Effects of GSM 1800 MHz on dendritic development of cultured hippo-campal neurons1
Introduction
Due to ubiquitous use in daily life, electromagnetic fields (EMF) radiated by global system for mobile communications (GSM) equipment have attracted attention as a potentially hazardous environmental factor. Debates on whether GSM microwaves may induce “non-thermal” effects on biological tissues are increased. Epidemiological research has indicated that the brain is likely to be affected by mobile phones[1,2]. Numerous in vivo and in vitro studies have been performed to investigate the biological consequences and to assess the health risks of GSM microwaves on the nervous system.
Until now, the results from the studies concerning the effects of GSM on the nervous system were highly controversial and failed to reveal an unequivocal correlation between the use of mobile phones and brain function. However, increasing studies indicate that mobile phones may affect cognitive processes, as well as learning and memory. GSM microwaves have been found to produce a significant decrease in choice reaction time[3], slower response and decision speed[4], and alters brain potential in humans performing a visual monitoring task[5,6]. Mobile phones have also been reported to induce a loss of mental regeneration and impair cognitive performance[7,8]. The exposure of the left side of the human brain to GSM microwaves was found to slow down the left-hand response time, which was apparent in 3 of the 4 distinct cognitive tasks, including spatial item recognition, verbal item recognition, and 2 spatial compatibility tasks[9]. A recent study also indicated that GSM-type radiation could induce seizures in rats following their facilitation by subconvulsive doses of picrotoxin [10].
The potential influence of the radiation frequency (RF) field on brain development has also been studied. Treatment with 10 mW/cm2 of 2450 MHz microwave radiation did not significantly affect the gross and histological development of the rat brain[11]. Mice offspring irradiated in the uterus with 2450 MHz RF radiation at a specific absorption rate (SAR) of 0.4 W/kg did not produce significant alterations in brain organogenesis, including weight of fetal brains and brain RNA and DNA and protein levels[12], while exposure at 28 mW/cm2 induced a lower brain weight[13]. A study on the cerebella of Japanese quails whose eggs were continuously exposed to 5.0 mW/cm2 of 2.45 GHz microwave radiation showed a slight developmental retardation in the cerebellar cortices[14]. An in vitro investigation that studied the effects of GSM microwaves on synaptic function during the development period revealed that GSM 1800 MHz microwaves may reduce excitatory synaptic activity and the number of excitatory synapses in cultured rat hippocampal neurons[15]. Apparently, the conclusion can not be drawn yet whether low level microwaves have biological effects on brain development in animals. Furthermore, the cellular and molecular mechanisms underlying the biological effects of microwaves on brain function and development are still unclear. The loss of excitatory drive on developing neurons with adaptive changes in neuronal morphology and connectivity has been proposed as a plausible mechanism involved in behavioral alterations[16]. Thus, the morphological study on developing neurons in vitro in living cells is preferred for further investigating the cellular and molecular mechanisms underlying the effects of microwaves on brain function.
In our experiments, we attempted to determine whether and how exposure to GSM 1800 MHz microwaves at different intensities during neuronal development from 6 days in vitro (DIV6) to DIV14 interferes dendritic development in cultured hippocampal neurons by living cell imaging on a fluorescence microscope.
Materials and methods
Primary hippocampal neuronal cultures and neuronal transfections Primary hippocampal cultures were prepared based on the method described previously[17]. Briefly, newborn rats (P0) were careful dissected, and then the hippocampi were chopped and digested in 0.25% trypsin (Sigma, St Louis, MO, USA) for 15 min at 37 oC with gentle shaking. Dissociated cells were plated at a density of 5?105 cells/cm2 in a 35 mm dish (Nunc, Roskilde, Denmark) with poly-L-lysine-coated coverslips in Dulbecco’s modified Eagle’s media (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) and 2 mmol/L glutamine, and maintained at 37 oC in a humidified atmosphere with 5% CO2. After culturing in vitro for 24 h, the medium was replaced with Neurobasal medium containing 2% B27 supplement, 1% antibiotic, and 0.25% glutamine (Invitrogen, USA). At DIV5, cytosine arabinofuranoside was added at a final concentration of 10 µmol/L. Thereafter, half of the medium was replaced twice a week with Neurobasal medium containing 2% B27 supplement, 1% antibiotic, and 0.25% glutamine. Hippocampal neurons were routinely transfected with farnesylated enhanced green fluorescent protein (F-GFP) and GFP-actin by Lipofectamine 2000 (Invitrogen, USA) at DIV5[17].
