Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line1
Introduction
Cervical cancer is the second most common cancer in women worldwide, but it is the leading malignancy among women in many developing countries[1]. Traditional herb formulations have been re-evaluated by clinicians because these medicines have fewer side-effects and are more suitable for long-term use compared with chemically synthesized medicines. New therapeutic strategies must be evaluated to improve survival. Clinical responses can be improved by identifying therapies that are particularly effective in activating apoptosis in vivo among cervical carcinoma cells.
Oridonin, a traditional herb formulation, has been successfully used for women’s diseases. Oridonin is a natural, biologically active substance isolated from Rabdosia rubescens[2], one of several Chinese herbs in the once popular dietary supplement called PC–SPES. PC–SPES, which contains 7 Chinese and 1 North American herbal supplements, has potent inhibitory activities against a variety of cancer cells[3]. The use of the entire plant of this Chinese herb purportedly offers digestive benefits, and anecdotal evidence suggests its efficacy in the treatment of esophageal carcinoma with little toxicity[4]. Oridonin, the main active component of Rabdosia rubescens, is a tetracyclic diterpenoid compound[5]. Tetracyclic diterpenoids are natural, biologically-active substances with various physiological and pharmacological properties, including antitumor activities[6].
In recent years, apoptosis has emerged as the major mechanism by which anticancer agents eliminate preneo-plastic or neoplastic cells. Oridonin induces apoptosis in neoplastic cells from the breast[7], liver[8], lung[9]and osteosarcoma[10]. Oridonin causes apoptosis of cancer cells by inhibiting the activation of NF-κβ, signal transducer and activator of transcription 3 and protein kinase B (Akt)[11]. Oridonin also downregulates the expression of various genes that NF-κβ regulates, including Bcl-2, cyclooxygenase-2 (COX-2), cyclinD1, and adhesion molecules[12]. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway plays an important role in various cellular processes including cell growth, survival, and motility[13]. In addition, the abnormal expression of the PI3K/Akt pathway has been reported for many human tumors[14]. Phosphatase and tensin homolog deleted on chromosome 10 and Src homology 2-containing 5' phosphoinositol phosphatases, which serve as negative regulators of the PI3K/Akt pathway, are frequently mutated in a number of human cancers[15,16]. The principal target of the PI3K pathway is the activation of Akt (protein kinase B), which directly effects apoptosis by targeting the pro-apoptotic Bcl-2-related protein Bad. In addition, PI3K plays a role in regulating the proliferation of many cell types[17]. Akt prevents apoptosis by generating anti-apoptotic signals by phosphorylating Bad, glycogen synthase kinase 3 (GSK3), and caspase-9, and by activating transcriptional factors, such as forkhead box class O (FOXO)-1 and NF-κβ[18–22]. Therefore, the deregulated signaling functions in the PI3K/Akt pathway make it a potential target for chemotherapeutic agents.
Cervical carcinoma accounts for about 10% of all newly diagnosed cancers in women worldwide[1]. Cervical carcinoma in patients with a poor prognosis is characterized by rapid cellular proliferation and strong expression of anti-apoptotic genes. These features may be due to incomplete cell cycle arrest and apoptosis resistance to conventional therapies. The purpose of this study was to explore the mechanism of the chemopreventive effects of oridonin in the human cervical carcinoma HeLa cell line. We examined the involvement of Akt and its substrates FOXO and GSK3 in HeLa cells, the role of members of the Bcl2 and inhibitor of apoptosis protein (IAP) families, the effect of oridonin on mitochondrial-mediated apoptosis involving cytochrome c release, and the activity of caspases-3 and -9 in propagating death signals.
