Heat shock protein 90 acts as a molecular chaperone in late-phase activation of extracellular signal-regulated kinase 1/2 stimulated by oxidative stress in vascular smooth muscle cells1
Introduction
Reactive oxygen species (ROS) is known to be involved in the pathological processes of many cardiovascular diseases, such as atherosclerosis, hypertension, and restenosis. Our previous study in cultured rat vascular smooth muscle cells (VSMC) showed that the intracellular increase of superoxide anion by exposure to LY83583 led to an early transient (10 min) and late sustained activation (120 min) of extracellular signal-regulated kinase (ERK) 1/2, and stimulated VSMC proliferation[1]. Further experiments demonstrated that the early phase was directly activated by ROS and the late was mediated via secreted oxidative-stressed factors involving heat shock protein 90 (HSP90)[1,2]. However, the mechanism of HSP90 mediating ERK1/2 activity needs to be explored further.
HSP90 is known as a molecular chaperone and associates with various proteins, including transcription factors and protein kinases, and plays an important role in the activity[3,4], stability[5-7], and intracellular distribution[8] of its associated partner molecules. It has been reported that HSP90 could associate with mitogen-activated protein kinases, such as MAPK/ MAK/ MRK overlapping kinase (MOK)[5] and Raf[6,8,9] which are up-streams of ERK1/2. In addition, HSP90 could also promote the solubility of its partner molecules and enhance the nuclear translocation of its partner mol-ecules.
ERK1/2 is ubiquitous cytosolic serine-threonine kinase and mediates cell growth and proliferation. ERK1/2 is activated by various stresses and growth stimuli and is involved in signaling transduction from cytoplasm into cell nuclei[5,11]. It is well known that the phosphorylation of ERK2 promotes its dimerization and nuclear translocation, and the increase of ERK1/2 solubility facilitates the nuclear translocation of itself[12]. Once entering cell nuclei, phosphor-ERK1/2 activates transcription factors Elk-1. Thus, the solubility level and nuclear-translocation ability of phosphor-ERK1/2 are 2 determinant events in cell growth[13].
Therefore, we propose that HSP90 acts as a molecular chaperone, mediates late-phase ERK1/2 activation and cell proliferation by associating with phosphor-ERK1/2, and increases the solubility and nuclear translocation of phosphor-ERK1/2.
Materials and methods
Materials Sprague-Dawley (SD) rats (150 g) were obtained from the Experimental Animal Center, Nanhua University (Hengyang, China, SPF grade, Certificate N
Cell culture and experiment grouping Rat VSMC were isolated from the aorta of 150 g male SD rats and cultured by an expellant method originally described by Campbell and Campbell[14]. The culture medium was DMEM containing 10 mmol/L sodium pyruvate supplemented with 20% FBS. Then the cells were maintained in 10% FBS and up to passage 12 when used. After confluence, the cells were inoculated on 100 mm dishes. The cells were growth-arrested by incubation in 0.1% FBS/DMEM for 24 h prior to use. VSMC were identified with an anti-actin antibody by immunohistochemical stain (S-P). First, the VSMC were treated with LY83583 [6-anilinoquinoline-5,8-quinoli-nedione, produce ROS, 1 µmol/L, dissolved in phosphate-buffered saline (PBS)] for different times (0, 5, 10, 30, 60, 90, 120, and 180 min) to determine the late peak of phosphor-ERK1/2. Then different treatments were classified as the following groups: control group (PBS 120 min), LY group (1 µmol/L LY83583 for 120 min), Gel+ DMSO+LY group (pretreated with geldanamycin which was dissolved in DMSO for 30 min, then treated with 1 µmol/L LY83583 for 120 min), and the DMSO+LY group (pretreated with DMSO, the vehicle of geldanamycin, for 30 min, then treated with 1 µmol/L LY83583 for 120 min).