Microwave exposure The modulated microwave exposure system was designed by the Foundation for Information Technologies in Society (Zurich, Switzerland)[18]. It mainly consists of a RF generator, an arbitrary function generator, a narrow band amplifier, and 2 rectangular wave-guides operating at a frequency of 1800 MHz. The 2 wave-guides, one for exposure and the other for sham exposure as the control, were placed inside a conventional incubator to ensure constant environmental conditions (37 ℃, 5% CO2, 95% humidity). A dish holder inside the waveguide guarantees that the dishes are placed exactly in the H field maximum of a standing wave and exposed simultaneously in E polarization inside a waveguide. The system enables the exposure of a monolayer of cells with a non-uniformity of SAR of less than 30%. Six Nunc dishes can be exposed simultaneously in 1 exposure waveguide. The entire setup is computer controlled, enabling the automated control of the exposure parameters, including exposure strength (SAR) and exposure time.
All experiments were performed with the GSM 1800 MHz signals. The signal is an amplitude modulated by rectangular pulses with a repetition frequency of 217 Hz and a duty cycle of 1:8 (pulse width 0.576 ms) corresponding to the dominant modulation component of GSM.
The system’s temperature remained at 37±0.1 ℃ during the whole exposure duration, and the temperature between the microwave-exposed and sham-exposed cultures never exceeded 0.1 ℃. Therefore, the results were observed under a condition of no obvious temperature change.
Hippocampal neurons were exposed to the GSM 1800 MHz microwaves at 2.4 or 0.8 W/kg for 15 min/d from DIV6 to DIV14.
Image processing and analysis The cultures were maintained in a recording chamber in extracellular solution for continuous perfusion. Nikon band pass filter cubes (Tokyo, Japan) were used for detecting GFP. Living neurons transfected with F-GFP and GFP-actin were imaged on a TE2000 inverted microscope (Nikon, Japan), equipped with 40×1.0 numerical aperture (N.A). objective lens. Digital images were acquired with a charged couple device (CCD) camera (CoolSNAP HQ, Roper Scientific, Tucson, AZ) controlled with MetaMorph 5.0 software (Universal Imaging, West Chester, PA, USA) capturing 200?2000 ms exposures. A single level of focus was maintained throughout each recording. Stacks were collected every 30 s. All images were captured 30 min after completing the last radiation of the GSM 1800 MHz microwaves.
When we imaged filopodia, 11 stacks for each neuron were collected for measuring their mobility. Motility was calculated as the absolute difference in length of protrusions from frame to frame, divided by the total imaging time. We defined filopodia in length as those whose length exceeded 3 µm; filopodia in mobility was defined as those extracting or retracting more than 0.8 µm/min, which were defined as mobile filopodia in our study. The density of filopodia was calculated based on the number of filopodia on all secondary and third branches and their total length in a neuron. The value is thus expressed as per 100 µm. The mobility of filopodia was measured based on the percentage of mobile filopodia in total filopodia more than 3 µm in length. The lengths of all filopodia were manually traced and measured with MetaMorph 5.0 (Universal Imaging Corporation, USA).
Our length and motility measurements for filopodia are likely to be slight underestimates of the actual values because the z-axis projection partially obscures the true lengths of filopodia going in and out of the final Z-plane. Motility measurements would be further underestimated at early ages by the fact that we did not take into account motility derived from the bending, branching, or sweeping behavior of filopodia.