Materials and methods
Reagents The Kunming Institute of Botany, the Chinese Academy of Sciences (Kunming, China) provided oridonin (purity 99.7%) isolated from the aerial parts of Rabdosia rubescens. The method for isolation of oridonin was the same as that described previously[23]. Stock solution for oridonin (10 mmol/L) was prepared in DMSO and protected from light at 4 oC, then diluted to a appropriate concentration with medium immediately before use. Bisbenzimide (Hoechst H33342), ethidium bromide, rhodamine, monoclonal mouse anti-β-actin antibody, and heat-inactivated fetal bovine serum were purchased from Sigma-Aldrich (St Louis, MO, USA). Z-D(OMe)-E(OMe)-V-D(OMe)-FMK (z-DEVD-fmk), (EMD Chemicals, Inc. San Diego, CA, USA) were prepared in 100 mmol/L in DMSO stock solution. Propidium iodide was purchased from Bio Basic (Toronto, Canada). L-Glutamine (200 mmol/L) and a mix of penicillin-streptomycin (10000 IU/mL and 10000 mg/mL, respectively) were purchased from Mediatech (Herdon, VA, USA). Antiphospho-Akt antibodies (Ser473, Thr308), anti-poly-adenosine diphosphate–ribose polymerase (PARP) and anti-caspase-3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-GSK3, anti-phospho-Forkhead Drosophila homolog rhabdomyosarcoma like 1 (FKHRL1), anti-cleaved caspase-3, anti-cIAP1, anti-X-linked inhibitor of apoptosis protein (XIAP), and antisurvivin antibodies were purchased from Cell Signaling Technologies (Beverly, MA, USA). The anticaspase-9 antibody was obtained from R&D Systems (Minneapolis, MN, USA). Secondary horseradish peroxidase-labeled goat antimouse and antirabbit antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The Polyvinylidene fluoride membrane was purchased from Millipore (Billerica, MA, USA). Plastic dishes were obtained from Corning-Costar (Cambridge, MA, USA), and all other tissue culture plastics were from Falcon Plastics (Los Angeles, CA, USA).The Bio-Rad protein assay based on the Bradford dye-binding procedure was obtained from Bio-Rad Laboratories (Hercules, CA, USA). The enhanced chemiluminescence detection system was purchased from Amersham (Piscataway, NJ, USA). All other reagents were of analytical reagent quality.
Cell culture and treatment The clonal human cervical carcinoma HeLa cells, obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal calf serum, 100 µg/mL streptomycin, and 100 U/mL penicillin. Cultures were maintained at 37 oC in a humidified incubator in an atmosphere of 5% CO2. The cells were exposed to oridonin at different concentrations and for different times. The cells grown in medium containing equivalent amounts of DMSO without oridonin served as the controls.
Cell viability assay The viability of the HeLa cells was assessed by using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, which was based on the reduction of MTT by the mitochondrial dehydrogenase of intact HeLa cells to a purple formazan product. A total of 2×104 HeLa cells were plated on a 96-well plate. After incubation for 24 h, they were treated with oridonin at different concentrations for different intervals. After treatment, the medium containing oridonin were carefully aspirated. 100 µL of 0.5 mg/mL MTT was added to the cell culture medium in the wells and incubated for 4 h, as described previously[24]. We added 100 µL of 1% SDS to each well after 4 h incubation. The plates were covered with aluminum foil and kept in an incubator for 12 h to allow the formed formazan crystals to dissolve. An ELISA reader was used to measure the absorbance at 570 nm, and the half maximal inhibitory concentration (the concentration that caused 50% inhibition of cell proliferation) was assessed by using the Bliss method[9].
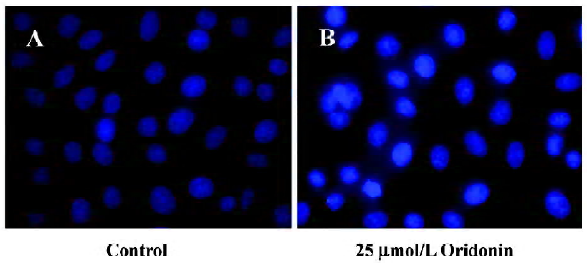
Hoechst 33342 staining Apoptotic morphology was studied by staining the cells with Hoechst 33342. The HeLa cells were seeded on coverslips on a 6-well plate and treated with 25 µmol/L oridonin. After 24 h, the cover glasses were carefully washed with phosphate-buffered saline (PBS) and stained with 20 µg/mL Hoechst 33342 for 10 min. Thereafter, the HeLa cells were washed in PBS and observed under a fluorescence microscope (Leica Microsystems AG, Wetzlar, Germany).