Extraction of the total, soluble, insoluble, and nuclear protein The cells were harvested following 2 quick rinses in PBS (pH 7.4) in ice-cold lysis buffer (50 mmol/L Tris-base, pH 7.4, 150 mmol/L NaCl, 10% glycerol, 1 mmol/L O,O'-Bis (2-aminoethyl) ethyleneglycol-N,N,N',N'-tetraacetic acid (EGTA), 1 mmol/L Na-or-thovanadate, 5µmol/L ZnCl2, 100 mmol/L NaF, 10 mmol/L aprotinin, 1 mmol/L leupeptin, 1µmol/L phenylmethylsulfonyl fluoride, and 1% Triton X-100) and homogenized by pulling through a 21 Ga needle 20 times. The detergent was omitted from the lysate buffer by centrifugation at 10 000×g at 4 ℃ for 20 min and the protein concentration of the supernatant was determined[16].
The growth-arrested VSMC were divided into 4 groups as described before. The total, soluble, insoluble and nuclear protein was extracted as described previously[2]. In brief, the cells were scraped in a buffer containing 137 mmol/L NaCl, 2.7 mmol/L KCl, 4.3 mmol/L Na2HPO4, 1.4 mmol/L KH2PO4, 20 µg/mL leupeptin, 1 mmol/L sodium orthovanadate, and 400 µmol/L phenylmethylsulfonyl fluoride, and frozen at -20 ℃ for 4 times, then centrifuged at 17 000×g for 60 min. The supernatants were recovered as soluble fractions, and the precipitates were redissolved by adding diluted (5:7) SDS sample buffer and then boiled for 5 min to obtain insoluble fractions.
The nuclear extracts were prepared as described by Backlund et al[16]. In brief, the cells were harvested in ice-cold PBS and pelleted by brief centrifugation (1000×g). The supernatant was discarded and the cell pellet was washed once in hypotonic buffer [10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.2 mmol/L phenylmethylsulfonyl fluoride, and 0.5 mmol/L 1,4-dithiothreitol (DTT)]. The cells were resuspended in hypotonic buffer and allowed to swell on ice for 10 min before homogenization in a glass Dounce homogenizer with 14 up-and-down strokes, using a B-type pestle. The nuclei were collected by centrifugation at 3300×g for 15 min and resuspended in 200 µL low salt buffer (20 mmol/L HEPES, pH 7.9, 25% glycerol, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.2 mmol/L EDTA, 0.2 mmol/L phenylmethyl-sulfonyl fluoride, and 0.5 mmol/L DTT). KCl (2.5 mol/L) was added dropwise to a final concentration of 0.4 mmol/L, and the nuclei were incubated for 30 min with continuous gentle mixing. The extracted nuclei were pelleted by centrifugation at 25 000×g for 30 min at 4 ℃. The resulting supernatant (nuclear extract) was aliquoted. The protein concentration was determined by bicinchoninic acid (BCA) protein assay. All of the lysates were immediately frozen at -80 ℃.
Western blotting analysis and immunoprecipitation For Western blotting, the cell lysates containing equal amounts of proteins were added with 5×sample buffer (0.31 mol/L Tris base, pH 6.8, 2.5% SDS, 50% glycerol, and 0.125% bromophenol blue) and boiled for 3 min. The protein was resolved on 10% SDS-PAGE. The protein was transferred to polyvinylid-ene fluoride (PVDF) film. Then the film were blocked in 5% skim milk in Tris-buffered saline Tween (TTBS) (50 mmol/L Tris-HCl, pH 8.3, 200 mmol/L NaCl, and 0.05% Tween-20). The filters were incubated with anti-rat HSP90 (1:1000), ERK1/2 (1:1200), and phosphor-ERK1/2 (1:1500) antibodies for 120 min at 37 ℃ or 4 ℃ overnight, detected by HRP-conjugated second antibodies, and then detected by an enhanced chemiluminescence reagent[17].
For immunoprecipitation, total cell lysates containing equal amounts of proteins were incubated with an anti-HSP90 antibody for 45 min at 4 ℃. The antibody-protein complexes were incubated with protein A — agarose for 20 min at 4 ℃, and the antibody-protein complexes that were bound to the beads were pelleted at 2000×g for 2 min. The beads were washed 3 times with lysis buffer and once with PBS, resuspended in the sample buffer, and immediately frozen at -80 ℃ [18].