For branches, we adopted the terminology used by Havton and Ohara[19], which indicates that the dendrite derived directly from the soma is the first-order branch or primary dendrite, and the daughter branches arising from primary dendrites are second-order branches, and so on. The lengths of all dendritic branches were manually traced and measured with MetaMorph 5.0.
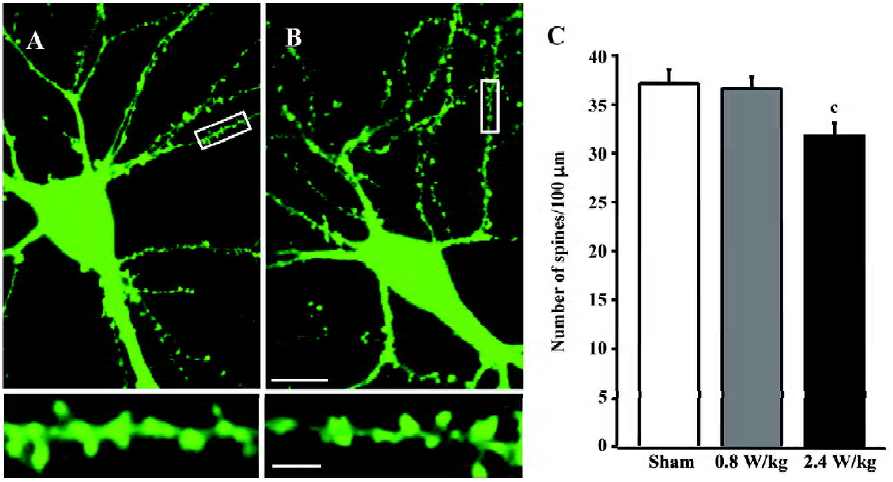
For spines, we collected pictures with 40? magnification so that all spines were displayed clearly. The density of spines was calculated based on the number of spines on all secondary and tertiary branches and their total length in a neuron, and the value was expressed as per 100 µm.
The lengths of all dendritic filopodia and branches in each picture were obtained by manually drawing skeleton versions of the dendrites. The analysis was done blindly on unprocessed images and there was no apparent variability between the 2 observers (Wei NING and Shu-jun XU). Filtering was used subsequently only for display purposes in the final figures shown here. Data were from 3 independent preparations of cell cultures and ensured independent radiation procedures. Data were expressed as mean±SEM and statistical significance was determined by ANOVA using SPSS version 13.0 (SPSS, Chicago, IL, USA).
Results
GSM 1800 MHz microwaves decrease the density and mobility of dendritic filopodia Dendritic filopodia of the sham-exposed neurons were arranged with high density along dendritic shafts (10.7±2.9/100 µm) and moved actively (85% mobile filopodia; (Figures 1,2). In the neurons treated with the chronic intermittent exposure of 2.4 W/kg GSM 1800 MHz microwaves from DIV6 to DIV8, the density of dendritic filopodia significantly decreased by 38.9%±5.8% (P<0.001, Figure 2A). To further evaluate the influence of the GSM microwaves on the density of filopodia, we measured the average number of mobile filopodia/100 µm dendrites by analyzing the time-lapse images and obtained a similar result: the density of mobile filopodia markedly decreased by 59%±4.1% (P<0.001, Figure 2B). In addition, the mobility of filopodia (repre-sented by the percentage of mobile/total filopodia) was reduced by 28%±6.0% (P<0.001, Figure 2C) in the neurons exposed to GSM microwaves at a SAR of 2.4 W/kg. However, the average length of filopodia was unaltered by microwave exposure (P>0.05, Figure 2D).