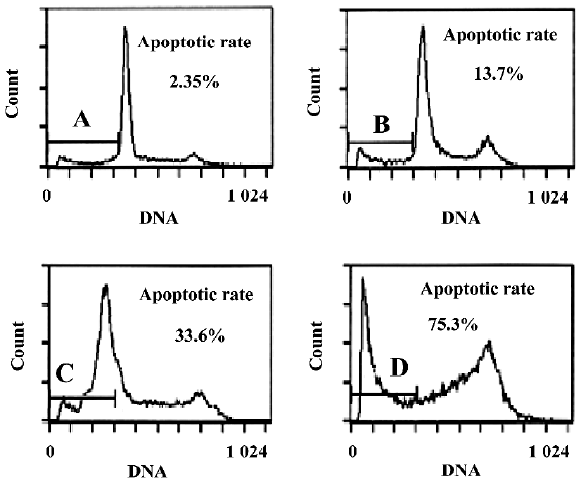
Flow cytometry analysis Flow cytometry (FCM) was used to quantitatively assess the rates of apoptosis. A total of 1×106 HeLa cells were plated onto 10 cm tissue culture dishes 1 d before treatment. They were then treated with 25 µmol/L oridonin for 6, 12, and 24 h. At the indicated time, floating and attached HeLa cells were harvested, washed with PBS, fixed in 70% ethanol overnight at 4 oC, and stained with 50 mg/mL propidium iodide. The sub-G1 peak (DNA content <2 N) was measured by means of FCM (FACScan; Becton Dickinson and Company, Franklin Lakes, NJ USA) and analyzed by using CellQuest software (BD, Franklin Lakes, NJ USA, USA).
Assay for cytochrome c release The release of cytochrome c from mitochondria was assayed as described elsewhere[4]. In brief, the HeLa cells were treated with and without oridonin and centrifuged at 1000×g. The cell pellets were resuspended in 5 volumes of a hypotonic buffer containing 20 mmol/L HEPES-KOH (pH 7.5), 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L ethylene glycol tetraacetic acid, 1 mmol/L DL-Dithiothreitol (DTT), 20 µg/mL leupeptin, 10 µg/mL aprotinin, and 250 mmol/L sucrose, and incubated for 15 min on ice. The HeLa cells were homogenized by passing them 15–20 times through a 22 gauge, 1.5 inch-long needle. The lysates were centrifuged at 1000×g for 5 min at 4 oC to create a pellet of nuclei and unbroken cells. The supernatants were collected and centrifuged at 12 000×g for 15 min. The resulting mitochondrial pellets were resuspended in lysis buffer. The supernatants were transferred to new tubes and centrifuged again at 12 000×g for 15 min, and the resulting supernatants, which represented cytosolic fractions, were separated. We analyzed 20–25 µg of protein from the cytosolic fraction of each sample by performing immunoblotting using an antibody against cytochrome c.
Measurement of mitochondrial membrane potential After a fluorescent probe was incorporated, the HeLa cells were incubated for up to 4 h with or without 25 µmol/L oridonin. At first, 1×106 HeLa cells/mL was incubated with 10 µmol/L rhodamine 123 for 10 min at 37 oC. At the end of the incubation, the cells were washed twice with PBS, harvested by means of centrifugation, and resuspended in 1.5 mL PBS. The fluorescent intensity of each cell suspension was measured at an excitation wavelength of 480 nm and an emission wavelength of 530 nm in a L15B fluorescence spectrophotometer (Perkin-Elmer, Waltham, MA, USA). The fluorescence intensity was recorded in arbitrary units representing the mitochondrial transmembrane potential.
Western blot analysis The HeLa cells were treated with oridonin and lysed, as previously described[25]. In brief, the cell pellets were resuspended in phosphorylation lysis buffer (0.5%–1.0% TritonX-100, 150 mmol/L NaCl, 1 mmol/L ethylenediamine tetraacetic acid, 200 µmol/L sodium orthovanadate, 10 µmol/L sodium pyrophosphate, 100 µmol/L sodium fluoride, 1.5 mmol/L magnesium chloride, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 µg/mL aprotinin). Protein concentrations were assessed using the Bradford assay before the samples were loaded. Equal amounts of proteins were separated by means of SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Immuno-blotting was performed with different antibodies and visualized using an enhanced chemiluminescence method.
Statistical analysis Data were expressed as mean±SD. Statistical analysis was performed by applying a one-way ANOVA followed by the Student’s t-test. P-values of less than 0.05 indicated statistical significance.