Immunofluorescence The prepared cells were washed in PBS 3 times for 5 min, permeabilized with 0.01% Triton X-100 in PBS for 1 min and 100% methanol for 3 min at -20 ℃, and then blocked with 1% goat serum and PBS (pH 7.5) for 30 min . The cells were incubated with a phosphor-ERK1/2 antibody in 1% goat serum and PBS (pH 7.5) for 60 min. After washing with PBS (pH 7.5) 3 times for 10 min, the cells were incubated with the FITC second antibody in 1% goat serum and PBS (pH 7.5) for 60 min and washed with PBS (pH 7.5) 3 times for 10 min[15].
Cell counting and MTT assay For determining number of living cells, a modified MTT assay was performed as described previously before[1]. Briefly, VSMC (1?105 cells/mL) were grown in 96-well plates for 24 h. After incuba-tion with agonists for 2 h, cells were treated with MTT (0.5 mg/mL) for 4 h at 37 ℃. The cell culture medium was removed, and cells were lysed by addition of 100 μL of isoamyl alcohol. The metabolized MTT was evaluated by optical dencity (OD) in an enzyme-linked immuno-sorbent assay reader at 570 nm. The proliferation ratio=(cell counting or OD of the experimental group cell counting or OD of the control group)/cell counting or OD of control group?100%.
Statistical analysis All data were expressed as mean±SD. The statistical analysis of the data was performed using ANOVA as appropriate. Values with P<0.05 were considered to be statistically significant.
Results
Oxidative stress increased cytosolic HSP90 in rat VSMC Brief exposure of VSMC to LY83583 stimulated ERK1/2 activity with peaks at 10 and 120 min. The late peak was responsible for secreted oxidative stress-induced factors in the conditioned medium that included HSP90, cyclophilin A, and cyclophilin B[1]. In this present experiment, the VSMC were exposed to LY83583 for 0-180 min. Figure 1 shows that 1µmol/L LY83583 treatment stimulated ERK1/2 activity and HSP90 expression in the total cell lysates. The ERK1/2 activation peaked at 10 and 120 min, respectively. However, the LY83583-induced HSP90 expression peaked only at 120 min, which was consistent with the late peak of phosphor-ERK1/2 (Figure 1).
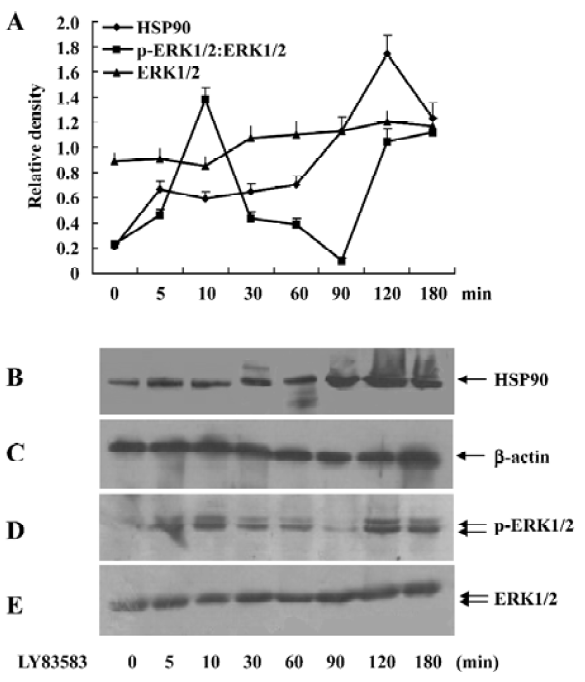
HSP90 mediates LY83583-induced ERK1/2 activation To examine the role of cytosolic HSP90 in LY83583-induced ERK1/2 activation, the VSMC were pretreated with a specific HSP90 inhibitor geldanamycin (1, 5, and 10 µmol/L) for 30 min and then exposed to LY83583 for 120 min. The data showed that LY83583 significantly increased total phosphor- ERK1/2 by 2.5-fold, and geldanamycin inhibited the effect of LY83583 in a dose-dependent manner (Figure 2).