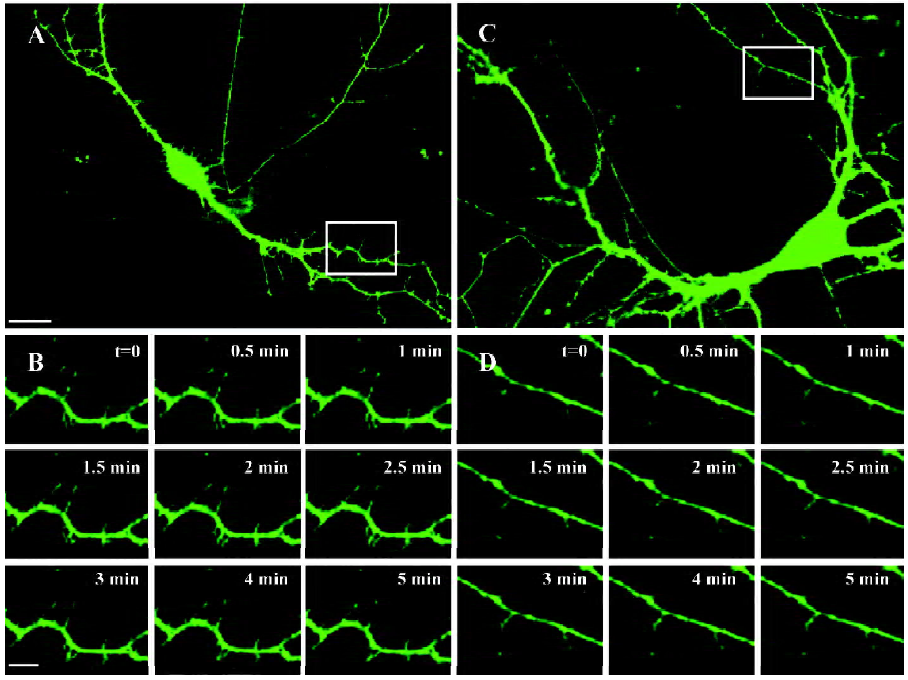
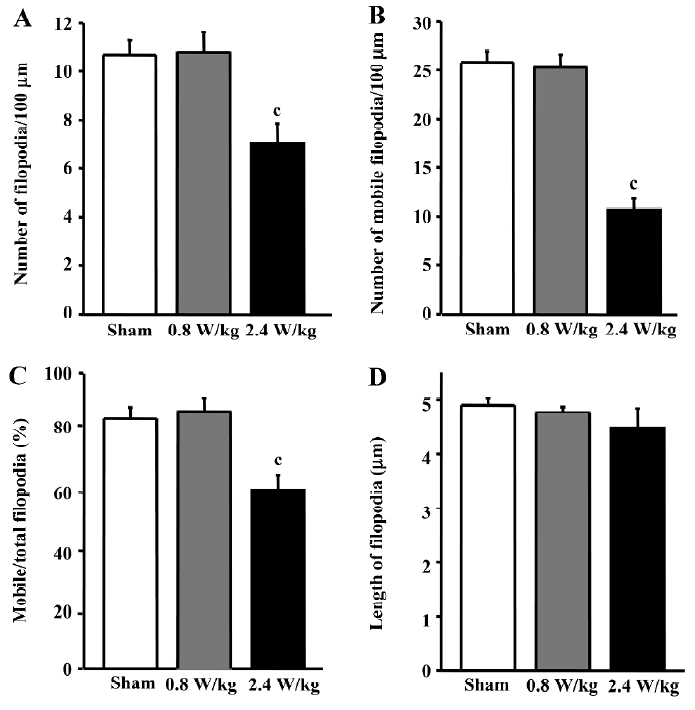
In contrast, the 0.8 W/kg GSM 1800 MHz microwave exposure failed to induce any significant alteration in density, mobility, or length of filopodia in the cultured hippocampal neurons (P>0.05, Figure 2).