Results
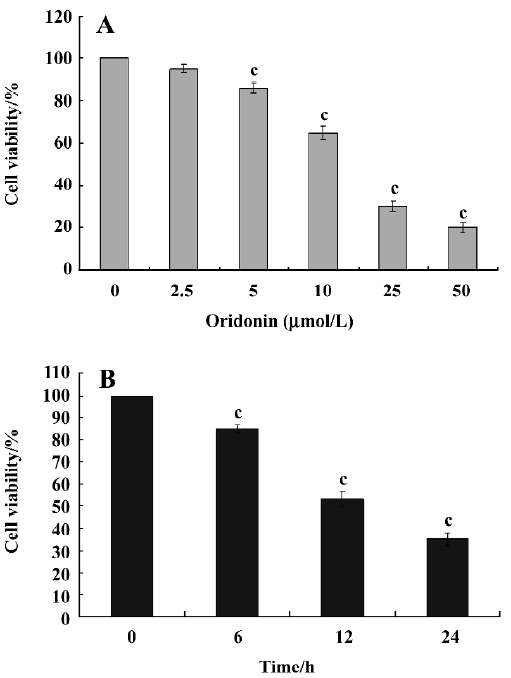
Cytotoxicity of oridonin in HeLa cells and apoptosis of HeLa cells When we investigated the effect of oridonin on HeLa cell survival, the cells were treated for 24 h in medium containing various oridonin concentrations (5–50 µmol/L). The cells were counted by performing MTT studies. Oridonin had a potent cytotoxic effect in HeLa cells in a dose- and time-dependent manner and was expressed as a percentage of cell viability (Figure 1A). The survival rate of the HeLa cells treated with 25 µmol/L oridonin started to decrease when treated for 6 h and sharply dropped after 12 h incubation (Figure 1B).When we examined several hallmarks of apoptosis (nuclear chromatin condensation and fragmentation of DNA by Hoechst 33342), oridonin induced apoptosis in human HeLa cells. In contrast to the control cells, the cells exposed to 25 µmol/L oridonin provoked nuclear chromatin condensation and fragmentation (Figure 2). On morphological examination, the oridonin-treated cells had changes typical of apoptosis, such as cell shrinkage, nuclear fragmentation, and formation of apoptotic bodies. When we quantified the apoptotic cells after oridonin treatment with FCM and propidium iodide staining, we identified apoptotic nuclei in the subdiploid region (sub-G1 peak) of the histo-grams. Figure 3 shows the rates of apoptosis in the control group compared with the cells treated with 25 µmol/L oridonin for 6, 12, and 24 h. These results suggested that oridonin treatment may suppress the growth of cervical carcinoma cells by inducing apoptosis.
Induced dephosphorylation of Akt and its substrates in HeLa cells Several lines of evidence appear to suggest that Akt may be a critical target for the discovery of novel anticancer drugs[26]. Oridonin inhibits the activation of Akt in other cell systems[27]. When we determined the expression and phosphorylation level of Akt in cervical carcinoma cells after treatment with various concentrations of oridonin, Akt was constitutively activated in HeLa cells, as determined by the expression of phosphorylated Akt at Ser473 and Thr308 (Figure 4A). Oridonin treatment dephosphorylated Akt at both sites. These findings suggested that Akt plays an important role in the survival of cervical carcinoma HeLa cells and their inactivation lead to their growth inhibition and apoptosis.
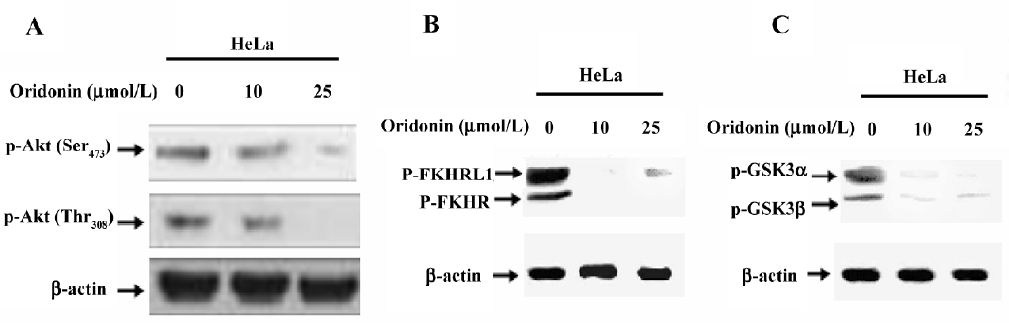
The forkhead family of transcription factors is reported as a downstream target of Akt-mediated apoptosis in other systems. Active transcription factors in the FKHR family promote the transcription of genes in cell cycle arrest and apoptosis[28]. One mechanism by which Akt promotes cell survival is by phosphorylating the transcription factors of the FKHR family, inactivating them, and preventing apoptosis. Because forkhead proteins are implicated as both Akt targets and a potential regulator of Fas ligand[29], we studied the status of forkhead phosphorylation in oridonin-treated and non-treated cervical carcinoma cell lines by performing Western blotting. The constitutive phosphorylation of FKHR was seen in the HeLa cell line (Figure 4B). Treatment with oridonin inhibited this phosphorylation in a dose-dependent manner.