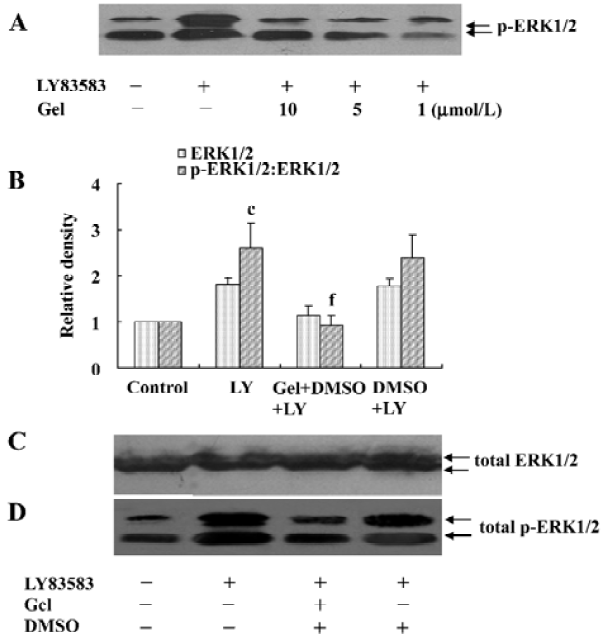
LY83583 increased HSP90 binding with phosphor-ERK1/2 To identify cytosolic HSP90 as a molecular chaperone binding with ERK1/2 or phosphor-ERK1/2, the total cell lysates were co-immunoprecipitated with an anti-HSP90 antibody and blotted with anti-ERK1/2 and phosphor-ERK1/2 antibodies, respectively. We observed that LY83583 significantly increased HSP90 association with phosphor-ERK1/2 (1.8-fold), but not with ERK1/2, as compared with the control. Pretreatment with geldanamycin attenuated the effects of LY83583 (Figure 3).
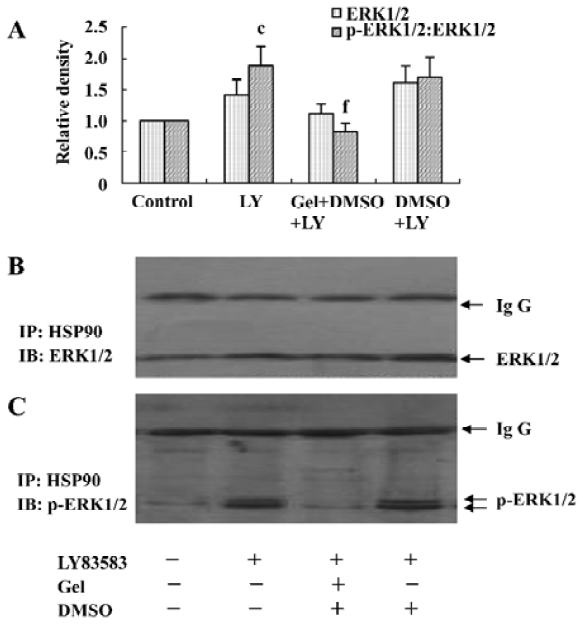
Roles of HSP90 in increasing the solubility of phosphor-ERK1/2 To further explore the effects of HSP90 on phosphor-ERK1/2, we collected the soluble and insoluble extracts of VSMC treated with LY83583 or/and geldanamycin, and analyzed the levels of ERK1/2 and phosphor-ERK1/2 by Western blotting. As shown in Figure 4, LY83583 obviously increased the phosphor-ERK1/2 level of soluble extracts and decreased the phosphor-ERK1/2 level of insoluble extracts (Figure 4, 5). Geldanamycin attenuated the effect of LY83583.