Mobile phone microwaves reduce the density of dendritic spines It was previously reported that filopodia occurred predominantly during the early development period of neurons and metamorphose into spines[20,21]. Since 2 important parameters of filopodia (density and mobility) were affected by GSM microwaves, we observed whether those factors would cause changes in the density of dendritic spines as a consequence. The cultured neurons were exposed to GSM microwaves 15 min daily from DIV6 to DIV9 and were observed at DIV14. Concomitantly, the density of dendritic spines showed an overall decrease of 14.3%?3.7% (P<0.01, Figure 3C) in the neurons exposed to 2.4 W/kg GSM 1800 MHz microwaves. In addition, the spine density was not affected by 0.8 W/kg GSM microwaves (Figure 3C).
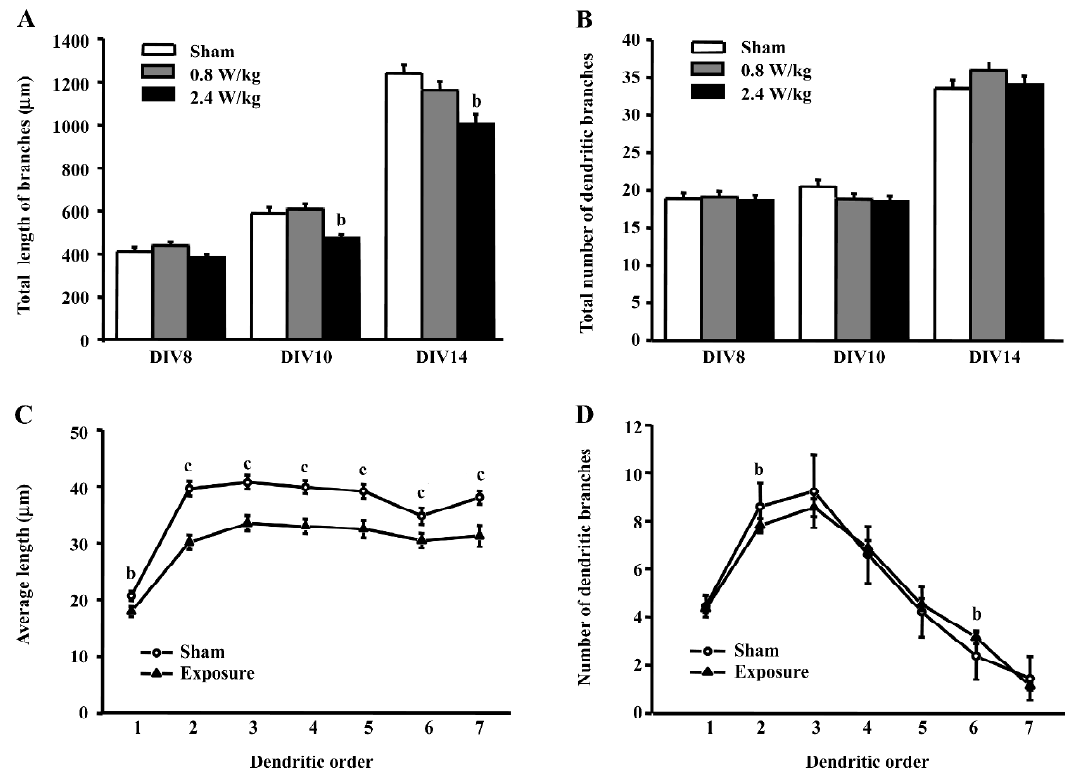
GSM 1800 MHz microwaves cause different effects on dendritic growth and arborization The exposure to GSM 1800 MHz microwaves at 2.4 W/kg for more than 5 d decreased the total length of dendritic branches, but did not change the arborization of dendritic trees, but the 0.8 W/kg microwave exposure did not alter both the growth and arborization of dendritic trees (Figure 4). After exposure to 2.4 W/kg microwaves for 5 d (at DIV10) and 9 d (at DIV14), the total length of the neurons was reduced by 19.6%?4.6% and 18.9%?3.4%, respectively (P<0.05, Figure 4A), while no change was observed after 15 min daily exposure for 3 d (at DIV8). To examine the influence of 2.4 W/kg microwave treatment on the growth and branching of dendritic arbors in detail, we chose the neurons at DIV14 for further analysis, because neurons at this age have passed the crucial developmental period and have acquired their mature characters. For each order of the dendritic arbors, we plotted the number and the average length of dendritic branches (Figure 4C, 4D). For all the dendritic orders, the average segment length in the exposed neurons was shorter than the sham-exposed neurons, while the number of dendritic branches for most dendrites did not change after exposure.