We further investigated the effect of oridonin on the activation of GSK3 in the HeLa cell line. GSK3 is reported as a target of Akt and is involved in the promotion of cell survival[30]. The cervical carcinoma HeLa cell line treated with oridonin showed dephosphorylation of GSK3 (Figure 4C). These results suggested that HeLa cells expressed a high level of constitutive Akt and its downstream targets FOXO transcription factors and GSK3. The treatment of oridonin inactivated Akt and its downstream targets and thereby inhibited HeLa cell growth.
Changes of mitochondrial membrane potential and release of cytochrome c from mitochondria Previous studies have demonstrated that Akt activity inhibits the release of cytochrome c from mitochondria after UV irradiation[25]. Therefore, we sought to determine whether oridonin treatment causes the release of cytochrome c from mitochondria to the cytosol. This release was examined with Western blot analysis in HeLa cells after treatment with 25 µmol/L oridonin for 24 h. Cytosolic-specific, mitochondria-free lysates were prepared. Equal amounts of protein from the control and oridonin-treated samples were separated on SDS-PAGE and immunoblotted with an antibody against cytochrome c.
Oridonin induced the translocation of cytochrome c from the mitochondria to the cytosol in the HeLa cells (Figure 5A). When we further tested the effect of oridonin on the mitochondrial membrane potential in the HeLa cells, oridonin induced mitochondrial transmembrane depolarization, which was represented as a decrease in the potential. Oridonin-induced cytochrome c release was also observed in the HeLa cells; this was represented as a significant increase in the cytosolic cytochrome c concentration (Figure 5B). Cytochrome c release and mitochondrial membrane potential was analyzed spectrophotometrically. The data suggested that a loss of mitochondrial membrane potential may be required for oridonin-induced cytochrome c release into the cytosol, which later triggered the cleavage and activation of mitochondrial downstream caspases and the onset of apoptosis.
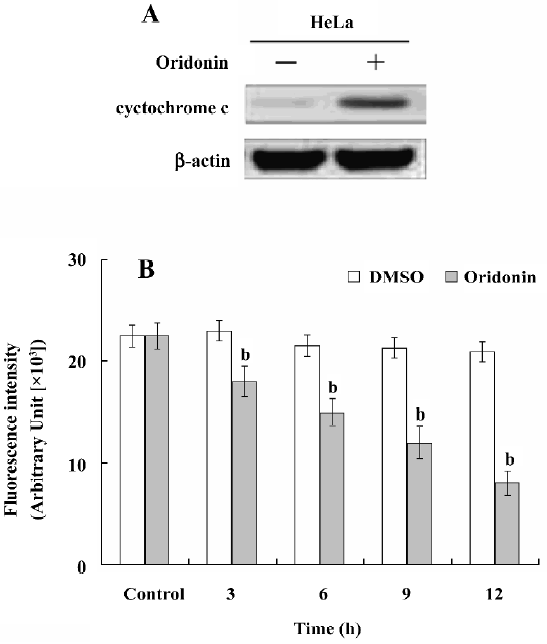
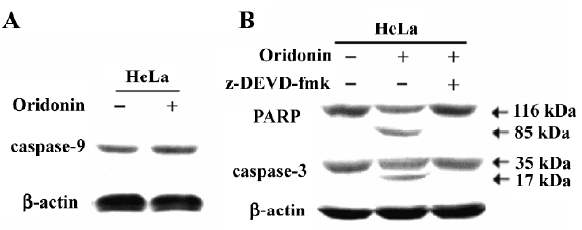
Activation of caspases-9 and -3 and PARP cleavage in HeLa cells We investigated whether the oridonin treatment of HeLa cells activates caspases-9 and -3 and promotes cleavage of PARP further downstream in the apoptotic pathway. Oridonin treatment activated caspases-9 and -3 in HeLa cells (Figure 5). On caspase-3 activity assay after treatment for 24 h, 25 µmol/L oridonin activated caspase-3 in HeLa cells. These results were consistent with the data on cytochrome c release and indicated that the activation of effector caspases participate in oridonin-induced apoptosis in HeLa cells. When we used the cell permeable caspase-3 inhibitor (z-DEVD-fmk), oridonin activated caspase-3, and cleaved PARP. However z-DEVD-fmk pretreatment abolished this induced activation and cleavage (Figure 6).
