Heart Lung Transplant 2002;21:251-9.
- Rega FR, Wuyts WA, Vanaudenaerde BM, Jannis NC, Neyrinck AP, Verleden GM, et al. Nebulized N-acetyl cysteine protects the pulmonary graft inside the non-heart-beating donor. J Heart Lung Transplant 2005;24:1369-77.
- Thabut G, Vinatier I, Stern JB, Leseche G, Loirat P, Fournier M, et al. Primary graft failure following lung transplantation. Predictive factors of mortality. Chest 2002;121:1876-82.
- Hertz MI, Taylor DO, Trulock EP, Boucek MM, Mohacsi PJ, Edwards LB, et al. The registry of the international society for heart and lung transplantation: nineteenth official report — 2002. J Heart Lung Transplant 2002;21:950-70.
- Perrot M, Liu M, Weddel K, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med 2003;167:490-511.
- Haydock DA, Trulock EP, Kaiser LR, Knight SR, Pasque MK, Cooper JD. Management of dysfunction in the transplanted lung: experience with 7 clinical cases. Washington University Lung Transplant Group. Ann Thorac Surg 1992;53:635-41.
- Mathias MA, Tribble CG, Dietz JF, Nguyen RP, Shockey KS, Kern JA, et al. Aprotinin improves pulmonary function during reperfusion in an isolated lung model. Ann Thorac Surg 2000;70:1671-4.
- Schmidt C, Peng B, Li Z, Sclabas GM, Fujioka S, Niu J. Mechanisms of pro-inflammatory cytokine-induced biophasic NF-κB activation. Mol Cell 2003;12:1287-300.
- Gao ZG, Zang AC, Bai RX. Radix, Tripterygium wilfordii Hook F in rheumatoid arthritis, ankylosing spondylitis and juvenile rheumatoid arthritis. Chin Med J 1986;99:317-20.
- Jiang X. Clinical observations on the use of the Chinese herb Tripterygium wilfordii Hook for the treatment of nephrotic syndrome. Pediatr Nephrol 1994;8:343-4.
- Wang J, Xu R, Jin R, Chen Z, Fidler JM. Immunosuppressive activity of the Chinese medicinal plant Tripterygium wilfordii. I. Prolongation of rat cardiac and renal allograft survival by the PG27 extract and immunosuppressive synergy in combination therapy with cyclosporine. Transplantation 2004;70:447-55.
- Gu WZ, Chen R, Brandwein S, McAlpine J, Burres N. Isolation, purification, and characterization of immunosuppressive compounds from Tripterygium: triptolide and tripdiolide. Int J Immunopharmacol 1995;17:351-6.
- Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, . Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin- 2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-κB transcriptional activation. J Biol Chem 1999; 274: 13 443-50.
- Lu H, Hachida M, Enosawa S, Li X, Suzuki S, Koyanagi H. Immunosuppressive effect of triptolide in vitro. Transplant Proc 1999;31:2056-7.
- Chan M, Kohlmeier JE, Branden M, Jung M, Benedict SH. Triptolide is more effective in preventing T cell proliferation and interferon-gamma production than is FK506. Phytother Res 1999;13:464-7.
- He X, Verran D, Hu C, Wang C, Li L, Wang L, et al. Synergistic effect of Tripterygium wilfordii Hook F) (TWHF) and cyclosporin A in rat liver transplantation. Transplant Proc 2000;32:2054.
- Fidler JM, Ku GY, Piazza D, Xu R, Jin R, Chen Z. Immunosuppressive activity of the Chinese medicinal plant Tripterygium wilfordii III suppression of graft-versus-host disease in murine allogeneic bone marrow transplantation by the PG27 extract. Transplantation 2002;74:445-57.