Discussion
This study demonstrates that chronic 2.4 W/kg GSM 1800 MHz microwave exposure is able to induce: (1) a significant reduction in the density and mobility of dendritic filopodia; (2) a notable decrease in the density of dendritic spines; and (3) a decline in the segment length of dendrites, whereas the 0.8 W/kg microwaves do not show similar influences on dendritic development in the cultured hippocampal neurons of rats.
Our primary finding is that chronic 2.4 W/kg GSM microwave exposure reduces the density and mobility of dendritic filopodia. Dendritic filopodia, long and thin protrusions that occur predominantly during early development of the mammalian central nervous system, are thought to be responsible for the formation of spines[20-22]. Rapid extensions and movements of filopodia were described as a necessary step for neurons to find new contact sites that can then evolve into nascent synapses and mature into functional synaptic connections[23,24]. Thus, the decrease in the density and mobility of dendritic filopodia induced by microwaves may affect the procedure resulting in the decreased formation of mature synapses.
Consistently, our study shows that the density of spines is decreased after 9 d exposure for 15 min/d. The result was validated by another experiment conducted by our group indicating that post synaptic density (PSD95) clusters were reduced after the 2.4 W/kg GSM microwave exposure[15]. Generally, the spines increase the surface area of dendrites, the excitatory synaptic density, and the number of connections between neurons[25], and vice versa. Given the decreased total length of dendritic branches, the absolute number of excitatory synapses per neuron should be much less than that reflected by the reduction of spine density. The hippocampus is one of the most plastic parts of the adult mammalian brain[26,27], and hippocampal changes, including fluctuations in dendritic complexity, spine density, and soma size may be associated with altered learning and memory performance[28]. In rats, spine density of the dentate gyrus and cornu ammonis fields CA1 region of the hippocampus has been positively correlated with water-maze learning and memory performance[29,30]. Therefore, the density of hippocampal spines may be an indication of the efficiency of the synaptic network involved in spatial learning and memory, and the microwave-induced alterations in dendritic development and synaptic formation might have a potential influence on animal behavior.
Interestingly, we observed that the growth of dendrites was affected by the 2.4 W/kg GSM microwave exposure, while dendritic branching was unaffected. There have been numerous studies that have found that most intracellular and extracellular factors affected dendritic growth and branching simultaneously[31-33], while our results showed that they were not necessarily affected at the same time by a certain factor. Our study suggests that dendritic growth and branching may be associated with different molecular motors or second-messenger cascades[34]. Consistent with our finding, Jones et al found that Abl tyrosine kinase activation promoted both dendritic branching and growth, while affecting dendritic branching more than its elongation[35]. The complex and highly branched architecture of the dendritic arbor is an important determinant of how a neuron accepts, receives, integrates, and transmits inputs from other neurons. A decrease in dendrite length might be sufficient to impair intercellular signal transmission, change local circuit function, and further influence connectivity patterns between brain regions, leading to developmental and behavioral disorders[36].