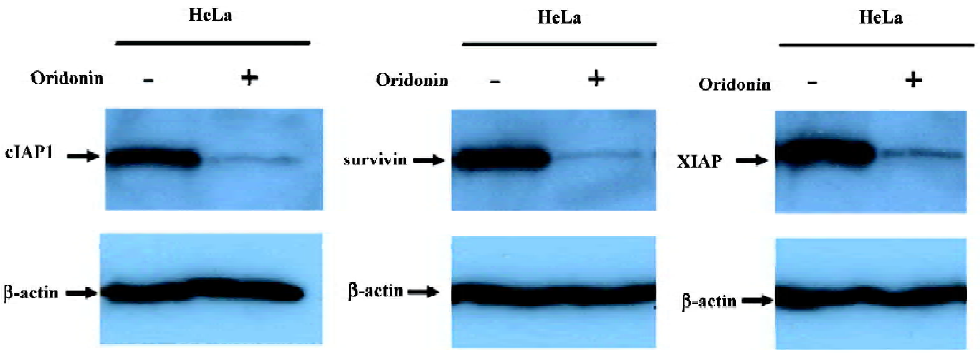
Modulation of the IAP protein family in oridonin-induced apoptosis in the HeLa cell line IAP are physiological substrates of Akt that are stabilized to inhibit programmed cell death. IAP have direct effects on caspases-9 and -3[31]. Therefore, we also determined whether oridonin induced cell death by modulating the expression of IAP family members that ultimately determine the cellular response to apoptotic stimuli. The HeLa cells lines were treated with 25 µmol/L oridonin for 24 h, and the expression of cIAP1, XIAP, and survivin were determined by Western blotting. Oridonin treatment caused the downregulation of cIAP1, XIAP, and survivin (Figure 7). These results suggested that Akt may modulate these survival proteins for the survival of HeLa cells.
Discussion
Apoptosis plays an important role in the maintenance of tissue homeostasis by selectively eliminating excessive cells. At present, many researchers focus on the ability of natural and dietary agents to induce cancer cell apoptosis to seek new anticancer drugs. In the present study, we demonstrated that oridonin significantly inhibited HeLa cell growth and induced apoptosis in a time- and dose-dependent manner. HeLa cells treated with oridonin exhibited characteristic morphological features of apoptosis, such as membrane shrinkage, chromosomal condensation, and increased caspase-3 activity. Moreover, the pre-incubation of cells with z-DEVD-fmk, a specific caspase-3 inhibitor, effectively inhibited caspase-3 activity and prevented oridonin-induced cell death. Oridonin induced apoptosis in HeLa cells in a caspase-dependent apoptotic pathway.
Signal transduction pathways involved in growth and apoptosis are potential targets for chemopreventive agents. Oridonin, which is potently antitumorigenic, has well-documented pro-apoptotic properties in a variety of cell types[6–9]. The oridonin inhibition of NF-κβ activity is accompanied by apoptosis in various types of cancer cells[13]. Apoptosis is a multistep process, and an increasing number of genes involved in the control or execution of apoptosis has been identified. Our data showed that oridonin-induced HeLa cell growth inhibition was accompanied by the inhibition of Akt activity. Oridonin inhibited the Akt phosphorylation at Ser473 and Thr308. For the full activation of Akt, cytokines and growth factors must phosphorylate Akt at these sites[32]. This observation further supports our hypothesis that the inhibition of Akt activity by oridonin plays a critical role in inducing growth inhibition by means of apoptosis. Studies have also demonstrated that Akt activity inhibits the release of cytochrome c from mitochondria after UV irradiation[25]. In addition, constitutively activated Akt significantly protected distal hereditary motor neuronopathy (HMN1) cells from apoptosis induced by C2-ceramide[26].Our results are in agreement with the finding that oridonin inhibited the constitutive activity of Akt in HeLa cells accompanied with the release of cytochrome c from mitochondria into the cytoplasm. This result suggested a link between mitochondria and oridonin-induced cell growth inhibition of cervical carcinoma HeLa cells.