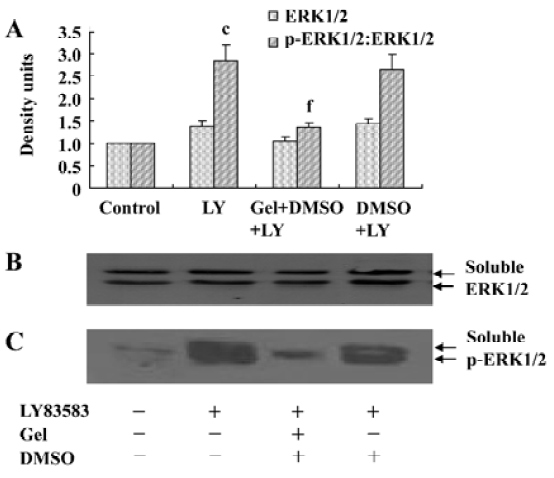
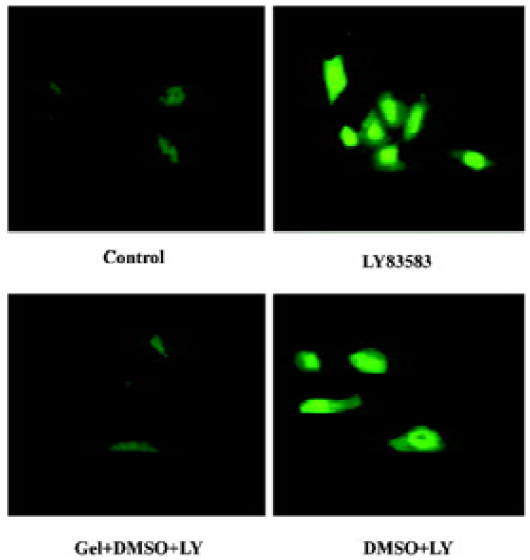
Roles of HSP90 in enhancing the nuclear translocation of phosphor-ERK1/2 It has been reported that an increase of phosphor-ERK1/2 solubility might enhance its function. In the present study, we investigated the effect of HSP90 on the nuclear translocation of phosphor-ERK1/2. The nuclear extracts were harvested from VSMC treated with LY83583 or/and geldanamycin, and the level of phosphor-ERK1/2 was measured by Western blotting. The results showed that LY83583 increased the level of phosphor-ERK1/2 in nuclei by 7.6-fold as compared with the control, and geldanamycin abolished the effect of LY83583 (Figure 6). An immunofluorescence analysis demonstrated that the obvious nuclear translocation of phosphor-ERK1/2 was observed in LY83583-treated VSMC as compared with untreated VSMC. Pretreatment with geldanamycin reduced the LY83583-stimulated nuclear translocation of phosphor-ERK1/2 (Figure 7). DMSO alone had no effect on the solubility and nuclear translocation of phosphor-ERK1/2 (Figures 5, 7).

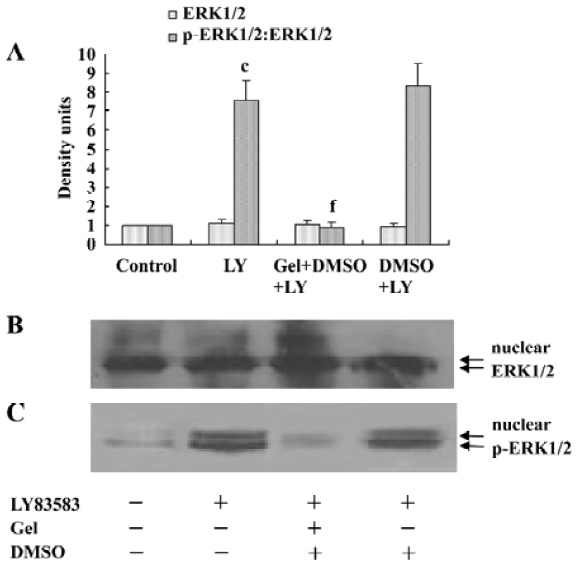
HSP90 mediates VSMC proliferation induced by oxidative stress To further confirm the relationship between the increased HSP90 expression and oxidative stress-stimulated cell proliferation, we observed the effects of HSP90 inhibitors, geldanamycin and radicicol (another HSP90 inhibitor without influence on ROS), on LY83583-induced VSMC. MTT assay and cell counting showed (Table 1) that LY83583 treatment accelerated VSMC proliferation by 90%. Geldanamycin and radicicol significantly inhibited LY83583-stimulated cell growth.