The alterations of dendritic growth and development induced by GSM microwave treatment may be associated with the changes in cytoskeleton–related molecules, such as actin and microtubule-associated protein-2 (MAP-2). Mobile phone radiation was found to affect the stability of F-actin stress fibers[37] and increase the expression of the F-actin in human endothelial cells[38,39]. The expression of MAP-2 in hippocampal neurons was reported to decrease after sustained exposure to static magnetic fields[40]. Actin is highly enriched in dendritic spines[41,42] and dendritic filopodia, and its assembly is important for filopodial extension and retraction[43]. MAP-2 is a major constituent of crossbridges between microtubules in dendrites and is essential for growth through the selective stabilization of dendritic microtu-bules[44], thus, these 2 molecules could be candidates of targeting proteins that mediate the effects of GSM microwaves on neurons. It would be interesting to further investigate the underlying molecular mechanisms, such as identifying other associated proteins and changes of the upstream signal pathway.
In our study, all changes occurred at a relatively low intensity and appeared time dependent, which may reflect the non-thermal and accumulative effects of GSM microwaves on dendritic development in cultured hippocampal neurons. However, the direct transposition of our results to mobile phone users is difficult to achieve since our experiments were performed in the cultured hippocampal neurons of rats. Therefore, the non-thermal effects of microwaves on filopodia and branch development in cultured neurons in our study may suggest its possible influence on the brain and may be considered a potential health hazard of mobile phones. The results derived from this in vitro study need to be confirmed by future in vivo studies. This study provides insights for further understanding some basic questions in neuroscience, as well as molecular and cellular mechanisms of GSM microwaves on learning and memory.
Acknowledgement
We are grateful to De-qiang LU and Guang-di CHEN for help with the operation and maintenance of the exposure apparatus.
References
- Leventhal A, Karsenty E, Sadetzki S. Cellular phones and public health. Harefuah 2004;143:614-20.
- Santini R, Santini P, Danze JM, Le Ruz P, Seigne M. Investigation on the health of people living near mobile telephone relay stations: I/Incidence according to distance and sex. Pathol Biol 2002;50:369-73.
- Preece AW, Iwi G, Davies-Smith A, Wesnes K, Butler S, Lim E, et al. Effect of a 915 MHz simulated mobile phone signal on cognitive function in man. Int J Radiat Biol 1999;75:447-56.
- Hladky A, Musil J, Roth Z, Urban P, Blazkova V. Acute effects of using a mobile phone on CNS functions. Cent Eur J Public Health 1999;7:165-7.
- Eulitz C, Ullsperger P, Freude G, Elbert T. Mobile phones modulate response patterns of human brain activity. Neuroreport 1998;9:3229-32.
- Freude G, Ullsperger P, Eggert S, Ruppe I. Effects of microwaves emitted by cellular phones on slow brain potentials. Bioelectro-magnetics 1998;19:384-7.
- Maier R. Is CNS activity modified by pulsed electromagnetic fields? Biomed Tech (Berl) 2001;46:18-23.
- Maier R, Greter SE, Maier N. Effects of pulsed electromagnetic fields on cognitive processes — a pilot study on pulsed field interference with cognitive regeneration. Acta Neurol Scand 2004;110:46-52.
- Eliyahu I, Luria R, Hareuveny R, Margaliot M, Meiran N, Shani G. Effects of radiofrequency radiation emitted by cellular telephones on the cognitive functions of humans. Bioelectromagnetics 2006;27:119-26.
- Lopez-Martin E, Relova-Quinteiro JL, Gallego-Gomez R, Peleteiro-Fernandez M, Jorge-Barreiro FJ, Ares-Pena FJ. GSM radiation triggers seizures and increases cerebral c-Fos positivity in rats pretreated with subconvulsive doses of picrotoxin. Neurosci Lett 2006;398:139-44.
- Inouye M, Galvin MJ, McRee DI. Effect of 2,450 MHz microwave radiation on the development of the rat brain. Teratology 1983;28:413-9.
- Merritt JH, Hardy KA, Chamness AF. In utero exposure to microwave radiation and rat brain development. Bioelectromagnetics 1984;5:315-22.
- Berman E, Carter HB, House D. Growth and development of mice offspring after irradiation in utero with 2,450-MHz microwaves. Teratology 1984;30:393-402.