On exposure to oridonin, we also observed a progressive decrease in the mitochondrial membrane potential and release of cytochrome c into the cytosol in HeLa cells. In many in vitro systems, apoptosis is associated with a loss of mitochondrial membrane potential, which may correspond to the opening of an outer membrane pore. Therefore, some have suggested that this event is responsible for the cytosolic release of cytochrome c from mitochondria[33]. In this study, the accumulation of cytosolic cytochrome c in oridonin-induced human HeLa cells was probably the consequence of the loss of mitochondrial membrane potential, which finally led to cell death. Members of the Bcl-2 homology family of proteins facilitate mitochondrial-mediated apoptosis[34]. Reduced mitochondrial membrane potential leads to the release of intermembrane proteins, such as cytochrome c and apoptosis-inducing factors into the cytosol and induces apoptosis[35].
In the cytosol, cytochrome c binds to Apaf-1 and triggers Apaf-mediated caspase-9 activation, which activates the death signal by activating caspase-3 and other caspases[36]. Our data demonstrated that oridonin-mediated cytochrome c release activated caspases-9 and -3 and cleaved PARP. The expression of IAP is correlated with resistance to apoptosis in transformed cell types and in a variety of human tumors cells[37,38]. Akt regulates the expression of many survival genes, including those encoding IAP[39]. Our results showed that cervical carcinoma cells expressed IAP, including cIAP1, XIAP, and survivin, and that oridonin treatment decreased the expression level of these molecules. This finding suggested that the downregulation of IAP may be involved in the activation of caspases-9 and -3 in oridonin-induced apoptosis.
In conclusion, oridonin induced apoptosis in cervical carcinoma HeLa cells. The treatment of HeLa cells with oridonin activated a cell-death pathway that regulated mitochondrial membrane permeability by downregulating Akt kinase signaling, triggering the release of cytochrome c from the mitochondria into the cytosol. Loss of mitochondrial membrane potential may be required for oridonin to induce the release of cytochrome c, which led to the activation of the downstream caspases-9 and -3 and the downregulation of the survival proteins (cIAP1, XIAP, and survivin), resulting in the death of cervical carcinoma cells. Taken together, our data suggests that oridonin may be a promising candidate as an antitumor agent against human cervical carcinoma.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden:Globoscan 2000. Int J Cancer 2001;94:153-6.
- de la Taille A, Hayek OR, Burchardt M, Burchardt T, Katz AE. Role of herbal compounds (PC-SPES) in hormone-refractory prostate cancer: two case reports. J Altern Complement Med 2000;6:449-51.
- Darzynkiewicz Z, Traganos F, Wu JM, Chen S. Chinese herbal mixture PC SPES in treatment of prostate cancer Int J Oncol 2000;17:729-36. (review).
- Leung CH, Grill SP, Lam W, Han QB, Sun HD, Cheng YC. Novel mechanism of inhibition of nuclear factor-kappa B DNA-binding activity by diterpenoids isolated from Isodon rubescens. Mol Pharmacol 2005;68:286-97.
- Liu HM, Yan X, Kiuchi F, Liu Z. A new diterpene glycoside from Rabdosia rubescens. Chem Pharm Bull (Tokyo) 2000;48:148-9.
- Ikezoe T, Yang Y, Bandobashi K, Saito T, Takemoto S, Machida H, et al. Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits the proliferation of cells from lymphoid malignancies in association with blockade of the NF-kappa B signal pathways. Mol Cancer Ther 2005;4:578-86.
- Hsieh TC, Wijeratne EK, Liang JY, Gunatilaka AL, Wu JM. Differential control of growth, cell cycle progression, and expression of NF-kappaB in human breast cancer cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids from the Chinese herb Rabdosia rubescens. Biochem Biophys Res Commun 2005;337:224-31.