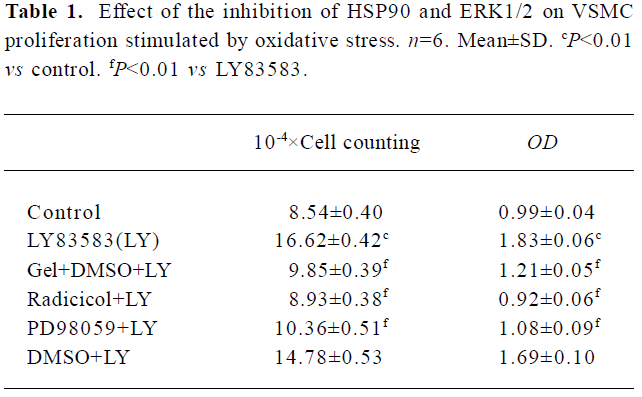
Full table
Discussion
HSP90 is known as a molecular chaperone and is associated with various proteins, such as pp60v-src[19], Raf-1[20], Cdk4[21], and Casein kinase II (CK2)[22]. Our previous studies demonstrated that oxidative stress increased HSP90 secretion and promoted VSMC growth[1,2]. Interestingly, the LY83583-induced generation of ROS peaked at 15 min and returned to baseline by 120 min[1,2]. However, the LY83583-induced activation of ERK1/2 showed 2 peaks at 10 min (early peak) and 120 min (late peak), respectively. Obviously, the late peak is not directly related with ROS induced by LY83583. Further experiments demonstrated that the late peak was mediated via secreted oxidative-stressed factors, which involve HSP90, cyclophilin A, and cyclophilin B[1,2]. However, the role of HSP90 in ERK1/2 activity needs to be further investigated. In the present study, we demonstrated that HSP90 was associated with phosphor-ERK1/2 when VSMC were challenged to oxidative stress. The association of phosphor-ERK1/2 with HSP90 promoted the solubility and nuclear translocation of phosphor-ERK1/2, indicating that HSP90 participated in the process of intracellular ERK1/2 activation.
Geldanamycin, a benzoquinone ansamycin, can specifically bind to HSP90[23] in vitro and in vivo and inhibit the function of HSP90. It also interferes indirectly with the functions of the other protein molecules with which HSP90 associates[24], often resulting in the disruption of HSP90-containing multimolecular complexes[9]. Miyata et al[5] demonstrated that HSP90 regulated stability and solubility of intracellular protein MOK by binding with it, and geldanamycin disrupted the effect of HSP90. Our data here showed that geldanamycin treatment altered the chaperone function of HSP90 by attenuating the interaction between HSP90 and phosphor-ERK1/2, abolishing oxidative stress-induced activation and decreasing the solubility and nuclear translocation of phosphor-ERK1/2.
The solubility of activated ERK1/2 is essential for their signal transduction. It has been reported that HSP90 increases the solubility of MOK, a novel member of mitogen-activated protein kinases (MAPK)[5]. Our experiments showed that oxidative stress increased the soluble phosphor-ERK1/2 and decreased insoluble phosphor-ERK1/2. Geldanamycin abolished the effects of oxidative stress, indicating that the solubility of phosphor-ERK1/2 requires the participation of HSP90.
Phosphor-ERK1/2 enters into nuclei to display its func-tion. The specific associations of non-catalytic proteins are important for catalytic subunits recognizing substrate or cellular localization. In the present experiment, we found that oxidative stress stimulated phosphor-ERK1/2 entering cell nuclei, and geldanamycin blocked the effect of oxidative stress.
Those results indicated that HSP90 could regulate ERK1/2 activity by promoting ERK1/2 phosphorylation, increasing the interaction of itself with phosphor-ERK1/2, and enhancing the solubility and nuclear translocation of phosphor-ERK1/2. It has been reported that HSP90 can bind with MOK, a member of MAPK and bind with mitogen-activated protein kinase kinase (MAPKK) kinases-Raf, which decrease the degradation and increase the phosphorylation of MOK and Raf[4-6]. Therefore, we propose that HSP90 might increase phosphor-ERK1/2 in 2 ways: first, HSP90 delays the degradation of phosphor-ERK1/2 by binding with it. Second, HSP90 increases the phosphorylation of ERK1/2 by binding with MAPKK and affecting MAPKK translocation.
It is well known that oxidative stress stimulates VSMC proliferation by activating the signal transduction pathway of growth factors, such as MAPK. Since HSP90 mediated the activation of ERK1/2 induced by oxidative stress, we propose that the function inhibition of HSP90 will inhibit cell proliferation. Our experiments showed that geldanamycin, radicicol (another HSP90 inhibitor), and PD98059 (an inhibitor of ERK1/2 activation) all significantly blocked the effects of oxidative stress on VSMC proliferation.