- Inouye M, Galvin MJ, McRee DI. Effects of 2.45 GHz microwave radiation on the development of Japanese quail cerebellum. Teratology 1982;25:115-21.
- Xu SJ, Ning W, Xu ZP, Zhou SY, Chiang H, Luo JH. Chronic exposure to GSM 1800-MHz microwaves reduces excitatory synaptic activity in cultured hippocampal neurons. Neurosci Lett 2006;398:253-7.
- Kolb B, Forgie M, Gibb R, Gorny G, Rowntree S. Age, experience and the changing brain. Neurosci Biobehav Rev 1998;22:143-59.
- Luo JH, Fu ZY, Losi G, Kim BG, Prybylowski K, Vissel B, et al. Functional expression of distinct NMDA channel subunits tagged with green fluorescent protein in hippocampal neurons in culture. Neuropharmacology 2002;42:306-18.
- Schonborn F, Pokovic K, Burkhardt M, Kuster N. Basis for optimization of in vitro exposure apparatus for health hazard evaluations of mobile communications. Bioelectromagnetics 2001;22:547-59.
- Havton LA, Ohara PT. Quantitative analyses of intracellularly characterized and labeled thalamocortical projection neurons in the ventrobasal complex of primates. J Comp neurol 1993;336:135-50.
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci 1996;16:2983-94.
- Ziv N, Smith S. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 1996;17:91-102.
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron 1998;20:847-54.
- Jontes D, Smith SJ. Filopodia, spines, and the generation of synaptic diversity. Neuron 2000;27:11-4.
- Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse 1989;3:255-85.
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus 2000;10:501-11.
- Breedlove SM, Jordan CL. The increasingly plastic, hormone-responsive adult brain. Proc Natl Acad Sci USA 2001;98:2956-7.
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci 1999;22:105-22.
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav 1998;34:140-8.
- Nylund R, Leszczynski D. Proteomics analysis of human endothelial cell line EA.hy926 after exposure to GSM 900 radiation. Proteomics 2004;4:1359-65.
- Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus). J Neurosci 2005;25:4521-6.
- Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci 1998;18:7836-46.
- Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron 2002;34:999-1010.
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci 2005;8:34-42.
- Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci 2003;23:7129-42.
- Jones SB, Lu HY, Lu Q. Abl tyrosine kinase promotes dendro-genesis by inducing actin cytoskeletal rearrangements in cooperation with Rho family small GTPases in hippocampal neurons. J Neurosci 2004;24:8510-21.
- Miller JP, Jacobs GA. Relationships between neuronal structure and function. J Exp Biol 1984;112:129-45.
- Leszczynski D, Joenvaara S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer- and blood朾rain barrier-related effects. Differentiation 2002;70:120-9.
- Leszczynski D, Nylund R, Joenvaara S, Reivinen J. Applicability of discovery science approach to determine biological effects of mobile phone radiation. Proteomics 2004;4:426-31.
- Ohara PT, Havton LA. Dendritic architecture of rat somatosensory thalamocortical projection neurons. J Comp Neurol 1994;341:159-71.
- Hirai T, Yoneda Y. Functional alterations in immature cultured rat hippocampal neurons after sustained exposure to static magnetic fields. J Neurosci Res 2004;75:230-40.
- Caceres A, Payne MR, Binder LI, Steward O. Immunocytochemical localization of actin and microtubule-associated protein MAP2 in dendritic spines. Proc Natl Acad Sci USA 1983;80:1738-42.
- Landis D, Reese T. Cytoplasmic organization in cerebellar dendritic spines. J Cell Biol 1983;97:1169-78.
- Sheetz MP, Wayne DB, Pearlman AL. Extension of filopodia by motor-dependent actin assembly. Cell Motil Cytoskeleton 1992;22:160-9.
- Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, a proper PKA signal transduction. J Cell Biol 2002;158:541-9.