- Zhang JF, Liu JJ, Liu PQ, Lin DJ, Li XD, Chen GH. Oridonin inhibits cell growth by induction of apoptosis on human hepatocellular carcinoma BEL-7402 cells. Hepatol Res 2006;35:104-10.
- Liu JJ, Huang RW, Lin DJ, Peng J, Wu XY, Pan XL, et al. Anti-proliferative effects of oridonin on SPC-A-1 cells and its mechanism of action. J Int Med Res 2004;32:617-25.
- Jin S, Shen JN, Wang J, Huang G, Zhou JG. Oridonin induced apoptosis through Akt and MAPKs signaling pathways in human osteosarcoma cells. Cancer Biol Ther 2007;6:261-8.
- Liu YQ, You S, Tashiro S, Onodera S, Ikejima T. Activation of phosphoinositide 3-kinase, protein kinase C, and extracellular signal-regulated kinase is required for oridonin-enhanced phagocytosis of apoptotic bodies in human macrophage-like U937 cells. J Pharmacol Sci 2005;98:361-71.
- Huang J, Wu L, Tashiro S, Onodera S, Ikejima T. Bcl-2 up-regulation and P-p53 down-regulation account for the low sensitivity of murine L929 fibrosarcoma cells to oridonin-induced apoptosis. Biol Pharm Bull 2005;28:2068-74.
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2002;2:489-501.
- Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, et al. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis 2002;23:201-5.
- Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA 1998; 95: 15 587–91.
- Leslie NR, Gray A, Pass I, Orchiston EA, Downes CP. Analysis of the cellular functions of PTEN using catalytic domain and C-terminal mutations: differential effects of C-terminal deletion on signalling pathways downstream of phosphoinositide 3-kinase. Biochem J 2000;346:827-33.
- Li BS, Ma W, Zhang L, Barker JL, Stenger DA, Pant HC. Activation of phosphatidylinositol-3-kinase (PI-3K) and extracellular regulated kinases (ERK1/2) is involved in muscarinic receptor mediated DNA synthesis in neural progenitor cells. J Neurosci 2001;21:1569-79.
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997;91:231-41.
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995;378:785-9.
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science 1998;282:1318-21.
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999;96:857-68.
- Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signaling. Nature 1999;401:86-90.
- Osawa K, Yasuda H, Maruyama T, Morita H, Takeya K, Itokawa H. Antibacterial trichorabdal diterpenes from Rabdosia trichocarpa. Phytochemistry 1994;36:1287-91.
- Qi YL, Liao F, Zhao CQ, Lin YD, Zuo MX. Cytotoxicity, apoptosis induction, and mitotic arrest by a novel podophyllotoxin glucoside, 4DPG, in tumor cells. Acta Pharmacol Sin 2005;26:1000-8.
- Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 1999;19:5800-10.
- Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol 2000;151:483-94.
- Cui Q, Tashiro S, Onodera S, Ikejima T. Augmentation of oridonin-induced apoptosis observed with reduced autophagy. J Pharmacol Sci 2006;101:230-9.
- Alvarez B, Martinez-A C, Burgering BM, Carrera AC. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature 2001;413:744-7.
- Ciechomska I, Pyrzynska B, Kazmierczak P, Kaminska B. Inhibition of Akt kinase signalling and activation of forkhead are indispensable for upregulation of FasL expression in apoptosis of glioma cells. Oncogene 2003;23:7617-27.
- Sawyers CL. Rational therapeutic intervention in cancer: kinases as drug targets. Curr Opin Genet Dev 2002;12:111-5.
- Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J Biol Chem 2004;279:5405-12.
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 1996;15:6541-51.
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 2002;9:505-12.
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004;305:626-9.
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2 release of cytochrome c from mitochondria blocked. Science 1997;275:1129-32.
- Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multi-meric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 1999; 274: 11 549–56.
- Adida C, Crotty PL, McGrath J, Berrebi D, Diebold J, Altieri DC. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol 1998;152:43-9.
- LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 1998;17:3247-59.
- Kornacker M, Verneris MR, Kornacker B, Scheffold C, Negrin RS. Survivin expression correlates with apoptosis resistance after lymphocyte activation and is found preferentially in memory T cells. Immunol Lett 2001;76:169-73.