In summary, HSP90 can mediate the oxidative stress-stimulated, late-phase activation of ERK1/2 and VSMC proliferation by promoting the ERK1/2 phosphorylation, the association with phosphor-ERK1/2, and the solubility and nuclear translocation of phosphor-ERK1/2.
Acknowledgements
The authors are indebted to Dr Zhi-hong ZHOU (Institute of Molecular and Cell Biology, Singapore) for her valuable help in the preparation of the manuscript.
References
- Liao DF, Jin ZG, Bass AS, Daum G, Gygi SP, Aebersold R, et al. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem 2000;275:189-96.
- Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, et al. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res 2000;87:789-96.
- Bamberger CM, Wald M, Bamberger AM, Schulte HM. Inhibition of mineralocorticoid and glucocorticoid receptor function by the heat shock protein 90-binding agent geldanamycin. Mol Cell Endocrinol 1997;131:233-40.
- Grammatikakis N, Lin JH, Grammatikakis A, Tsichlis PN, Cochran BH. p50(cdc37) acting in concert with HSP90 is required for Raf-1 function. Mol Cell Biol 1999; 19: 1661?72.
- Miyata Y, Ikawa Y, Shibuya M, Nishida E. Specific association of a set of molecular chaperons including HSP90 and Cdc37 with MOK, a member of the mitogen-activated protein kinase superfamily. J Biol Chem 2001; 276: 21 841?8.
- Stancato LF, Silverstein AM, Owens-Grillo JK, Chow YH, Jove R, Pratt WB. The HSP90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem 1997;272:4013-20.
- Akagami M, Morrison P, Welch WJ. Benzoquinoid ansamycins (herbimycin A and geldanamycin) interfere with the maturation of growth factor receptor tyrosine kinases. Cell Stress Chaperones 1999;4:19-28.
- Prima V, Depoix C, Masselot B, Formstecher P, Lefebvre P. Alteration of the glucocorticoid receptor subcellular localization by non steroidal compounds. J Steroid Biochem Mol Biol 2000;72:1-12.
- Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-HSP90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem 1995; 270: 24 585?8.
- Stancato LF, Chow YH, Hutchison KA, Perdew GH, Jove R, Pratt WB. Raf exists in a native heterocomplex with HSP90 and p50 that can be reconstituted in a cell-free system. J Biol Chem 1993; 268: 21 711-6.
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem 1995; 270: 14 843-6.
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, et al. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 1998;93:605-15.
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 1995;80:179-85.
- Campbell JH, Campbell GR. Culture techniques and their applications to studies of vascular smooth muscle. Clin Sci 1993;85:501-12.
- Setalo G Jr, Singh M, Guan X, Toran-Allerand CD. Estradiol-induced phosphorylation of ERK1/2 in explants of the mouse cerebral cortex: the roles of heat shock protein 90 (HSP90) and MEK2. J Neurobiol 2002;50:1-12.
- Backlund M, Johansson I, Mkrtchian S, Sundberg MI. Signal transduction-mediated activation of the aryl hydrocarbon receptor in rat hepatoma H4IIE cells. J Biol Chem 1997; 272: 31 755?63.
- Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 2001;50:2563-71.
- Wu LX, Xu JH, Huang XW, Zhang KZ, Wen CX, Chen YZ. Down-regulation of p210bcr/abl by curcumin involves disrupting molecular chaperone functions of HSP90. Acta Pharmacol Sin 2006;27:694-9.
- Perdew GH, Wiegand H, Vanden Heuvel JP, Mitchell C, Singh SS. A. 50 kilodalton protein associated with raf and pp60(v-src) protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry 1997;36:3600-7.
- Stancato LF, Chow YH, Hutchison KA, Perdew GH, Jove R, Pratt WB. Raf exists in a native heterocomplex with HSP90 and p50 that can be reconstituted in a cell-free system. J Biol Chem 1993; 268: 21 711?6.
- Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, Interaction between Cdc37 and Cdk4 in human cells. Oncogene 1997; 14: 1999?2004.
- Kimura Y, Rutherford SL, Miyata Y, Yahara I, Freeman BC, Yue L, Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev 1997; 11: 1775?85.
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA 1994;91:8324-8.
- Segnitz B, Gehring U. The function of steroid hormone receptors is inhibited by the HSP90-specific compound geldanamycin. J Biol Chem 1997; 272: 18 694?701.
